Abstract
1. The effect of temperature (20-37·5 °C) on the potentiation of twitch tension was examined during and after the staircase (250 stimuli, 5/sec) and after the tetanus (188 stimuli, 125/sec) in the extensor digitorum longus muscle of adult Lewis rats.
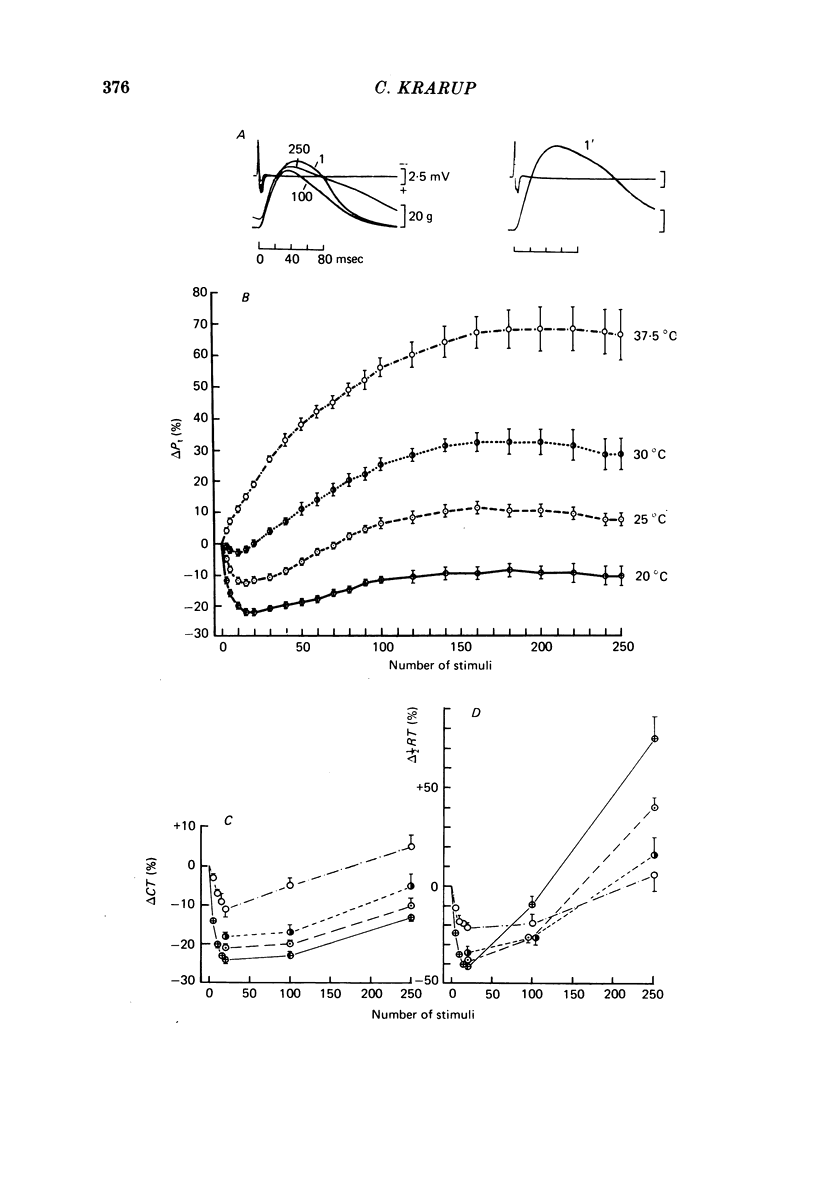
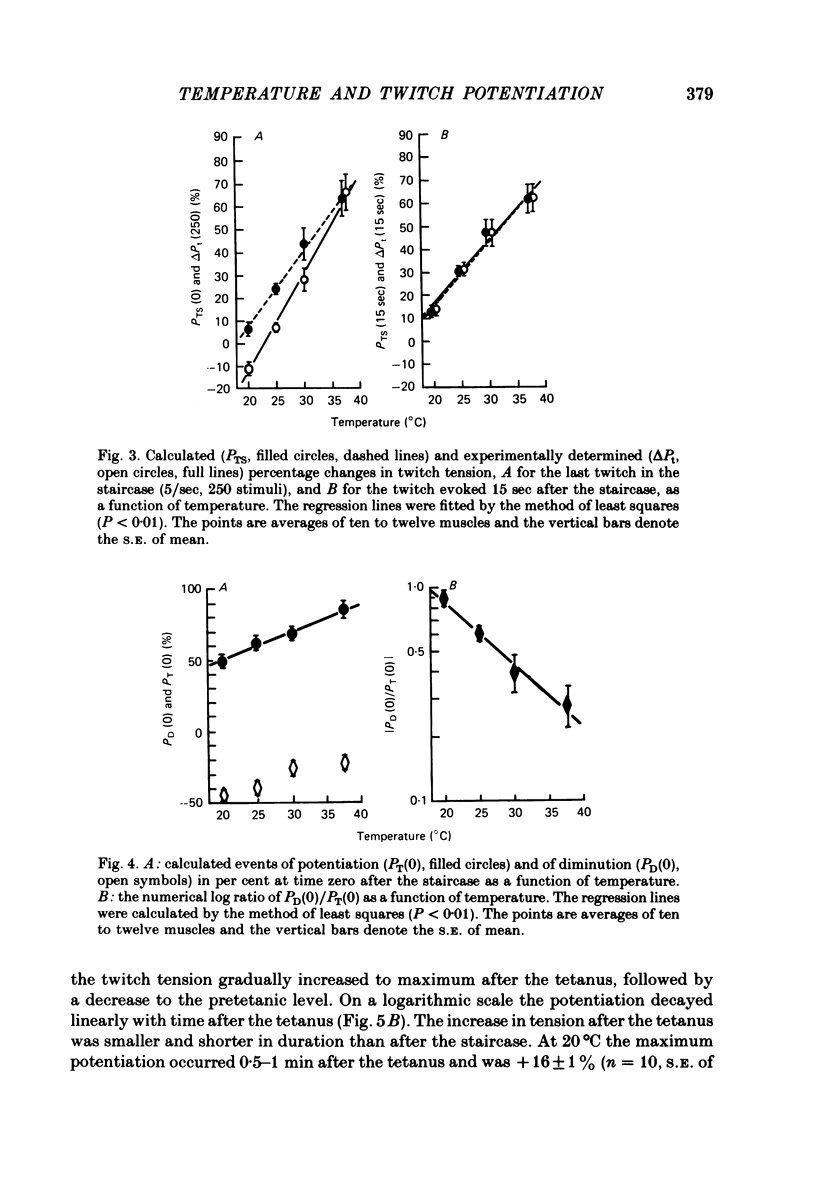
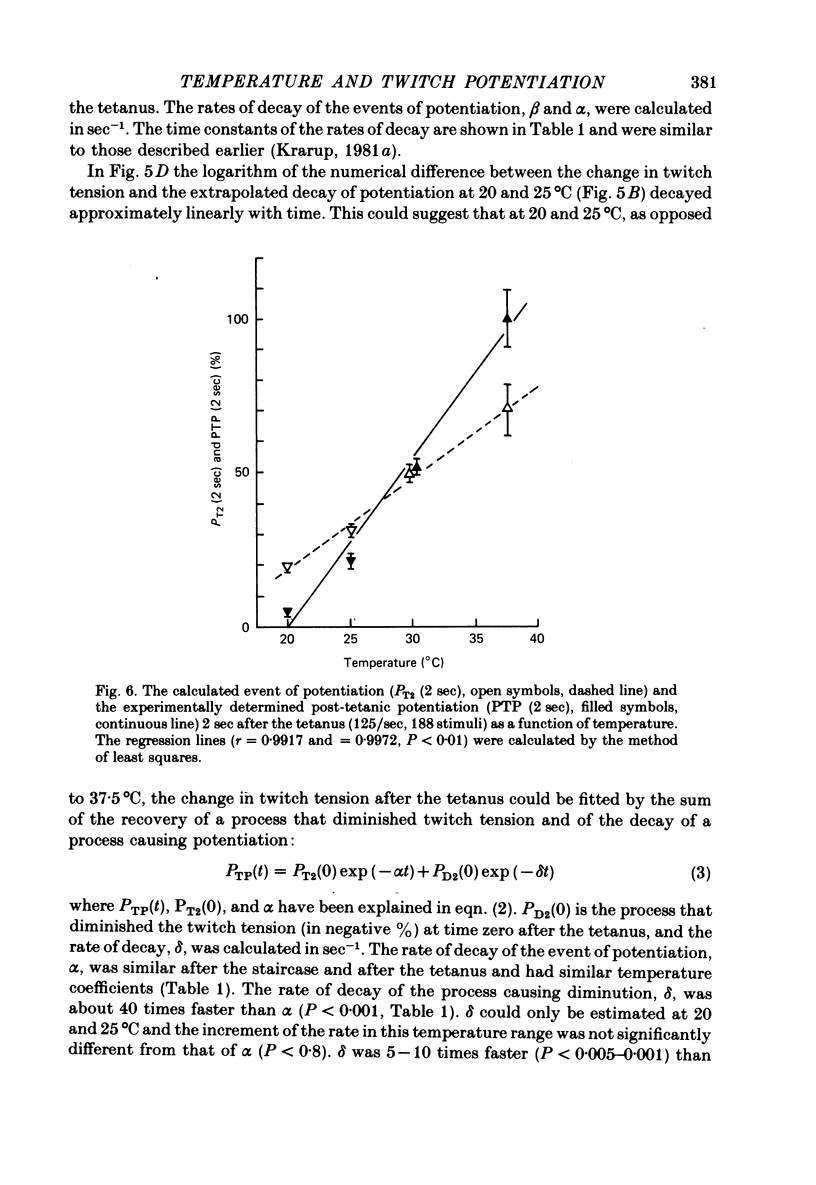
