Full text
PDF

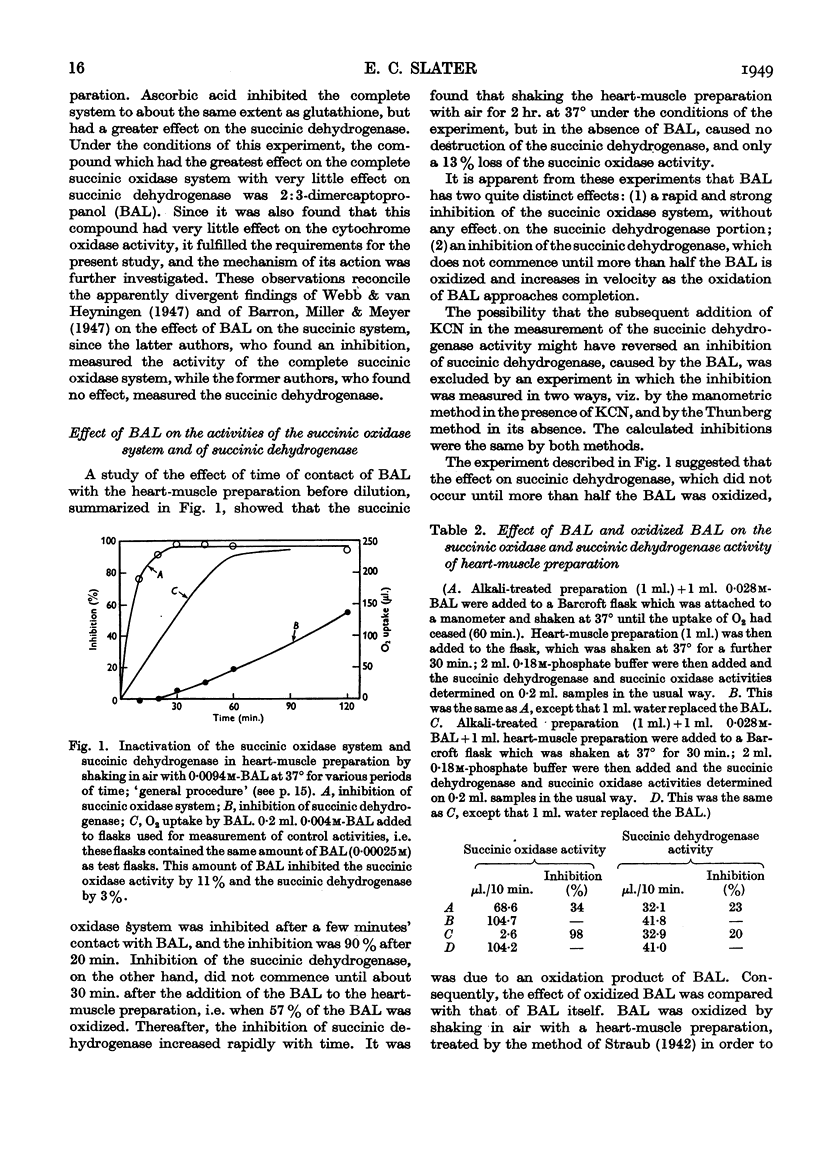
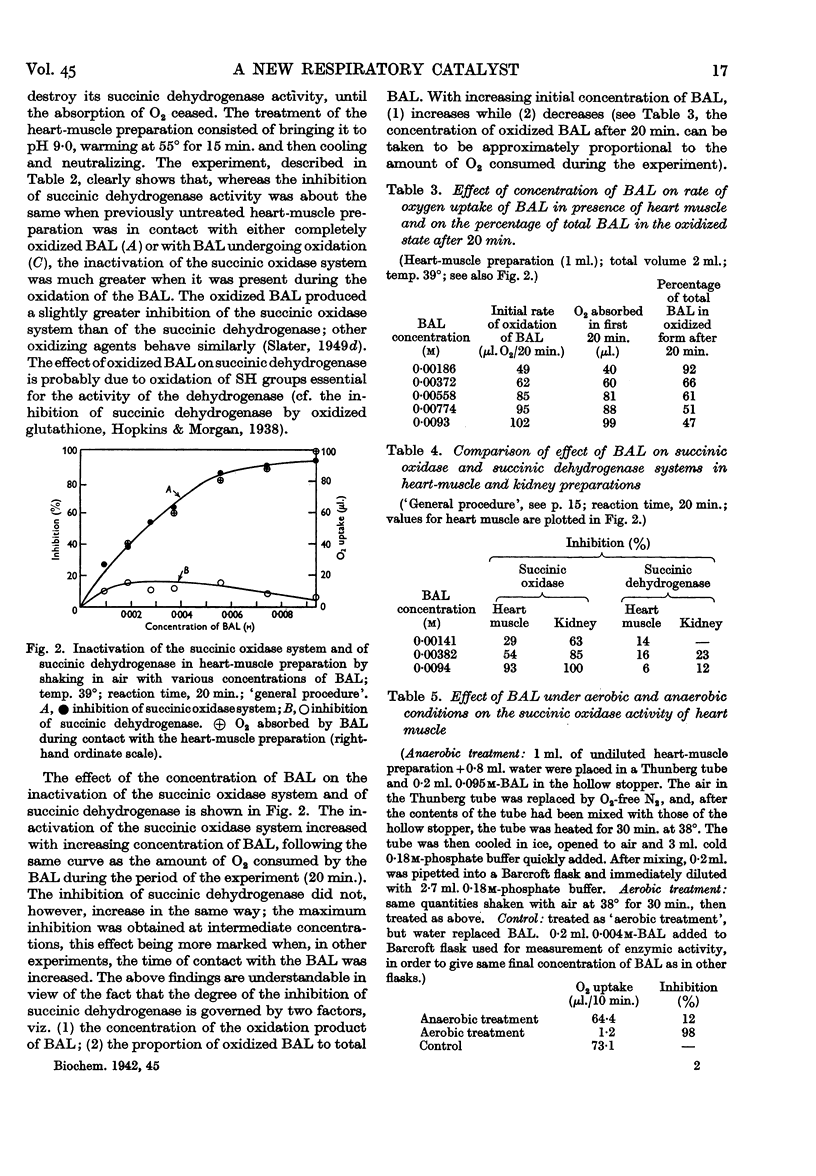
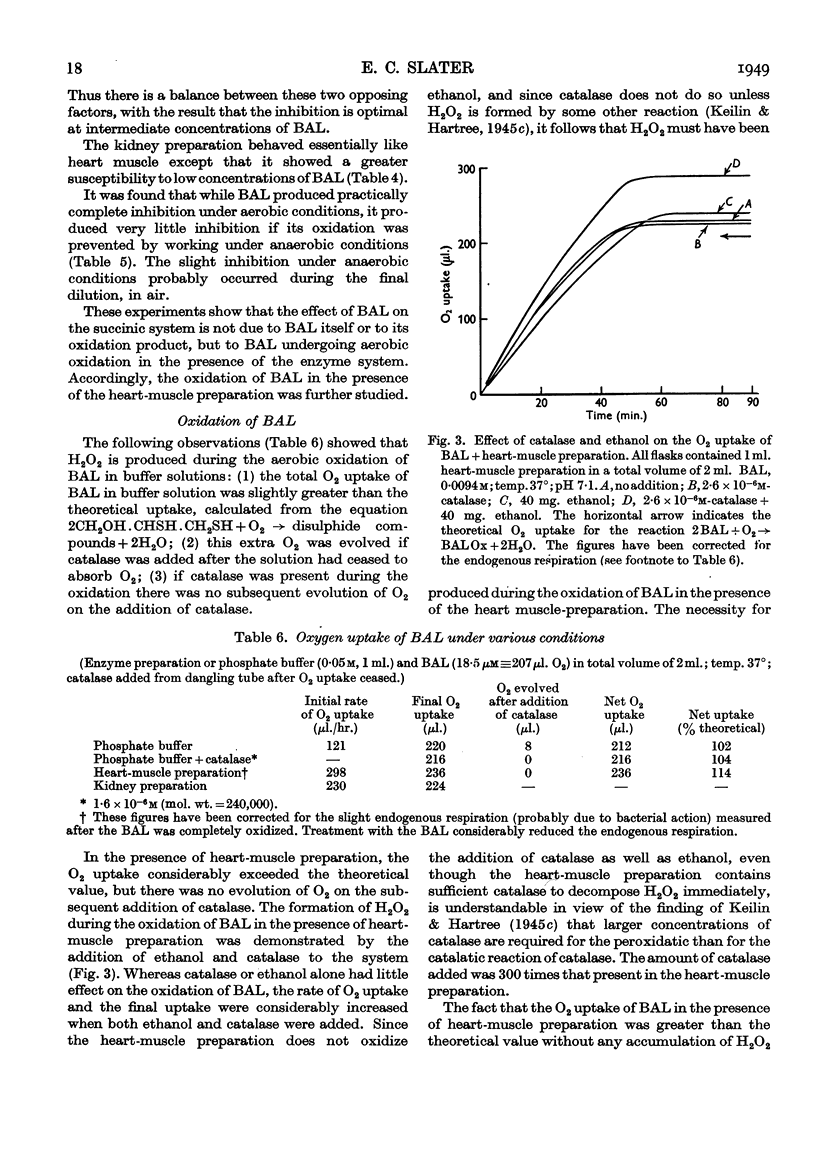
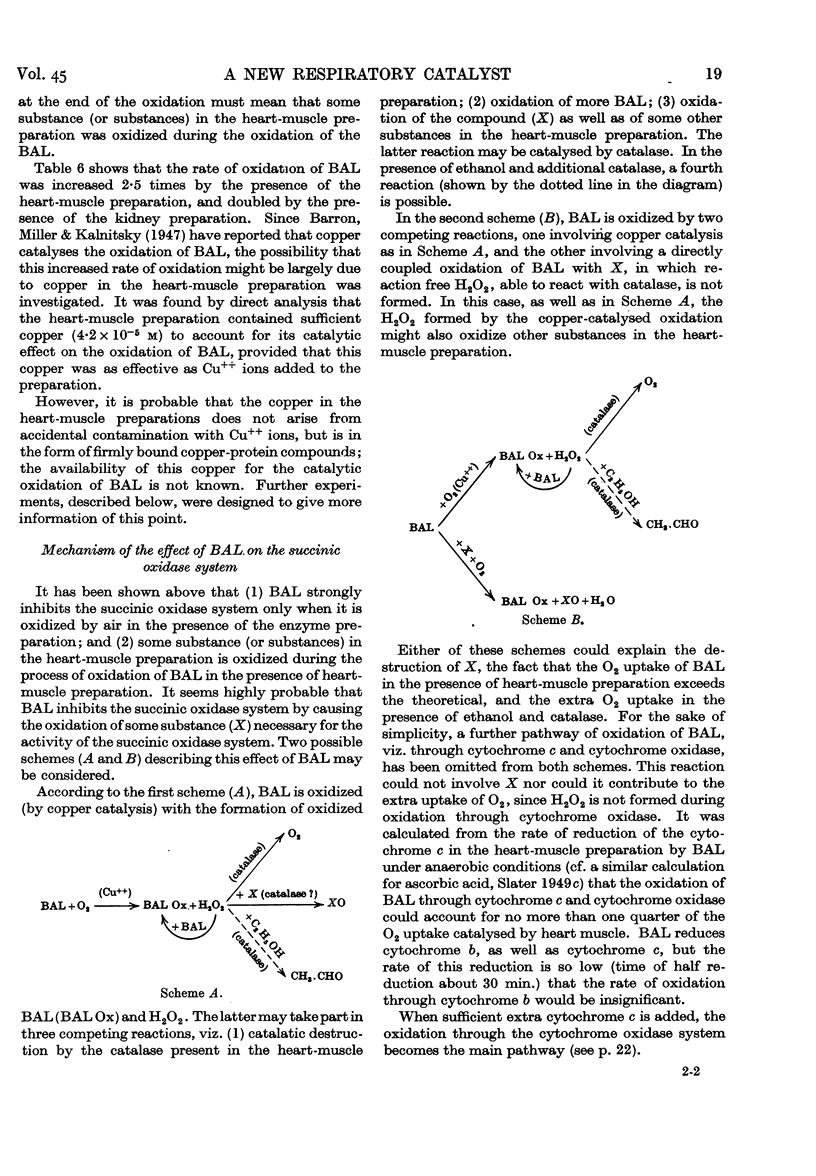







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach S. J., Dixon M., Zerfas L. G. Yeast lactic dehydrogenase and cytochrome b(2). Biochem J. 1946;40(2):229–239. [PMC free article] [PubMed] [Google Scholar]
- Barron E. S., Miller Z. B., Kalnitsky G. The oxidation of dithiols. Biochem J. 1947;41(1):62–68. doi: 10.1042/bj0410062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron E. S., Miller Z. B., Meyer J. The effect of 2:3-dimercaptopropanol on the activity of enzymes and on the metabolism of tissues. Biochem J. 1947;41(1):78–82. doi: 10.1042/bj0410078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins F. G., Morgan E. J., Lutwak-Mann C. The influence of thiol groups in the activity of dehydrogenases. II: