Abstract
1. We investigated the role of calcium and albumin on the perfusate flow in isolated perfused rat kidneys. The initial perfusion medium was Krebs-Henseleit bicarbonate solution with 1.82 mm-calcium without albumin. Perfusate flow autoregulation occurred above 100 mmHg.
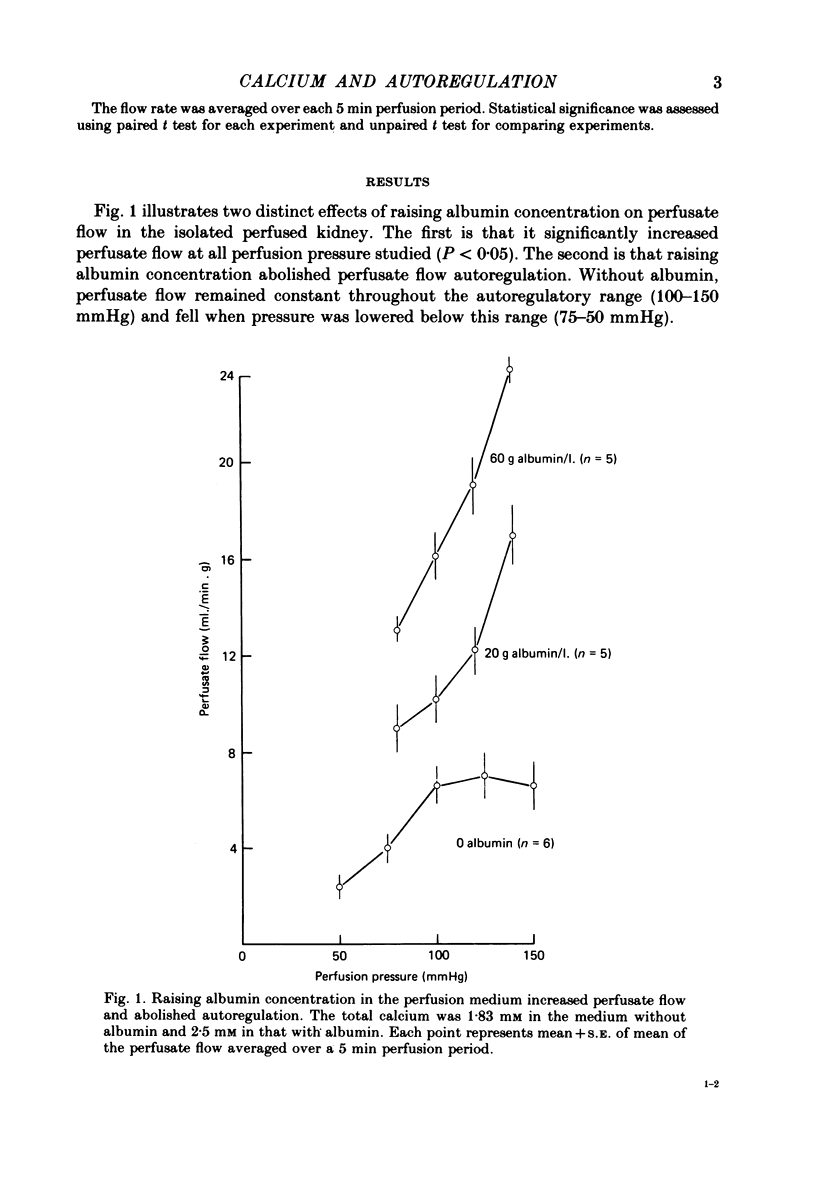