2. During the staircase at 20°C the twitch tension decreased (negative staircase) by 10-20%. At 25-30°C the staircase was initially negative and later positive. At 37·5°C the staircase was positive throughout the train. Both at the end of the staircase and 2 sec after the tetanus the potentiation increased linearly with increasing temperature.
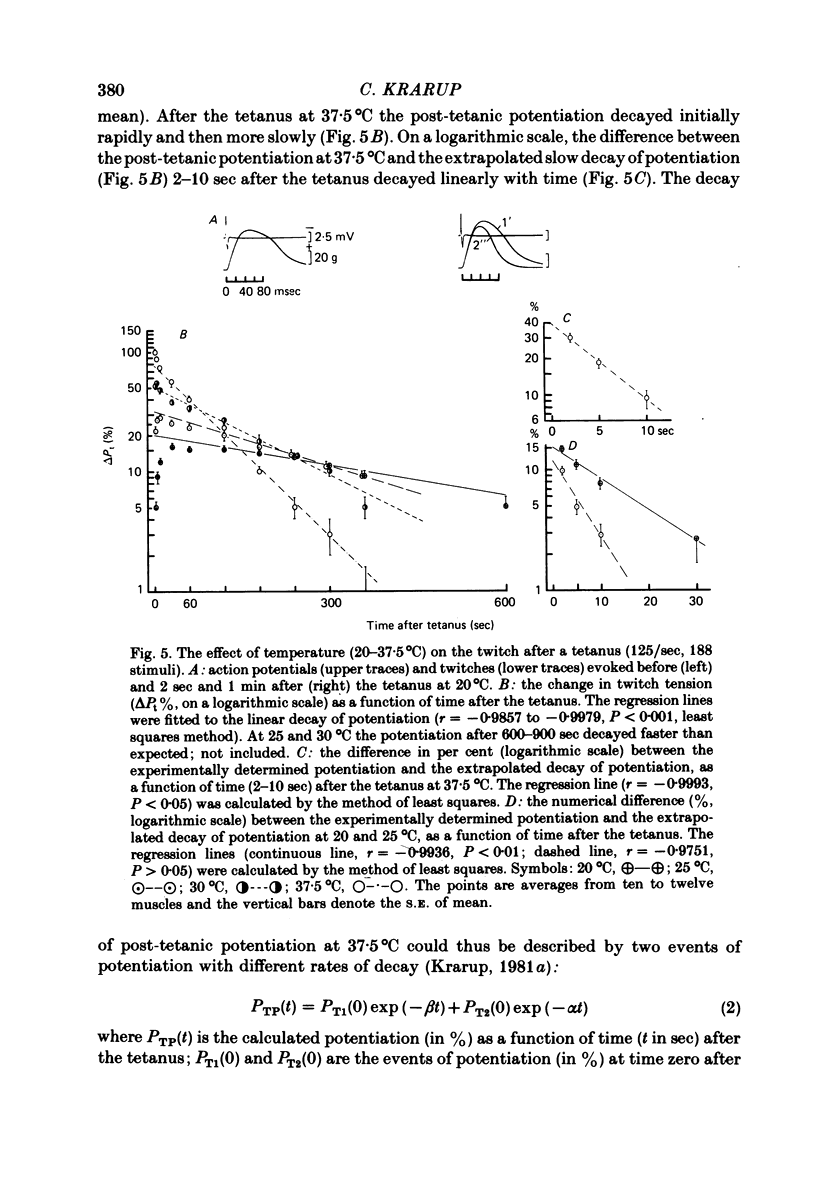
3. After the staircase and the tetanus at 20-30°C the twitch tension increased initially rapidly and later after the staircase at a slower rate. Maximal potentiation at 20°C was attained 3 min after the staircase (+ 30 ± 3%, n = 10, s.e. of mean) and 1 min after the tetanus (+ 16 ± 1%, n = 10, s.e. of mean). At 37·5°C the potentiation decayed rapidly after the staircase and the tetanus.
