Abstract
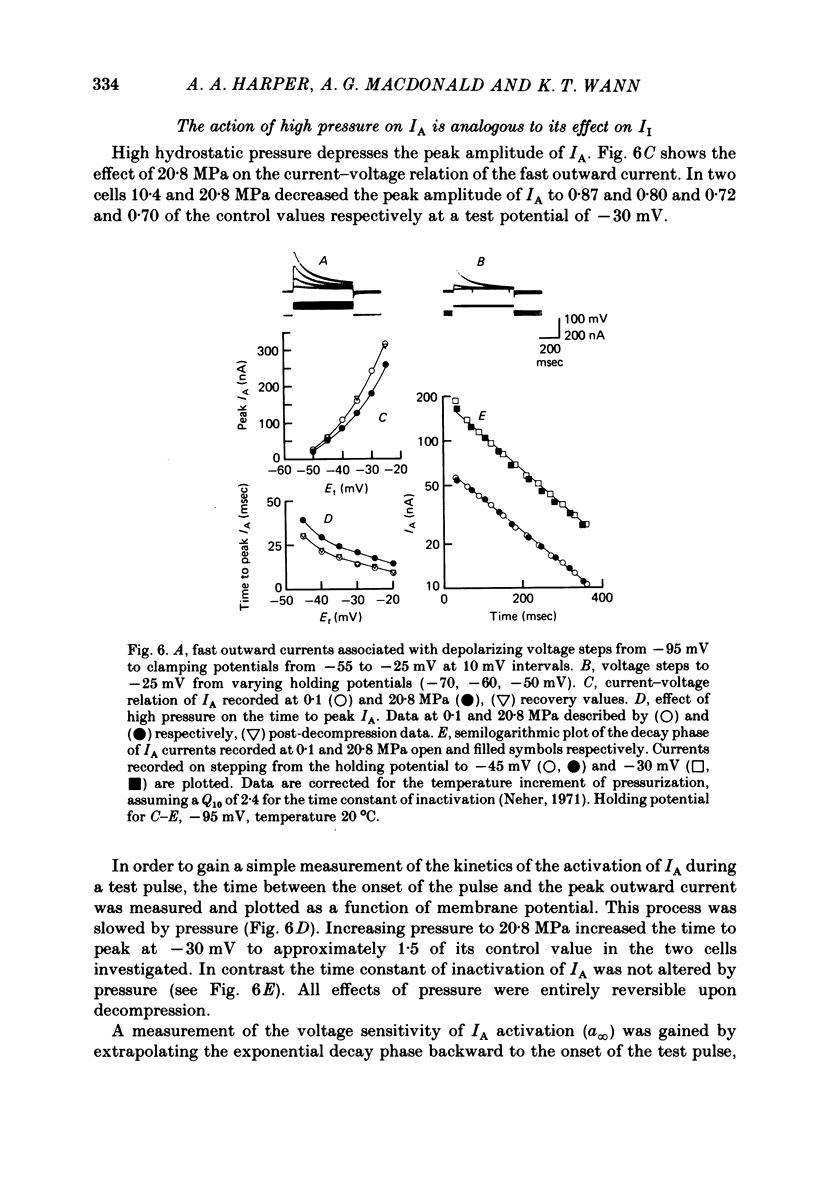
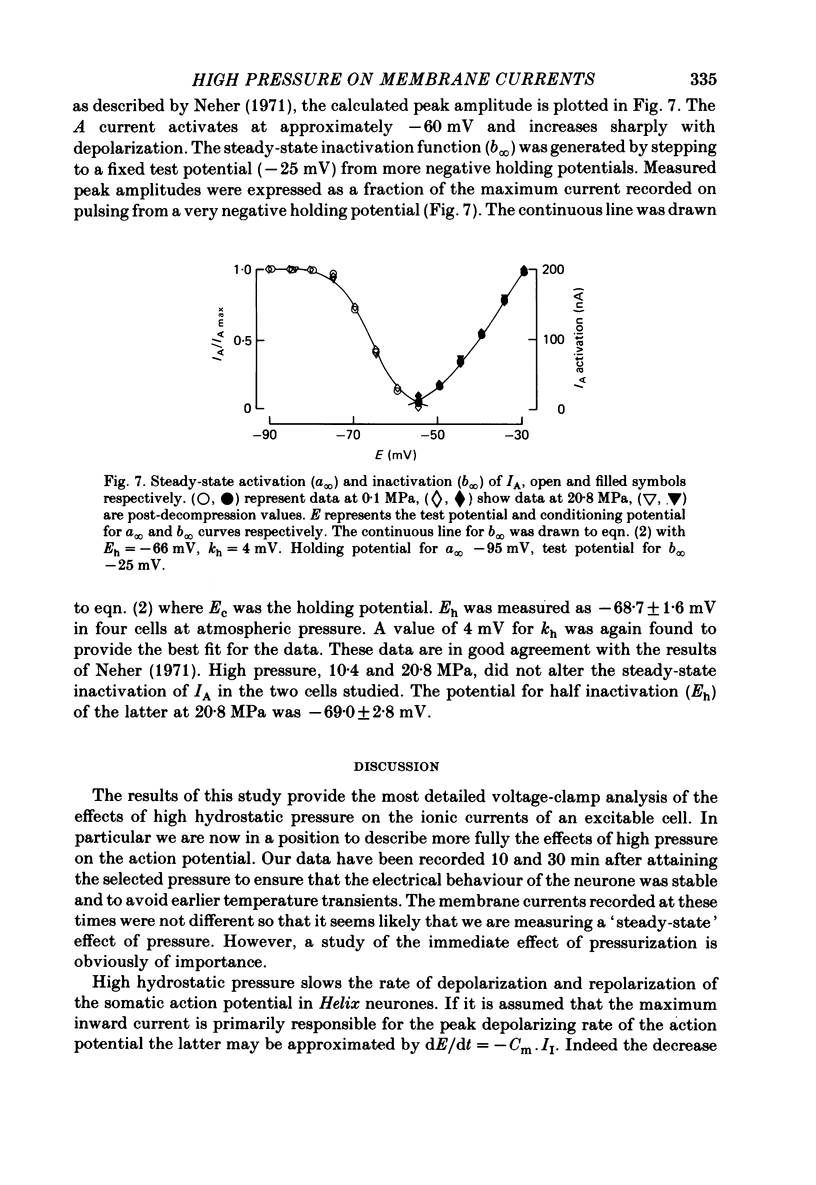
1. The actions of high hydrostatic pressure (10.4, 20.8 MPa) on the membrane currents of Helix neurones were examined under voltage clamp. 2. High hydrostatic pressure (20.8 MPa) reduced the maximum inward current to 0.78 and the delayed outward current, measured at the inward current reversal potential, to 0.75 of their value at atmospheric pressure. 3. High hydrostatic pressure shifted the curve relating the inward current conductance to membrane potential to more positive values but the maximum conductance was altered. 4. The rates of activation of the inward and delayed outward currents were slowed by pressure. 5. The steady-state level and time course of inactivation of the inward current was unaffected by high pressure over the investigated range. 6. The effects of high hydrostatic pressure on the fast outward current identified in gastropod neurones by Connor & Stevens (1971) were also examined. 20.8 MPa reduced the current measured at -30 mV to 0.71 of its control value. 7. The rate of activation of the fast outward current was slowed by high pressure but the time constant of inactivation was unchanged. 8. The majority of the effects of high hydrostatic pressure were completely reversible upon decompression. 9. These results are discussed with reference to the known effects of high hydrostatic pressure on the action potential and discharge frequency of gastropod neurones. Possible sites and mechanisms of pressure action on the excitable cell are briefly discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford M. L., Macdonald A. G., Wann K. T. Moderate hydrostatic pressures reduce the spontaneous release of transmitter in the frog [proceedings]. J Physiol. 1979 Jul;292:44P–44P. [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot R. B. The effects of high hydrostatic pressure on transmission at the crustacean neuromuscular junction. Comp Biochem Physiol B. 1975 Sep 15;52(1):133–140. doi: 10.1016/0305-0491(75)90128-5. [DOI] [PubMed] [Google Scholar]