2. Raising albumin concentration to 20 and 60 g/l. abolished autoregulation and increased perfusate flow.
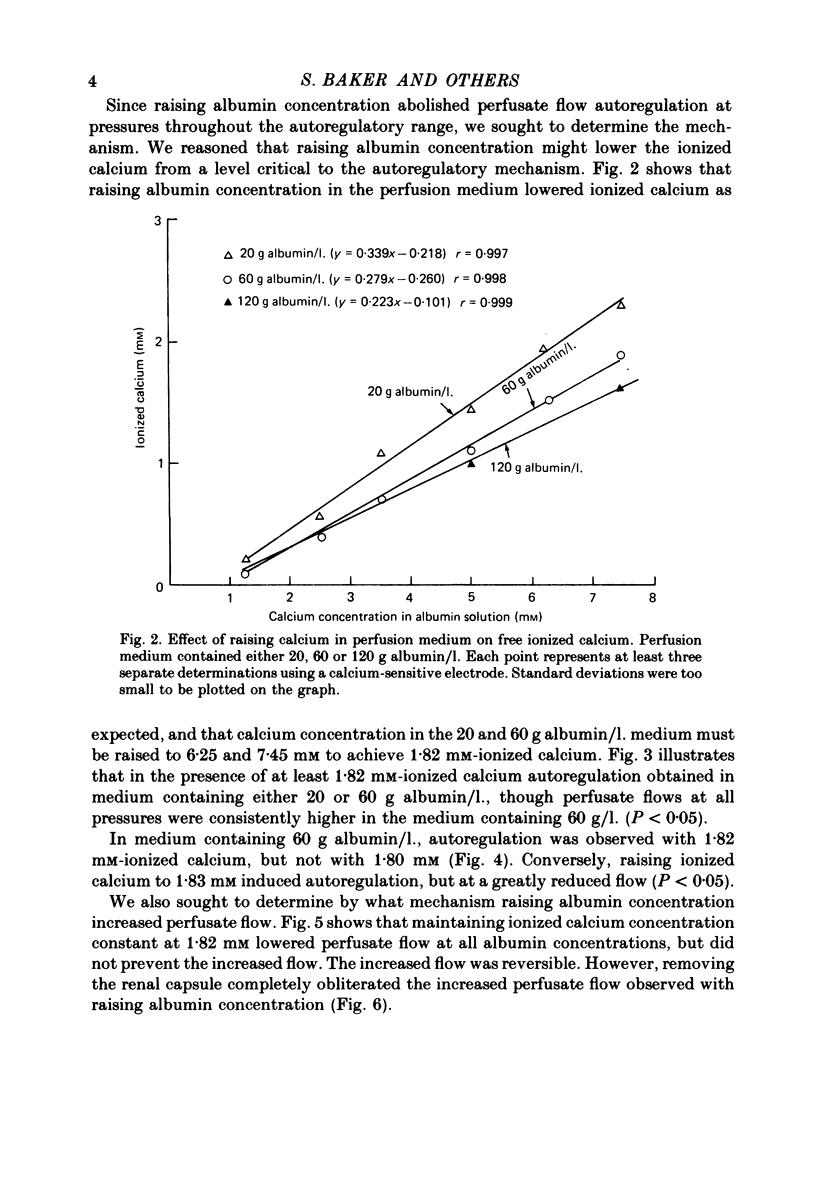
3. Keeping ionized calcium at 1.82 mm restored autoregulation in medium containing 20 and 60 g albumin/l. However, in 60 g albumin/l. autoregulation occurred at a significantly higher flow.