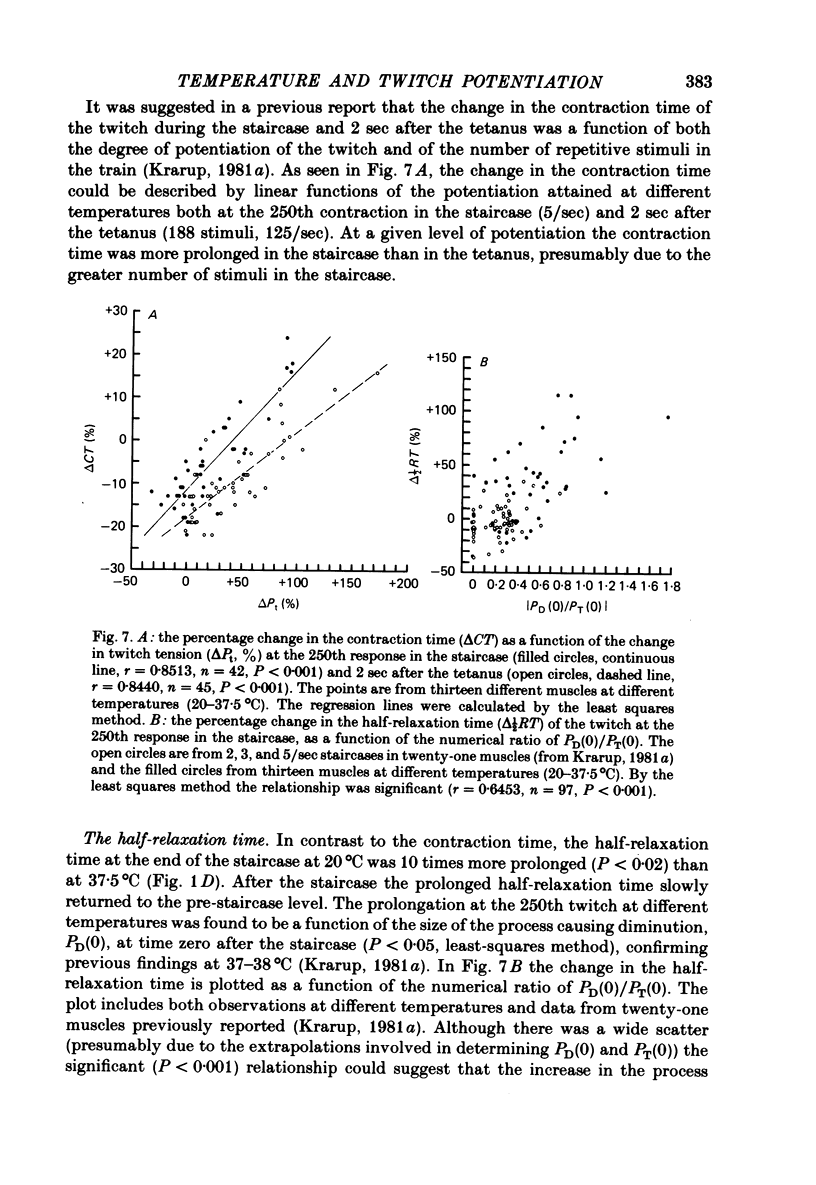
4. During the staircase the time course of the twitch was shortened twice as much at 20 as at 37·5°C. At the end of the staircase and 2 sec after the tetanus the contraction time was the more prolonged the greater the potentiation. At maximal potentiation the contraction time was prolonged three times as much at 20°C (+ 19 ± 3%, n = 10, s.e. of mean) as at 37·5°C (P < 0·005). The half-relaxation time at the end of the staircase was prolonged 10 times more at 20 than at 37·5°C (P < 0·02).
