Full text
PDF

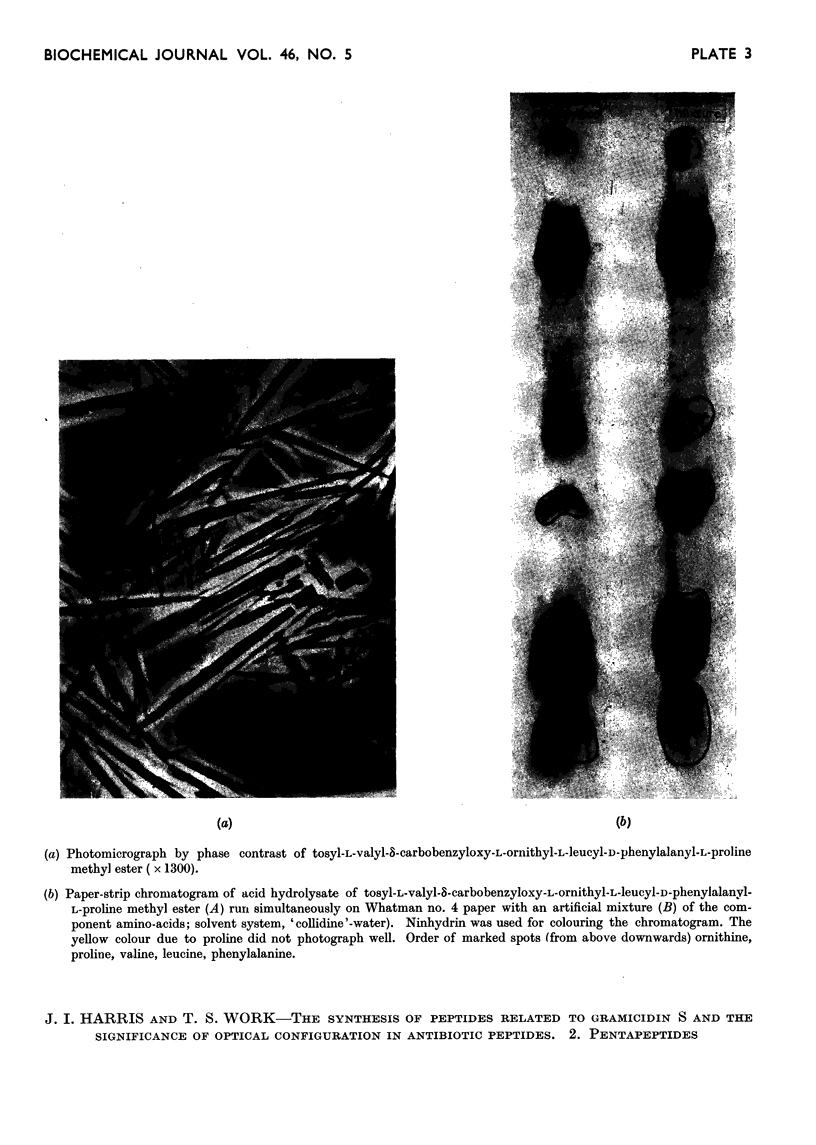





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Consden R., Gordon A. H., Martin A. J. Gramicidin S: the sequence of the amino-acid residues. Biochem J. 1947;41(4):596–602. [PMC free article] [PubMed] [Google Scholar]
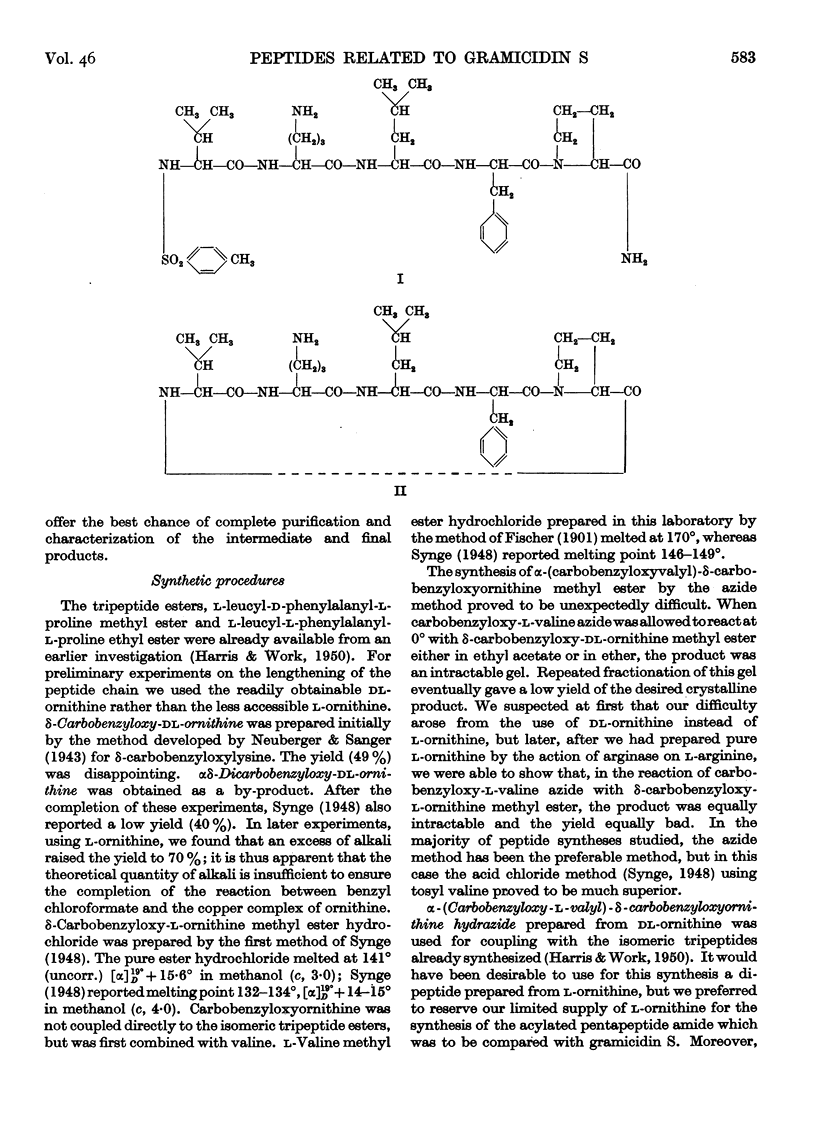
- Harris J. I., Work T. S. The synthesis of peptides related to gramicidin S and the significance of optical configuration in antibiotic peptides. 1. Tripeptides. Biochem J. 1950 Feb;46(2):196–199. doi: 10.1042/bj0460196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter A. Enzymic methods for the preparation of arginine and ornithine. Biochem J. 1939 Jan;33(1):27–35. doi: 10.1042/bj0330027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger A., Sanger F. The availability of the acetyl derivatives of lysine for growth. Biochem J. 1943 Oct;37(4):515–518. doi: 10.1042/bj0370515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synge R. L. The synthesis of some dipeptides related to gramicidin S. Biochem J. 1948;42(1):99–104. doi: 10.1042/bj0420099. [DOI] [PMC free article] [PubMed] [Google Scholar]