Abstract
1. Potentiation of the isometric twitch tension was compared during and after the staircase and after tetanic stimuli in the fast-twitch extensor digitorum longus muscle of adult Lewis rats at 37-38°C.
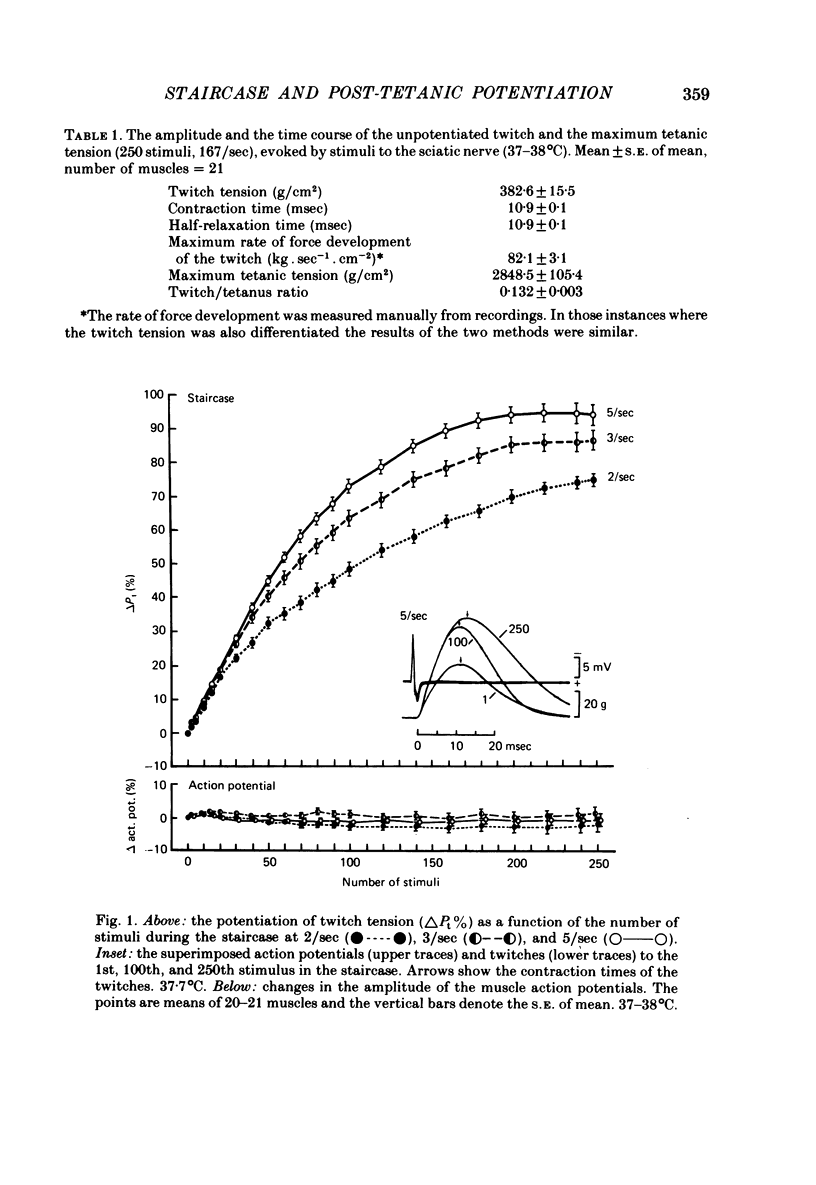