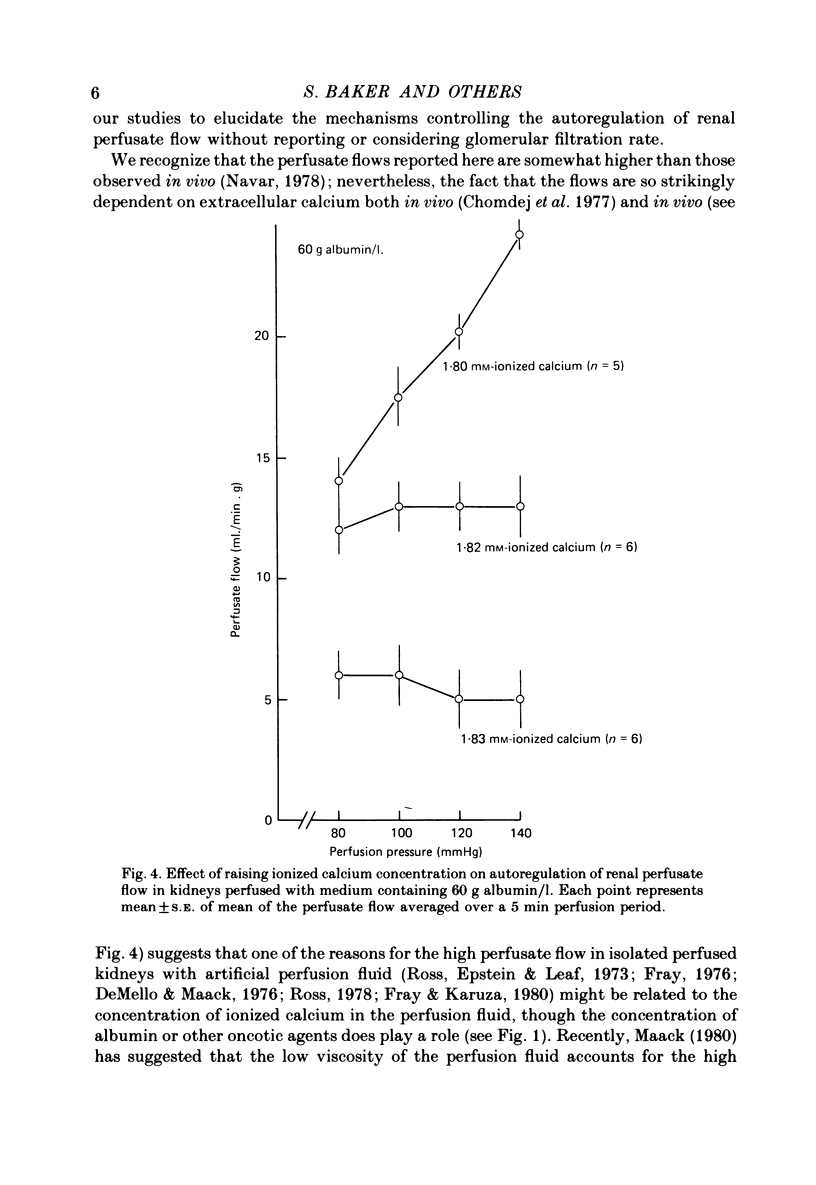
4. 1.82 mm-ionized calcium appears to be a critical level for autoregulation of flow in these experiments, for autoregulation was not obtained in 60 g albumin/l. medium containing 1.80 mm-ionized calcium. On the other hand, autoregulation occurred in medium containing 1.83 mm-ionized calcium, but at a lower perfusate flow.
5. Raising albumin concentration to 120 g/l. increased perfusate flow from 14.6±0.8 to 20.8±0.7 ml./min.g (n = 5, P < 0.01) in the presence of 1.82 mm-total calcium, and from 11.6±1.0 to 15.8±0.7 ml./min.g (n = 5, P < 0.01) in 1.82 mm-ionized calcium. The effect of raising albumin concentration was reversible.