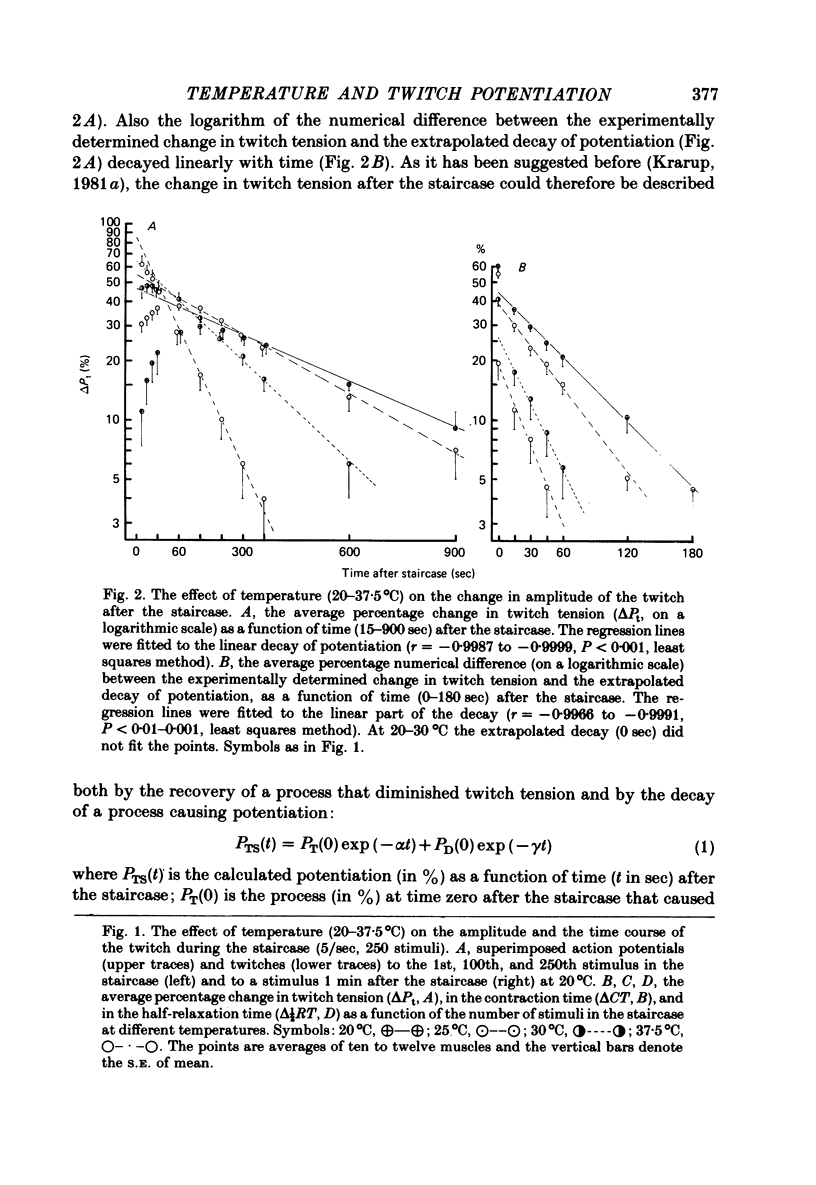
5. When extrapolated to time zero after the staircase and the tetanus the potentiation at 20°C was still marked (20-50%). The rate of decay of potentiation (time constant, 20°C, 561·2 ± 37·4 sec, n = 20, s.e. of mean) increased with increasing temperature (Q10 = 2·6). The event of potentiation with a fast rate of decay, present after the tetanus but not after the staircase at 37·5°C, was abolished below 30°C.