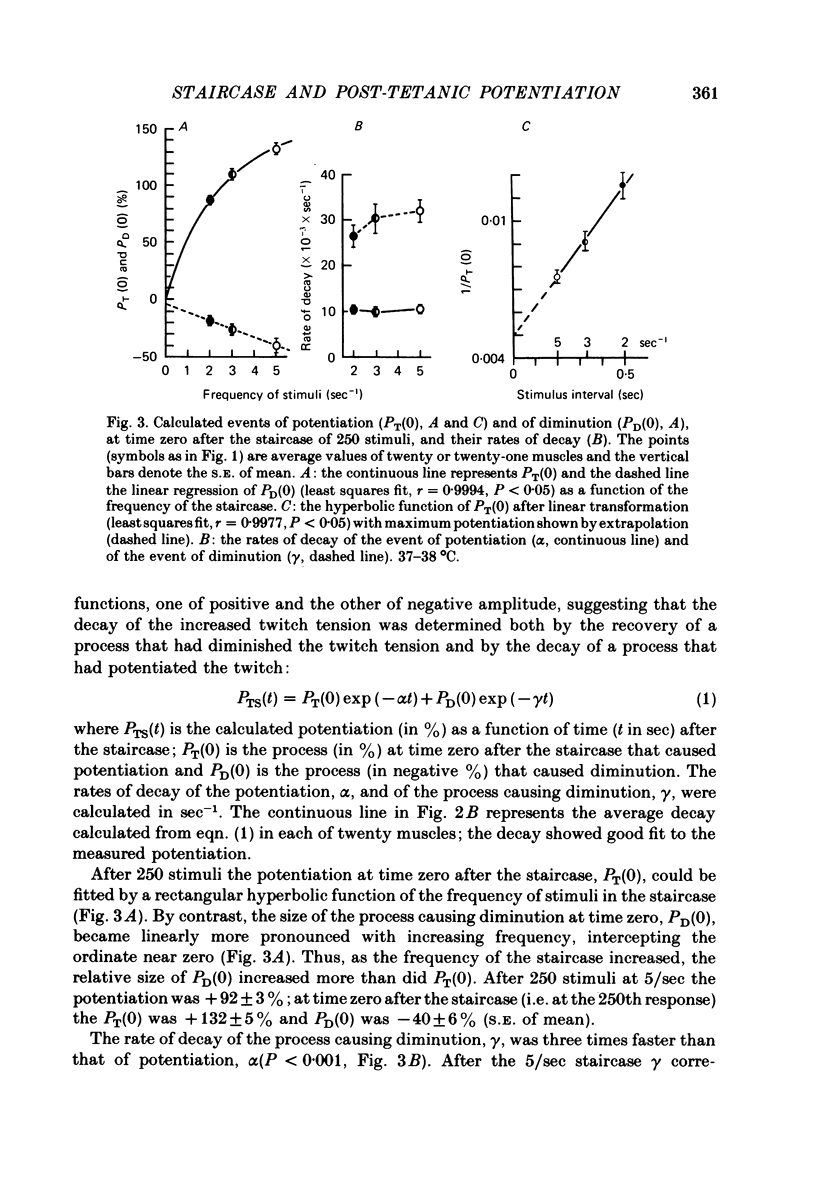
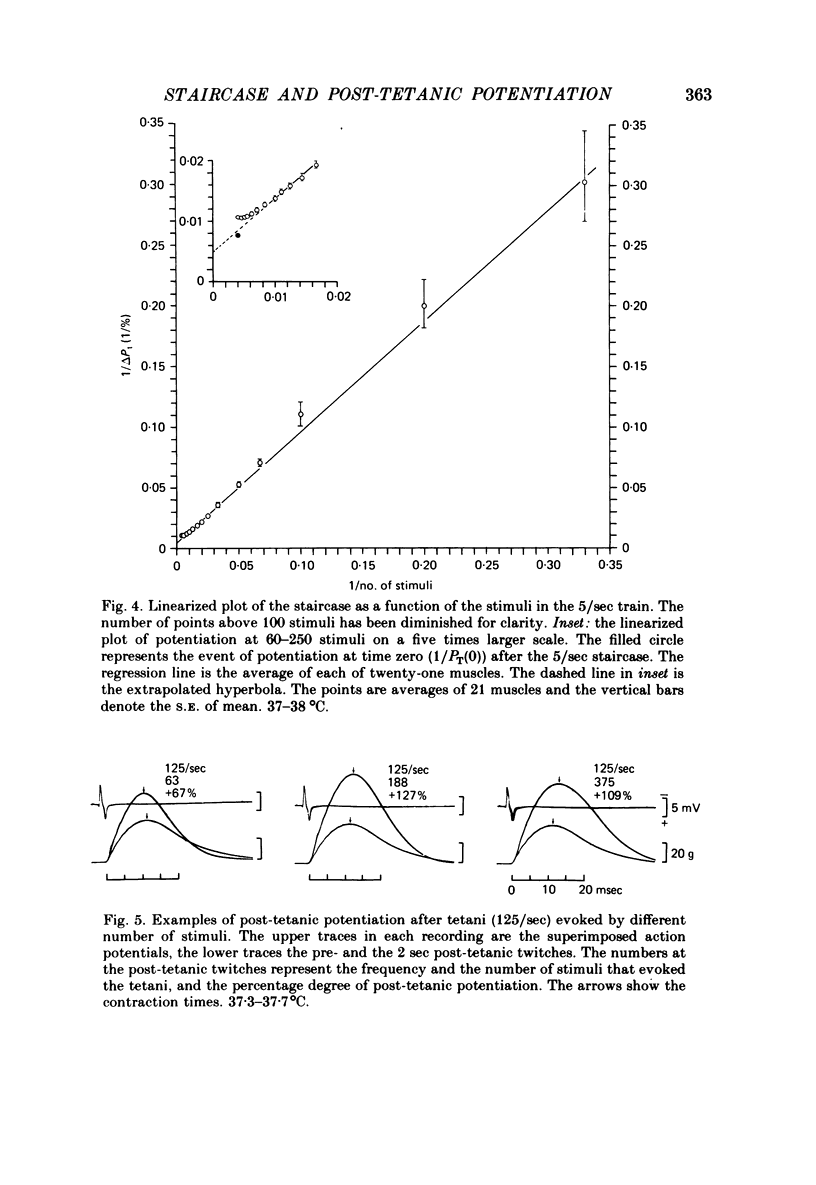
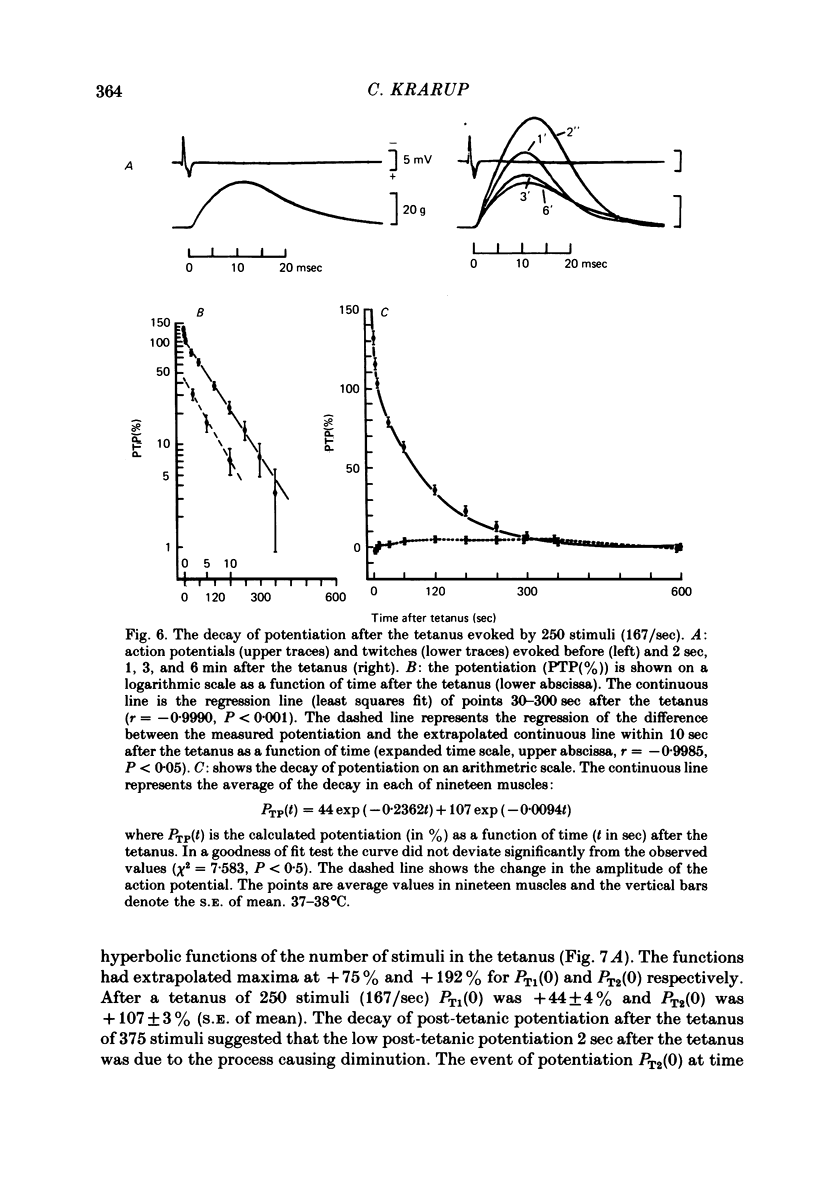
2. With up to 250 stimuli the potentiation rose with an increase in both the frequency and number of stimuli in the staircase (2-5/sec) and the tetanus (100-167/sec). After a tetanus of 375 stimuli (125/sec) the potentiation was smaller. The potentiation 2 sec after a tetanus of 250 stimuli (167/sec) was + 132 ± 5% (n = 21, s.e. of mean) which was greater (P < 0·001) than at the 250th stimulus at 5/sec, +92±3% (n = 21, s.e. of mean).