With an addendum on the location of dehydrogenases in muscle. Biochem J. 1938 Oct;32(10):1829–1848. doi: 10.1042/bj0321829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins F. G., Morgan E. J. The influence of thiol-groups in the activity of dehydrogenases. Biochem J. 1938 Mar;32(3):611–620. doi: 10.1042/bj0320611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Activity of the succinic dehydrogenase-cytochrome system in different tissue preparations. Biochem J. 1949;44(2):205–218. doi: 10.1042/bj0440205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of azide-catalase. Biochem J. 1945;39(2):148–157. doi: 10.1042/bj0390148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of catalase. Catalysis of coupled oxidation of alcohols. Biochem J. 1945;39(4):293–301. [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Purification and properties of cytochrome c. Biochem J. 1945;39(4):289–292. doi: 10.1042/bj0390289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg R., Legge J. W., Lockwood W. H. Coupled oxidation of ascorbic acid and haemoglobin: Formation and properties of choleglobin. Biochem J. 1941 Mar;35(3):328–338. doi: 10.1042/bj0350328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane W. D. Application of the sodium diethyldithiocarbamate reaction to the micro-colorimetric determination of copper in organic substances. Biochem J. 1932;26(4):1022–1033. doi: 10.1042/bj0261022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirie N. W. The preparation of glutathione from yeast and liver. Biochem J. 1930;24(1):51–54. doi: 10.1042/bj0240051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proceedings of the Biochemical Society. Biochem J. 1948;42(1):i.1–i.x. [PMC free article] [PubMed] [Google Scholar]
- Quastel J. H., Wheatley A. H. The relation of thiol compounds to glucose fermentation. Biochem J. 1932;26(6):2169–2176. doi: 10.1042/bj0262169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C. A comparative study of the succinic dehydrogenase-cytochrome system in heart muscle and in kidney. Biochem J. 1949;45(1):1–8. doi: 10.1042/bj0450001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C. The action of inhibitors on the system of enzymes which catalyse the aerobic oxidation of succinate. Biochem J. 1949;45(1):8–13. doi: 10.1042/bj0450008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C. The measurement of the cytochrome oxidase activity of enzyme preparations. Biochem J. 1949;44(3):305–318. doi: 10.1042/bj0440305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb E. C., Van Heyningen R. The action of British anti-lewisite (BAL) on enzyme systems. Biochem J. 1947;41(1):74–78. doi: 10.1042/bj0410074. [DOI] [PMC free article] [PubMed] [Google Scholar]


