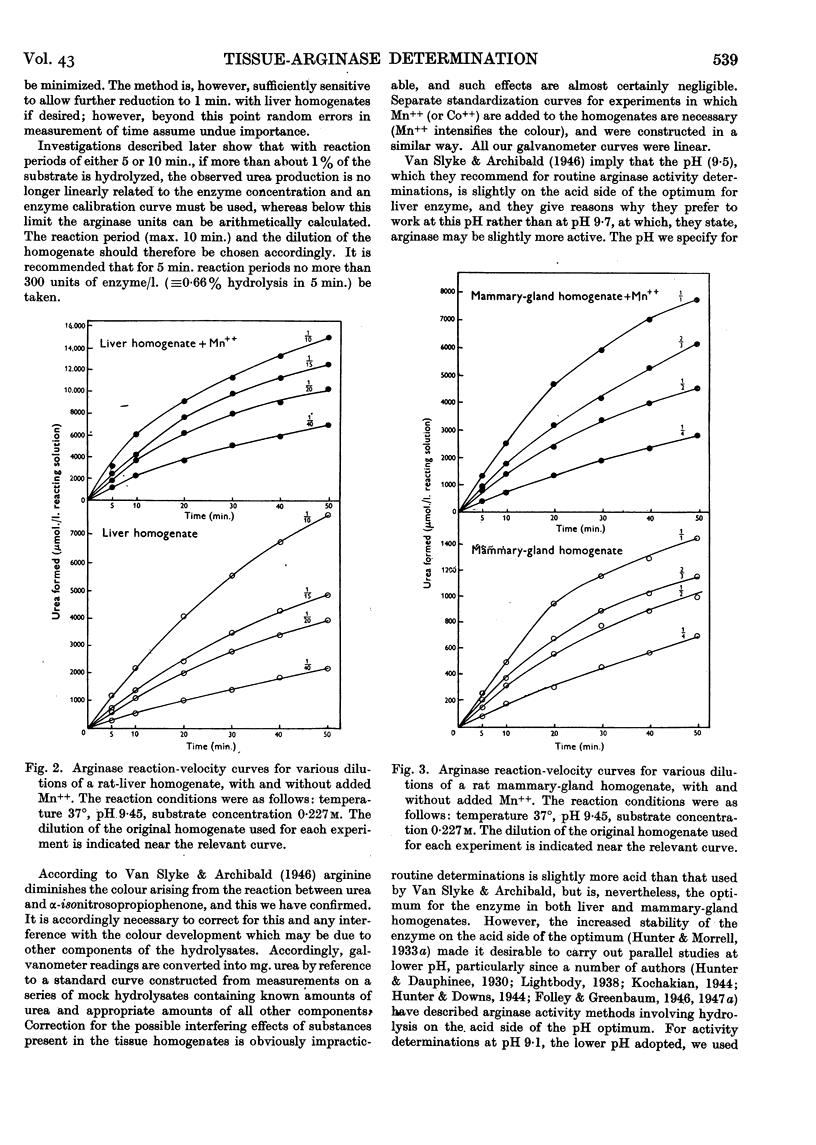
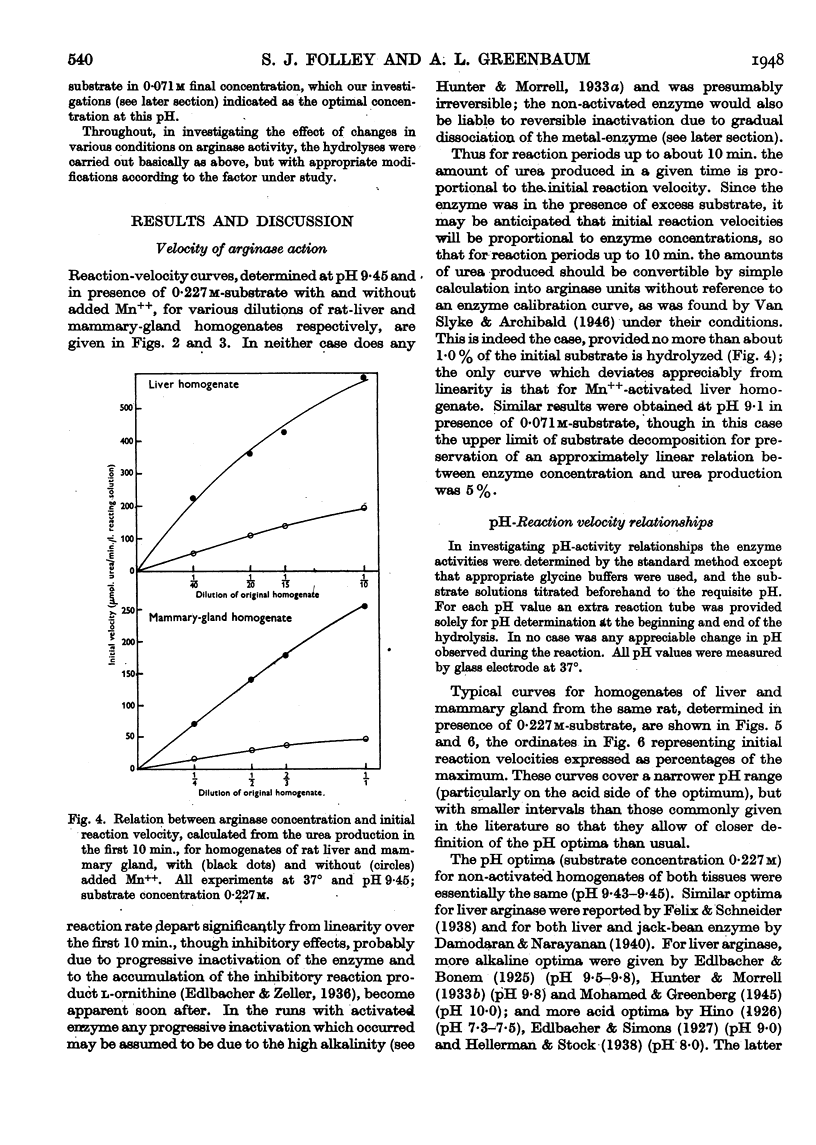
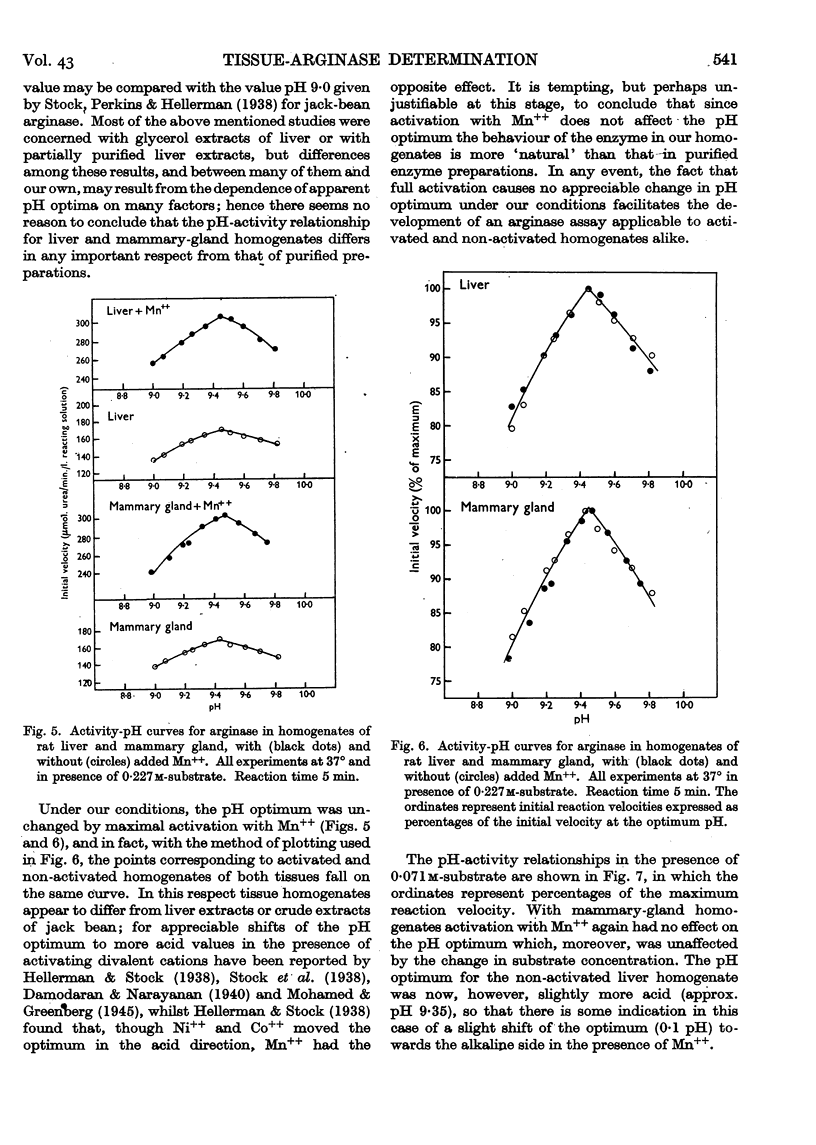
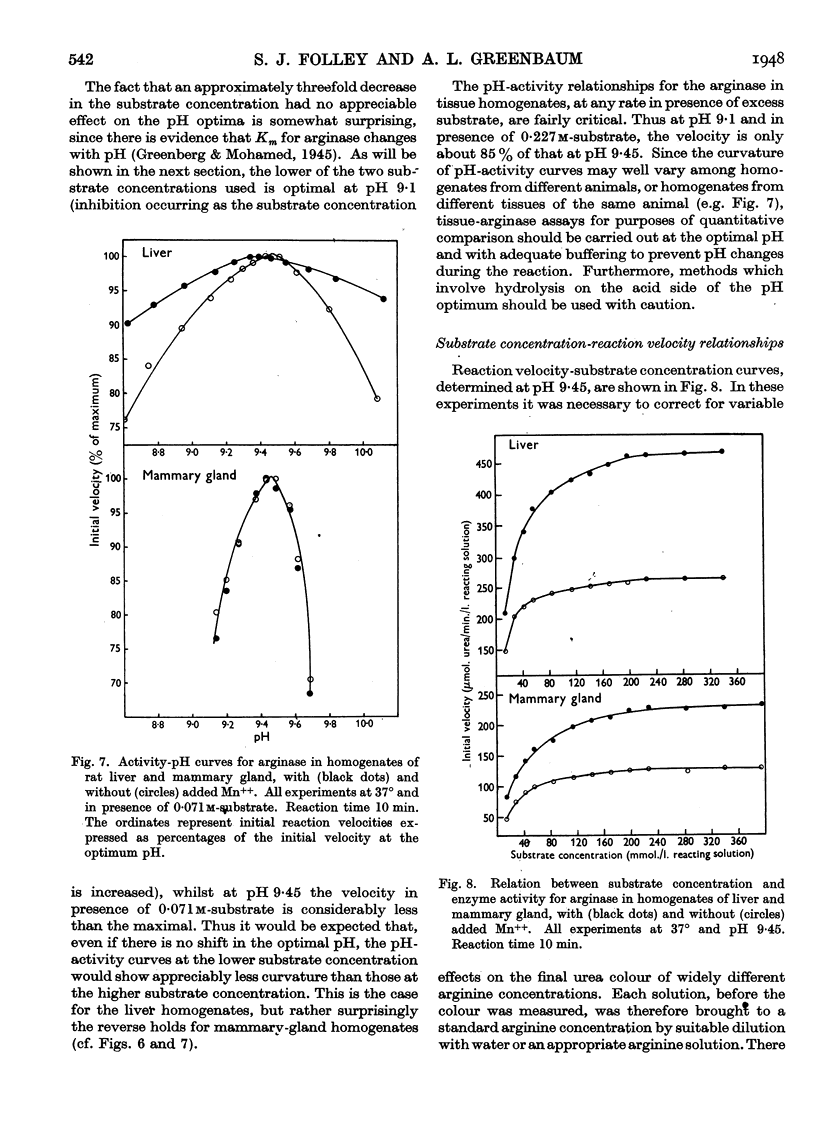
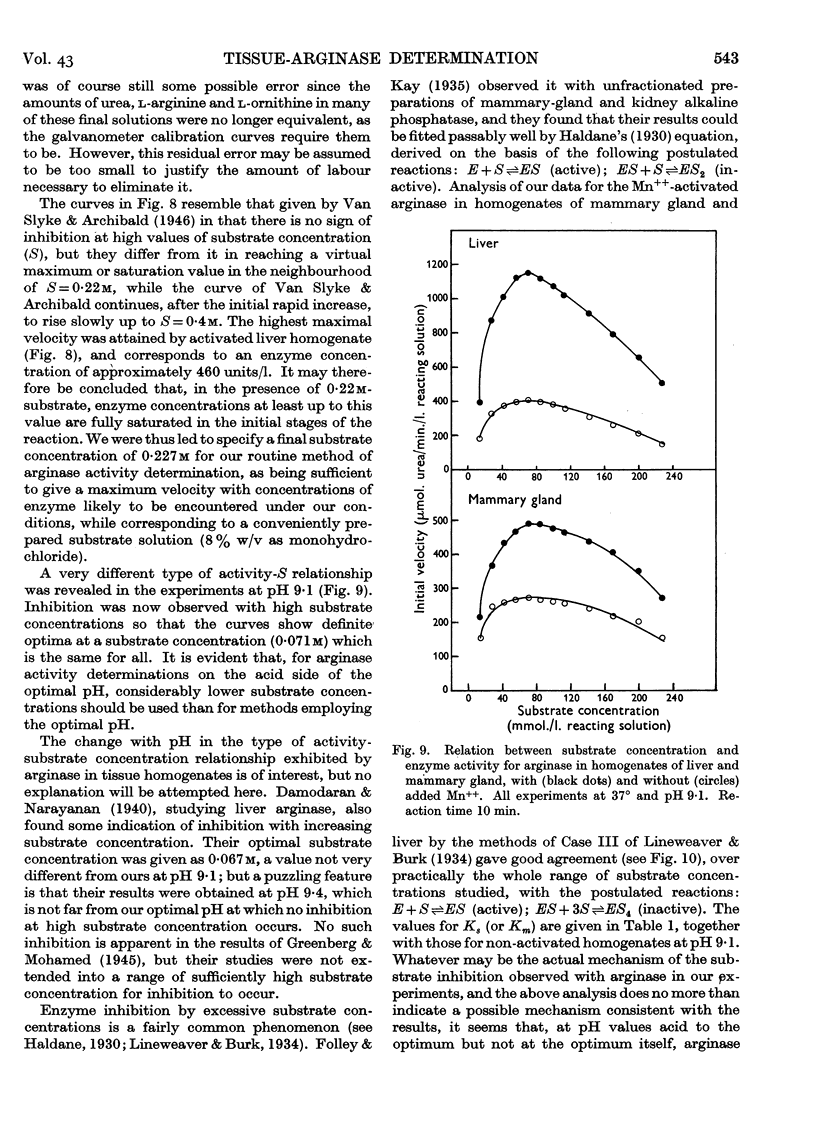
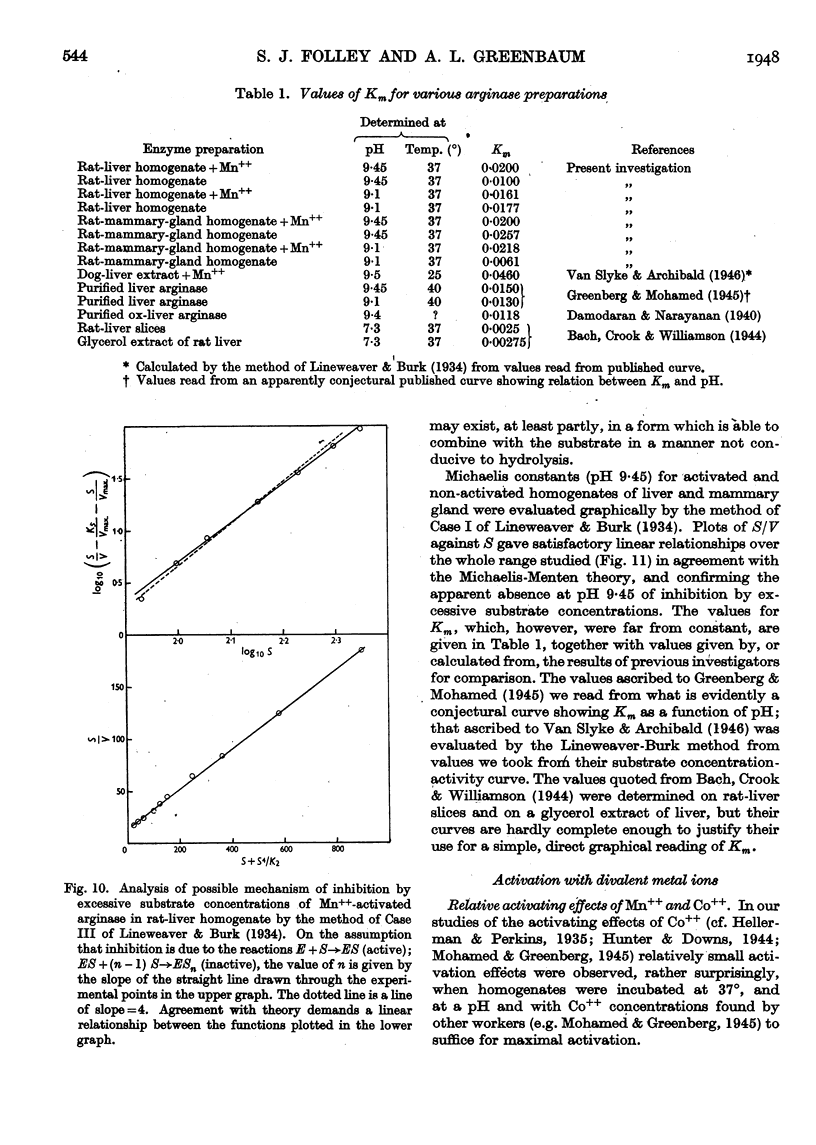
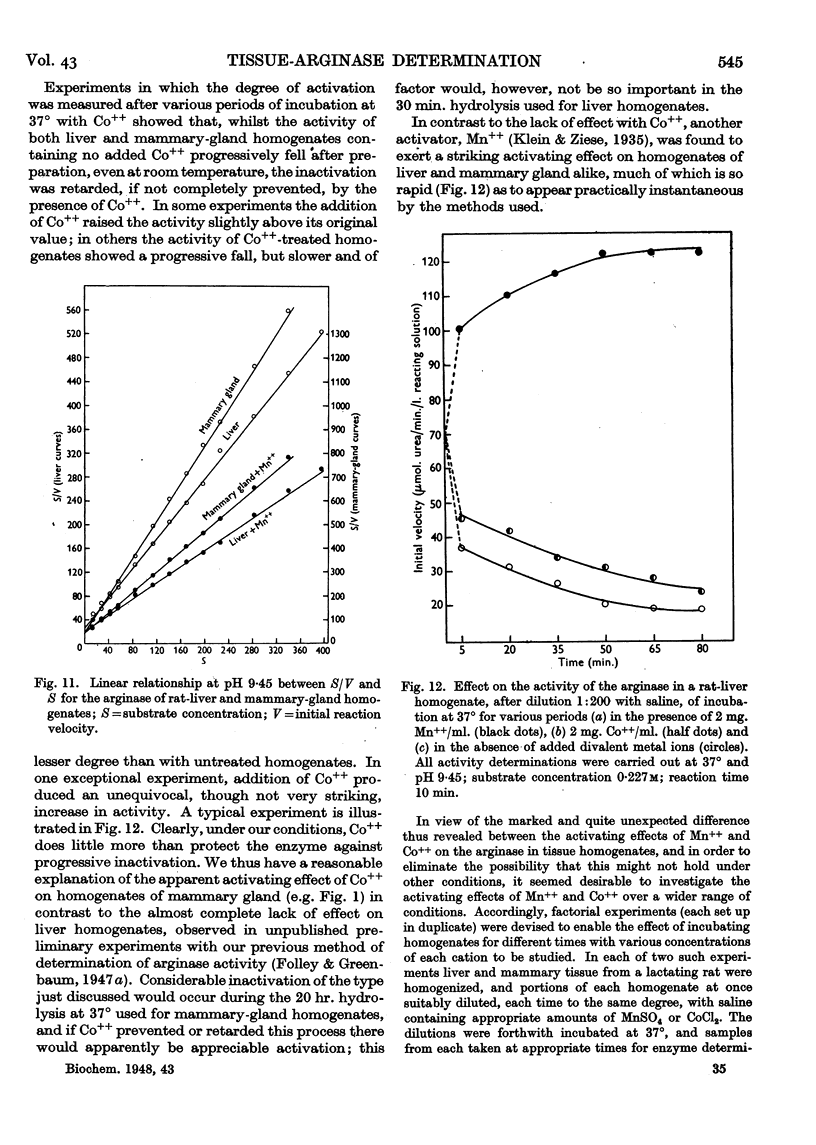