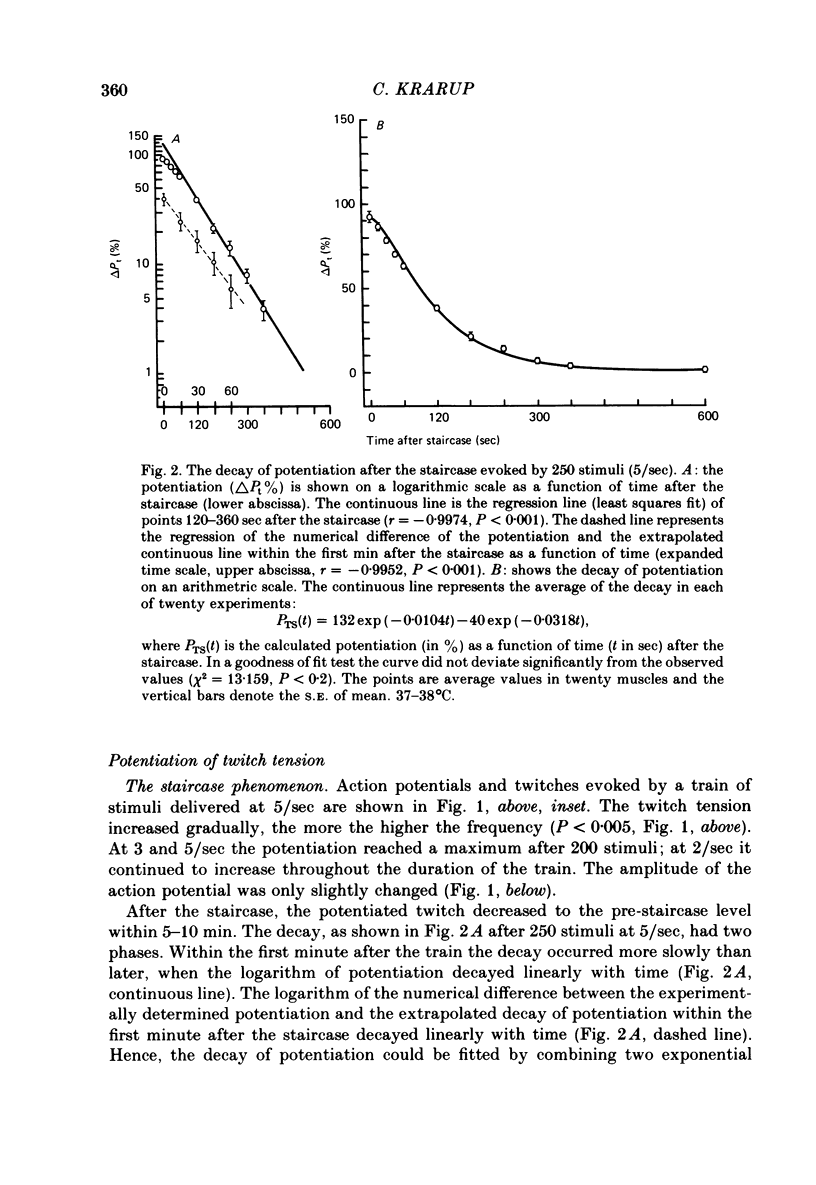
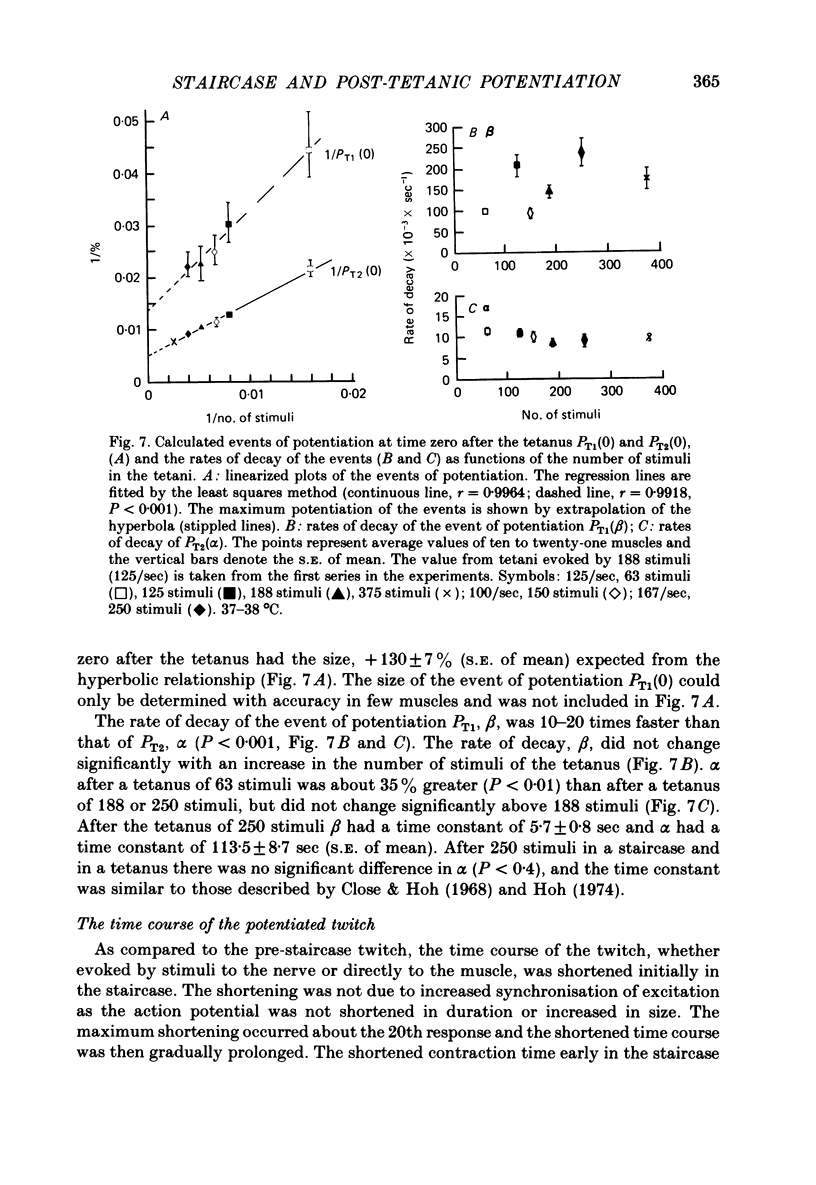
3. After the staircase the decay of potentiation was initially slow and later more rapid. This was taken to indicate both the recovery of a process that diminished twitch tension and the decay of a process causing potentiation. After 250 stimuli (5/sec) the rate of decay of the processes causing diminution and potentiation had time constants of 34·5 ± 3·8 sec (n = 18, s.e. of mean) and 102·2 ± 6·6 sec (n = 20, s.e. of mean) respectively. Compared with the potentiation, the process causing diminution became relatively more pronounced the greater the frequency of stimuli.
4. The decay of post-tetanic potentiation showed an initial rapid and a later slower phase of decay. After a tetanus of 250 stimuli (167/sec) the rates of decay had time constants of 5·7 ± 0·8 sec (n = 16, s.e. of mean) and 113·5 ± 8·7 sec (n = 19, s.e. of mean) respectively.