6. Removing the capsule from the kidneys abolished the increased flow in response to raising albumin concentration.
7. We conclude that (a) the mechanism for the autoregulation of renal perfusate flow in isolated perfused kidneys is critically dependent on an extremely narrow range of ionized calcium concentration in the perfusion medium, below this range, autoregulation is not achieved; above it, however, autoregulation is achieved, but during intense vasoconstriction; (b) raising albumin concentration in the perfusion medium increases perfusate flow and abolishes autoregulation by lowering extracellular ionized calcium and by raising intrarenal tissue pressure.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke T. J., Navar L. G., Clapp J. R., Robinson R. R. Response to single nephron glomerular filtration rate to distal nephron microperfusion. Kidney Int. 1974 Oct;6(4):230–240. doi: 10.1038/ki.1974.104. [DOI] [PubMed] [Google Scholar]
- Chomdej B., Bell P. D., Navar L. G. Renal hemodynamic and autoregulatory responses to acute hypercalcemia. Am J Physiol. 1977 Jun;232(6):F490–F496. doi: 10.1152/ajprenal.1977.232.6.F490. [DOI] [PubMed] [Google Scholar]
- De Mello G., Maack T. Nephron function of the isolated perfused rat kidney. Am J Physiol. 1976 Dec;231(6):1699–1707. doi: 10.1152/ajplegacy.1976.231.6.1699. [DOI] [PubMed] [Google Scholar]
- Fray J. C., Karuza A. S. Influence of raising albumin concentration on renin release in isolated perfused rat kidneys. J Physiol. 1980 Feb;299:45–54. doi: 10.1113/jphysiol.1980.sp013109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray J. C. Stretch receptor model for renin release with evidence from perfused rat kidney. Am J Physiol. 1976 Sep;231(3):936–944. doi: 10.1152/ajplegacy.1976.231.3.936. [DOI] [PubMed] [Google Scholar]
- Maack T. Physiological evaluation of the isolated perfused rat kidney. Am J Physiol. 1980 Feb;238(2):F71–F78. doi: 10.1152/ajprenal.1980.238.2.F71. [DOI] [PubMed] [Google Scholar]
- Navar L. G. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol. 1978 May;234(5):F357–F370. doi: 10.1152/ajprenal.1978.234.5.F357. [DOI] [PubMed] [Google Scholar]
- Ono H., Kokubun H., Hashimoto K. Abolition by calcium antagonists of the autoregulation of renal blood flow. Naunyn Schmiedebergs Arch Pharmacol. 1974;285(3):201–207. doi: 10.1007/BF00498990. [DOI] [PubMed] [Google Scholar]
- Ott C. E., Navar L. G., Guyton A. C. Pressures in static and dynamic states from capsules implanted in the kidney. Am J Physiol. 1971 Aug;221(2):394–400. doi: 10.1152/ajplegacy.1971.221.2.394. [DOI] [PubMed] [Google Scholar]
- Ross B. D., Epstein F. H., Leaf A. Sodium reabsorption in the perfused rat kidney. Am J Physiol. 1973 Nov;225(5):1165–1171. doi: 10.1152/ajplegacy.1973.225.5.1165. [DOI] [PubMed] [Google Scholar]
- Ross B. D. The isolated perfused rat kidney. Clin Sci Mol Med Suppl. 1978 Dec;55(6):513–521. doi: 10.1042/cs0550513. [DOI] [PubMed] [Google Scholar]
- SHIPLEY R. E., STUDY R. S. Changes in renal blood flow, extraction of inulin, glomerular filtration rate, tissue pressure and urine flow with acute alterations of renal artery blood pressure. Am J Physiol. 1951 Dec;167(3):676–688. doi: 10.1152/ajplegacy.1951.167.3.676. [DOI] [PubMed] [Google Scholar]
- Sanowski J., Wocial B. Renin release and autoregulation of blood flow in a new model of non-filtering non-transporting kidney. J Physiol. 1977 Apr;266(2):219–233. doi: 10.1113/jphysiol.1977.sp011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnermann J., Hermle M. Maintenance of feedback regulation of filtration dynamics in the absence of divalent cations in the lumen of the distal tubule. Pflugers Arch. 1975 Aug 12;358(4):311–323. doi: 10.1007/BF00580529. [DOI] [PubMed] [Google Scholar]
- Wright F. S., Briggs J. P. Feedback control of glomerular blood flow, pressure, and filtration rate. Physiol Rev. 1979 Oct;59(4):958–1006. doi: 10.1152/physrev.1979.59.4.958. [DOI] [PubMed] [Google Scholar]