- Cohen I., Van Der Kloot W. The effects of pH changes on the frequency of miniature end-plate potentials at the frog neuromuscular junction. J Physiol. 1976 Nov;262(2):401–414. doi: 10.1113/jphysiol.1976.sp011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M., Romey G. Responses anomales a des courants sous-liminaires de certaines membranes somatiques (neurones géants d'Helix pomatia). Analyse par la methode du voltage impose. Pflugers Arch. 1971;327(2):105–131. doi: 10.1007/BF00587365. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A. A., Macdonald A. G., Wann K. T. Proceedings: Apparatus for intracellular recording from excitable cells subjected to high hydrostatic pressure. J Physiol. 1975 Sep;251(1):7P–8P. [PubMed] [Google Scholar]
- Henderson J. V., Jr, Gilbert D. L. Slowing of ionic currents in the voltage-clamped squid axon by helium pressure. Nature. 1975 Nov 27;258(5533):351–352. doi: 10.1038/258351a0. [DOI] [PubMed] [Google Scholar]
- Henderson J. V., Jr, Morin R. A., Lanphier E. H. Apparatus for intracellular electrophysiological measurements at 200 ATA. J Appl Physiol. 1975 Feb;38(2):353–355. doi: 10.1152/jappl.1975.38.2.353. [DOI] [PubMed] [Google Scholar]
- Henderson J. V., Lowenhaupt M. T., Gilbert D. L. Helium pressure alteration of function in squid giant synapse. Undersea Biomed Res. 1977 Mar;4(1):19–26. [PubMed] [Google Scholar]
- Kendig J. J., Cohen E. N. Neuromuscular function at hyperbaric pressures: pressure-anesthetic interactions. Am J Physiol. 1976 May;230(5):1244–1249. doi: 10.1152/ajplegacy.1976.230.5.1244. [DOI] [PubMed] [Google Scholar]