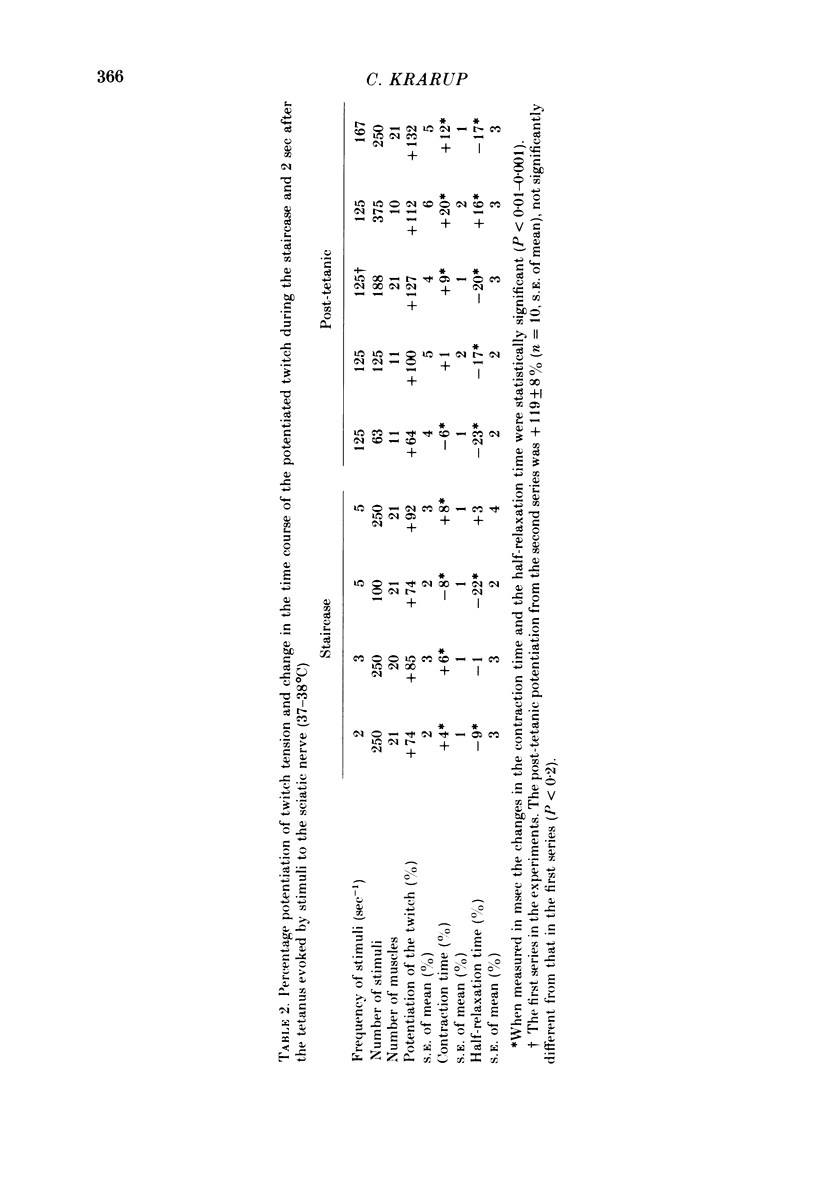
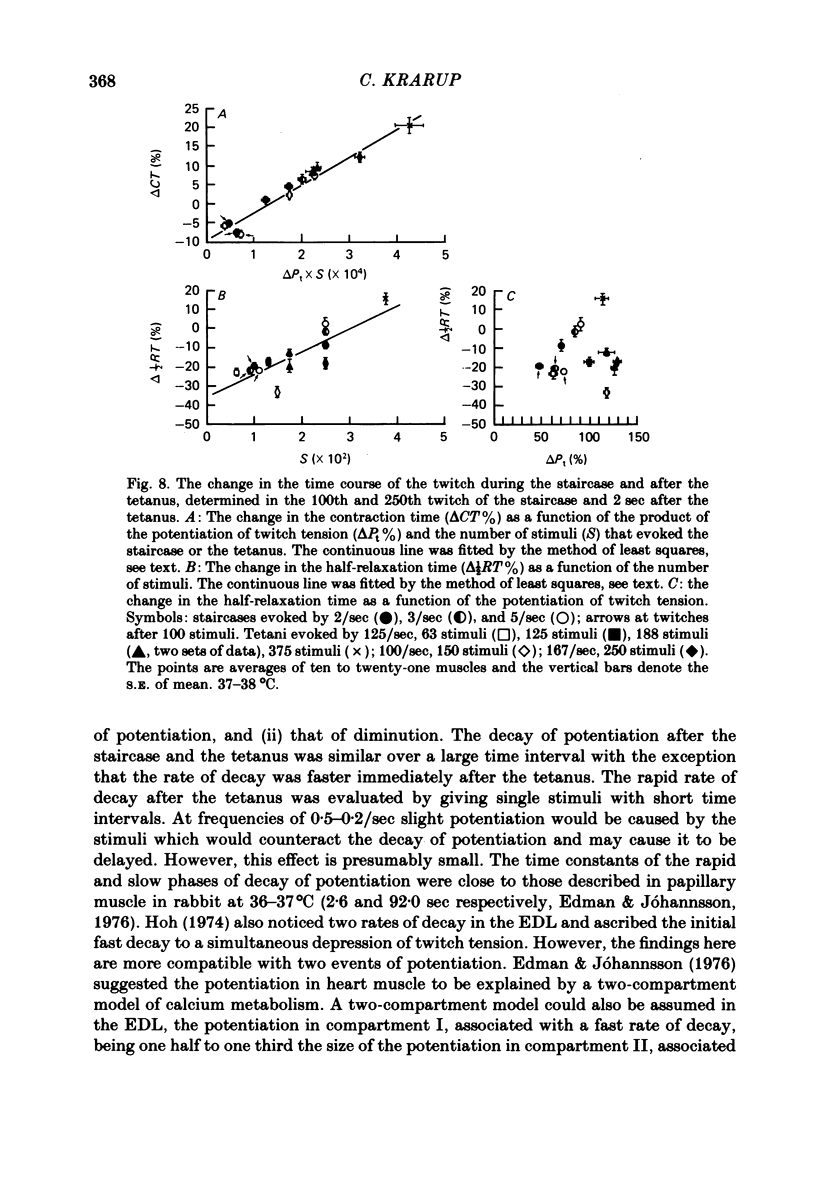
5. Compared with the unpotentiated response the time course of the twitch was shortened initially in the staircase and when the post-tetanic potentiation was low. The contraction time was then increasingly prolonged the greater the potentiation and the greater the number of stimuli in the staircase and in the tetanus. The half-relaxation time was the more prolonged the greater the number of stimuli.
6. Potentiation can be described in terms of a two-compartment model of processes which show saturation. Both compartments were activated in a tetanus whereas only the compartment with a slow rate of decay was activated in the staircase. It is speculated that the two compartments are related to the excitation—contraction coupling. The process that caused diminution of twitch tension during the staircase may be due to fatigue. It is suggested that the energy consumption in 250 twitches is about 10 times greater than in a tetanus of 250 stimuli which may explain the presence of fatigue after the staircase whereas it was absent after the tetanus.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asmussen G., Gaunitz U. Kontraktile Eigenschaften der quergestreiften Muskelfasern des Osophagus im Vergleich mit ausgewählten Skelettmuskeln der Ratte. Acta Biol Med Ger. 1978;37(2):335–346. [PubMed] [Google Scholar]
- Bagust J., Lewis D. M., Luck J. C. Post-tetanic effects in motor units of fast and slow twitch muscle of the cat. J Physiol. 1974 Feb;237(1):115–121. doi: 10.1113/jphysiol.1974.sp010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Dynamic properties of fast and slow skeletal muscles of the rat after nerve cross-union. J Physiol. 1969 Oct;204(2):331–346. doi: 10.1113/jphysiol.1969.sp008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R., Hoh J. F. Influence of temperature on isometric contractions of rat skeletal muscles. Nature. 1968 Mar 23;217(5134):1179–1180. doi: 10.1038/2171179a0. [DOI] [PubMed] [Google Scholar]
- Close R., Hoh J. F. Post-tetanic potentiation of twitch contractions of cross-innervated rat fast and slow muscles. Nature. 1969 Jan 11;221(5176):179–181. doi: 10.1038/221179a0. [DOI] [PubMed] [Google Scholar]