6. The increase in twitch tension after the staircase and the tetanus at 20-30°C was taken to indicate the recovery of events that diminished the twitch, occurring simultaneously with potentiation.
7. (i) One process of diminution, present after the staircase but not after the tetanus, increased on cooling and was assumed to be due to fatigue. The rate of recovery of the process (time constant, 20°C, 79·6 ± 7·4 sec, n = 10, s.e. of mean) increased with increasing temperature (Q10 = 1·9). The half-relaxation time of the last twitch in the staircase was the more prolonged the greater the process. (ii) Another process causing diminution was present after the staircase and the tetanus at 20-30°C. It recovered at 20°C with a time constant of 14·9 ± 2·2 sec (n = 10, s.e. of mean). This process, possibly responsible for the initially negative staircase, was not thought to be due to fatigue. It may reflect a diminished depolarization of the transverse tubules by repetitive stimuli.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian J., Nakajima S. Action potential in the transverse tubules and its role in the activation of skeletal muscle. J Gen Physiol. 1974 Feb;63(2):257–278. doi: 10.1085/jgp.63.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R., Hoh J. F. Influence of temperature on isometric contractions of rat skeletal muscles. Nature. 1968 Mar 23;217(5134):1179–1180. doi: 10.1038/2171179a0. [DOI] [PubMed] [Google Scholar]
- Connolly R., Gough W., Winegrad S. Characteristics of the isometric twitch of skeletal muscle immediately after a tetanus. A study of the influence of the distribution of calcium within the sarcoplasmic reticulum on the twitch. J Gen Physiol. 1971 Jun;57(6):697–709. [PubMed] [Google Scholar]
- Costantin L. L. Contractile activation in skeletal muscle. Prog Biophys Mol Biol. 1975;29(2):197–224. doi: 10.1016/0079-6107(76)90023-7. [DOI] [PubMed] [Google Scholar]
- Costantin L. L. The role of sodium current in the radial spread of contraction in frog muscle fibers. J Gen Physiol. 1970 Jun;55(6):703–715. doi: 10.1085/jgp.55.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Serratos H. Inward spread of activation in vertebrate muscle fibres. J Physiol. 1971 Feb;212(3):777–799. doi: 10.1113/jphysiol.1971.sp009356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski W., Lobsiger E. A., Lüttgau H. C. The effect of repetitive stimulation at low frequencies upon the electrical and mechanical activity of single muscle fibres. Pflugers Arch. 1972;334(3):222–239. doi: 10.1007/BF00626225. [DOI] [PubMed] [Google Scholar]
- Hanson J. The effects of repetitive stimulation on the action potential and the twitch of rat muscle. Acta Physiol Scand. 1974 Feb;90(2):387–400. doi: 10.1111/j.1748-1716.1974.tb05600.x. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Krarup C. The effect of dantrolene on the enhancement and diminution of tension evoked by staircase and by tetanus in rat muscle. J Physiol. 1981 Feb;311:389–400. doi: 10.1113/jphysiol.1981.sp013591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., WILKIE D. R. The effect of previous stimulation on the active state of muscle. J Physiol. 1955 Nov 28;130(2):488–496. doi: 10.1113/jphysiol.1955.sp005422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K. W. Changes produced by chronic denervation in the temperature-dependent isometric contractile characteristics of rat fast and slow twitch skeletal muscles. J Physiol. 1977 Dec;273(1):255–262. doi: 10.1113/jphysiol.1977.sp012092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUONG X. T., WALL B. J., WALKER S. M. EFFECTS OF TEMPERATURE ON ISOMETRIC CONTRACTION OF RAT MUSCLE. Am J Physiol. 1964 Aug;207:393–396. doi: 10.1152/ajplegacy.1964.207.2.393. [DOI] [PubMed] [Google Scholar]
- WALKER S. M. Failure of potentiation in successive, posttetanic, and summated twitches in cooled skeletal muscle of the rat. Am J Physiol. 1951 Aug;166(2):480–484. doi: 10.1152/ajplegacy.1951.166.2.480. [DOI] [PubMed] [Google Scholar]
- Winegrad S. The intracellular site of calcium activaton of contraction in frog skeletal muscle. J Gen Physiol. 1970 Jan;55(1):77–88. doi: 10.1085/jgp.55.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]