- Kendig J. J., Cohen E. N. Pressure antagonism to nerve conduction block by anesthetic agents. Anesthesiology. 1977 Jul;47(1):6–10. [PubMed] [Google Scholar]
- Kendig J. J., Trudell J. R., Cohen E. N. Effects of pressure and anesthetics on conduction and synaptic transmission. J Pharmacol Exp Ther. 1975 Nov;195(2):216–224. [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Shakhovalov Y. A. Separation of sodium and calcium currents in the somatic membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):545–568. doi: 10.1113/jphysiol.1977.sp011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M. J., Miller K. W., Paton W. D., Smith E. B. Pressure reversal of anaesthesia. Nature. 1971 Jun 11;231(5302):368–371. doi: 10.1038/231368a0. [DOI] [PubMed] [Google Scholar]
- MOORE J. W., ULBRICHT W., TAKATA M. EFFECT OF ETHANOL ON THE SODIUM AND POTASSIUM CONDUCTANCES OF THE SQUID AXON MEMBRANE. J Gen Physiol. 1964 Nov;48:279–295. doi: 10.1085/jgp.48.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier J. Mapping studies of a gastropod brain. Brain Res. 1973 Sep 14;59:201–210. doi: 10.1016/0006-8993(73)90261-8. [DOI] [PubMed] [Google Scholar]
- SPYROPOULOS C. S. The effects of hydrostatic pressure upon the normal and narcotized nerve fiber. J Gen Physiol. 1957 Jul 20;40(6):849–857. doi: 10.1085/jgp.40.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B. Properties of a calcium channel in snail neurones. Nature. 1974 Jul 26;250(464):340–342. doi: 10.1038/250340a0. [DOI] [PubMed] [Google Scholar]
- Standen N. B. Voltage-clamp studies of the calcium inward current in an identified snail neurone: comparison with the sodium inward current. J Physiol. 1975 Jul;249(2):253–268. doi: 10.1113/jphysiol.1975.sp011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]