- Close R., Hoh J. F. The after-effects of repetitive stimulation on the isometric twitch contraction of rat fast skeletal muscle. J Physiol. 1968 Jul;197(2):461–477. doi: 10.1113/jphysiol.1968.sp008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly R., Gough W., Winegrad S. Characteristics of the isometric twitch of skeletal muscle immediately after a tetanus. A study of the influence of the distribution of calcium within the sarcoplasmic reticulum on the twitch. J Gen Physiol. 1971 Jun;57(6):697–709. [PubMed] [Google Scholar]
- Dahl K., Buchthal F. Digital memory recorder in electromyography and nerve conduction studies. Electroencephalogr Clin Neurophysiol. 1978 Oct;45(4):538–544. doi: 10.1016/0013-4694(78)90298-5. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol. 1980 Feb;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J. E., Hainaut K. Kinetics of myofilament activation in potentiated contraction: staircase phenomenon in human skeletal muscle. Nature. 1968 Feb 10;217(5128):529–532. doi: 10.1038/217529a0. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Grieve D. W., Nilsson E. Studies of the excitation-contraction mechanism in the skeletal muscle and the myocardium. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;290(4):320–334. doi: 10.1007/BF00363311. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Jóhannsson M. The contractile state of rabbit papillary muscle in relation to stimulation frequency. J Physiol. 1976 Jan;254(3):565–581. doi: 10.1113/jphysiol.1976.sp011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A. Metabolic changes associated with the slowing of relaxation in fatigued mouse muscle. J Physiol. 1975 Oct;251(2):287–301. doi: 10.1113/jphysiol.1975.sp011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. The effects of repetitive stimulation on the action potential and the twitch of rat muscle. Acta Physiol Scand. 1974 Feb;90(2):387–400. doi: 10.1111/j.1748-1716.1974.tb05600.x. [DOI] [PubMed] [Google Scholar]
- Homsher E., Kean C. J. Skeletal muscle energetics and metabolism. Annu Rev Physiol. 1978;40:93–131. doi: 10.1146/annurev.ph.40.030178.000521. [DOI] [PubMed] [Google Scholar]
- Karis J. H., Gissen A. J., Nastuk W. L. The effect of volatile anesthetic agents on neuromuscular transmission. Anesthesiology. 1967 Jan-Feb;28(1):128–134. doi: 10.1097/00000542-196701000-00014. [DOI] [PubMed] [Google Scholar]