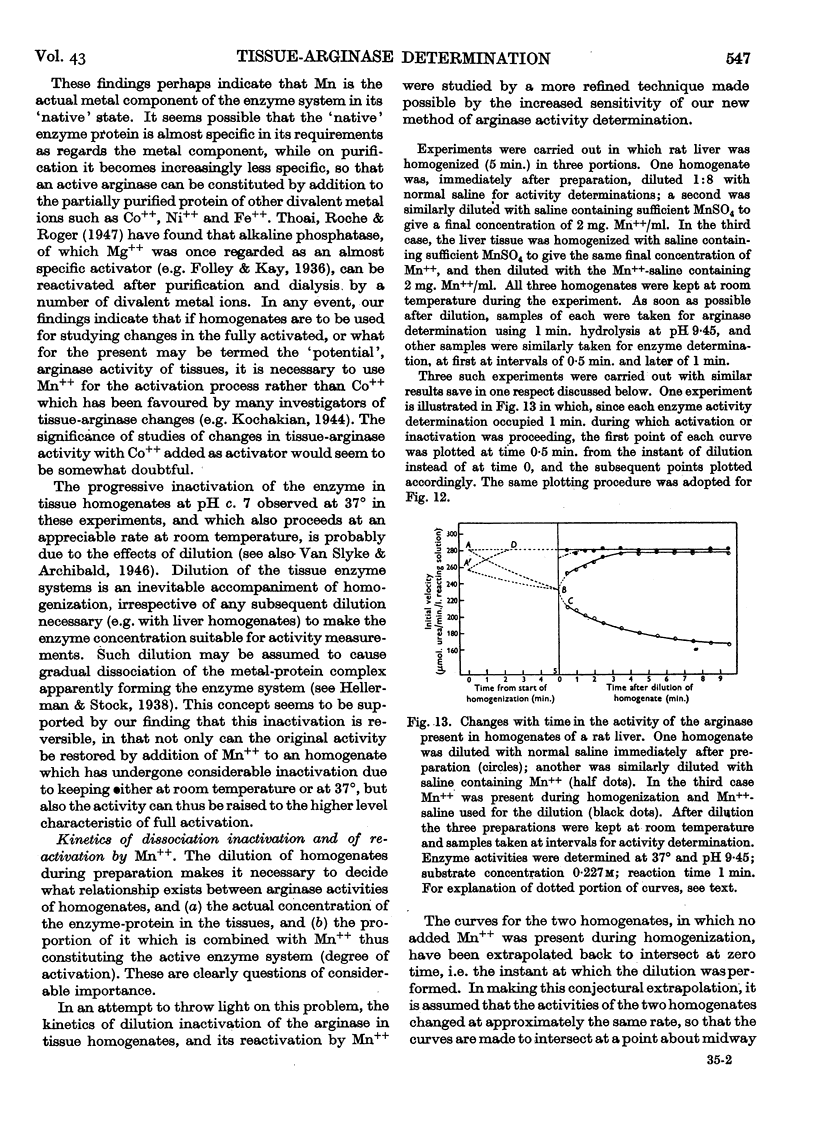
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach S. J., Crook E. M., Williamson S. On arginase and its participation in urea synthesis in the liver. Biochem J. 1944;38(4):325–332. doi: 10.1042/bj0380325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damodaran M., Narayanan K. G. A comparative study of arginase and canavanase. Biochem J. 1940 Nov;34(10-11):1449–1459. doi: 10.1042/bj0341449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folley S. J., Greenbaum A. L. Changes in the arginase and alkaline phosphatase contents of the mammary gland and liver of the rat during pregnancy, lactation and mammary involution. Biochem J. 1947;41(2):261–269. doi: 10.1042/bj0410261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folley S. J., Greenbaum A. L. Effects of adrenalectomy and of treatment with adrenal cortex hormones on the arginase and phosphatase levels of lactating rats. Biochem J. 1946;40(1):46–51. doi: 10.1042/bj0400046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folley S. J., Kay H. D. The alkaline phosphomonoesterase of the mammary gland. Biochem J. 1935 Aug;29(8):1837–1850. doi: 10.1042/bj0291837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folley S. J., Watson S. C. A high-speed tissue homogenizer. Biochem J. 1948;42(2):204–206. doi: 10.1042/bj0420204. [DOI] [PMC free article] [PubMed] [Google Scholar]