- Krarup C. Electrical and mechanical responses in the platysma and in the adductor pollicis muscle: in normal subjects. J Neurol Neurosurg Psychiatry. 1977 Mar;40(3):234–240. doi: 10.1136/jnnp.40.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krarup C., Horowitz S. H. Evoked responses of the elbow flexors in control subjects and in myopathy patients. Muscle Nerve. 1979 Nov-Dec;2(6):465–477. doi: 10.1002/mus.880020608. [DOI] [PubMed] [Google Scholar]
- Krarup C. The effect of dantrolene on the enhancement and diminution of tension evoked by staircase and by tetanus in rat muscle. J Physiol. 1981 Feb;311:389–400. doi: 10.1113/jphysiol.1981.sp013591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E., Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979 Mar;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Way W. L., Dolan W. M., Stevens W. C., Eger E. I., 2nd The dependence of pancuronium- and d-tubocurarine-induced neuromuscular blockades on alveolar concentrations of halothane and forane. Anesthesiology. 1972 Dec;37(6):573–581. doi: 10.1097/00000542-197212000-00001. [DOI] [PubMed] [Google Scholar]
- RITCHIE J. M., WILKIE D. R. The effect of previous stimulation on the active state of muscle. J Physiol. 1955 Nov 28;130(2):488–496. doi: 10.1113/jphysiol.1955.sp005422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K. W. Influence of temperature on the characteristics of summation of isometric mechanical responses of mammalian skeletal muscle. Exp Neurol. 1977 Mar;54(3):513–532. doi: 10.1016/0014-4886(77)90254-0. [DOI] [PubMed] [Google Scholar]
- Rosenfalck P. Change in active state during the staircase phenomenon of human muscle. Acta Physiol Scand. 1974 Sep;92(1):12–20. doi: 10.1111/j.1748-1716.1974.tb05718.x. [DOI] [PubMed] [Google Scholar]
- Slomić A., Rosenfalck A., Buchthal F. Electrical and mechanical responses of normal and myasthenic muscle. Brain Res. 1968 Aug 5;10(1):1–78. doi: 10.1016/0006-8993(68)90227-8. [DOI] [PubMed] [Google Scholar]
- Waud B. E., Cheng M. C., Waud D. R. Comparison of drug-receptor dissociation constants at the mammalian neuromuscular junction in the presence and absence of halothane. J Pharmacol Exp Ther. 1973 Oct;187(1):40–46. [PubMed] [Google Scholar]
- Wendt I. R., Gibbs C. L. Energy production of rat extensor digitorum longus muscle. Am J Physiol. 1973 May;224(5):1081–1086. doi: 10.1152/ajplegacy.1973.224.5.1081. [DOI] [PubMed] [Google Scholar]


