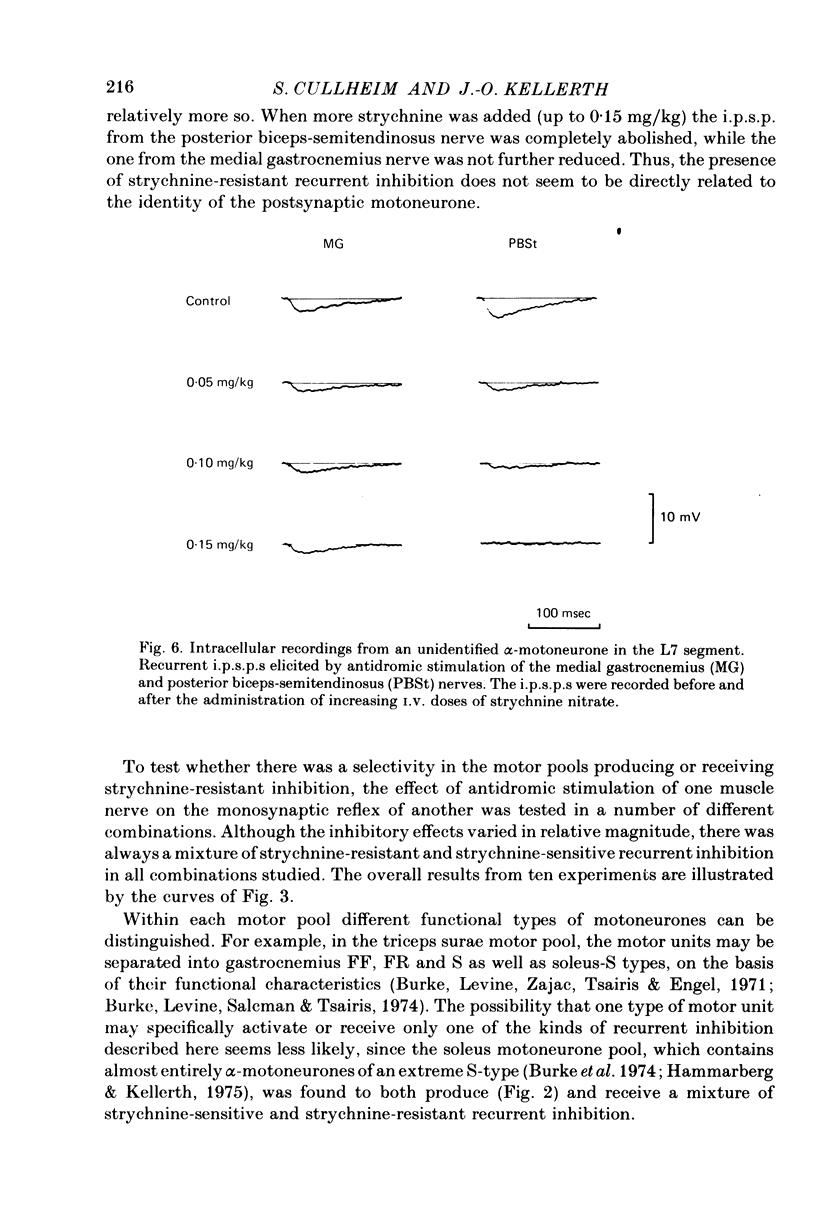
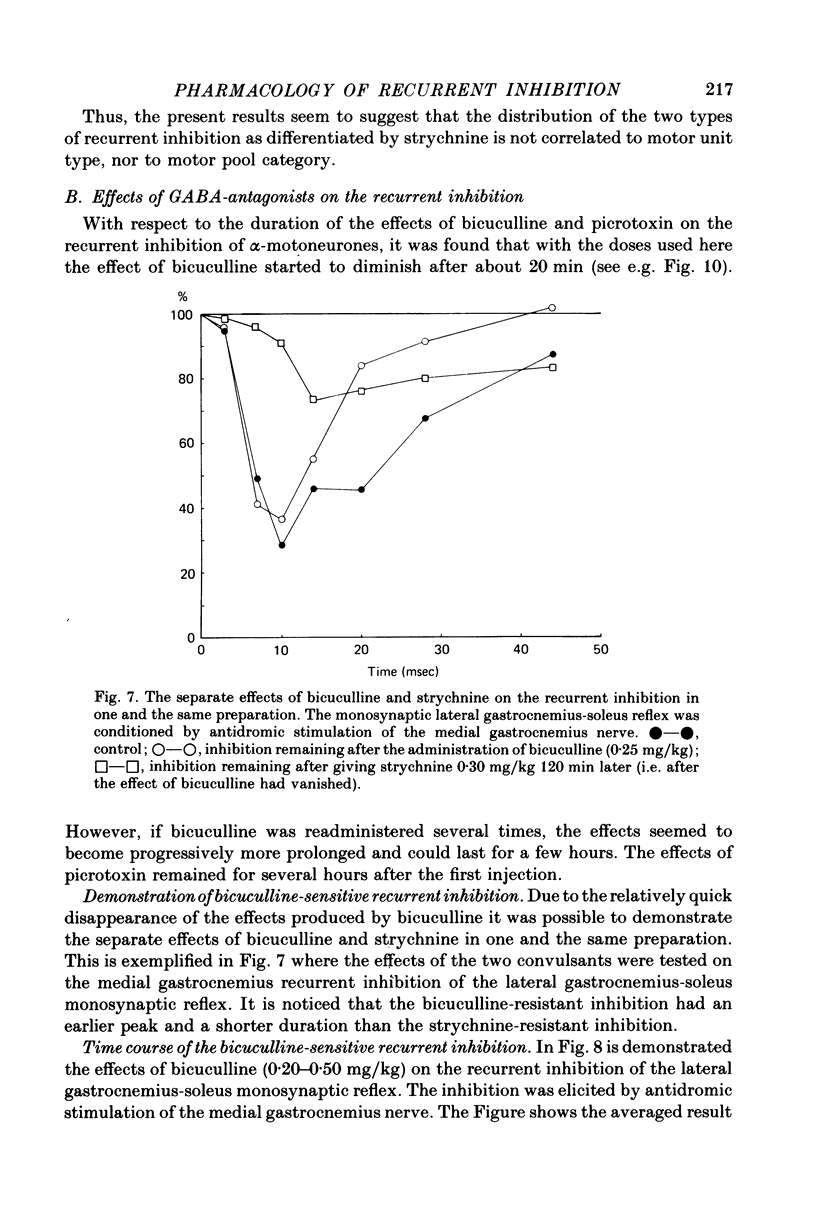
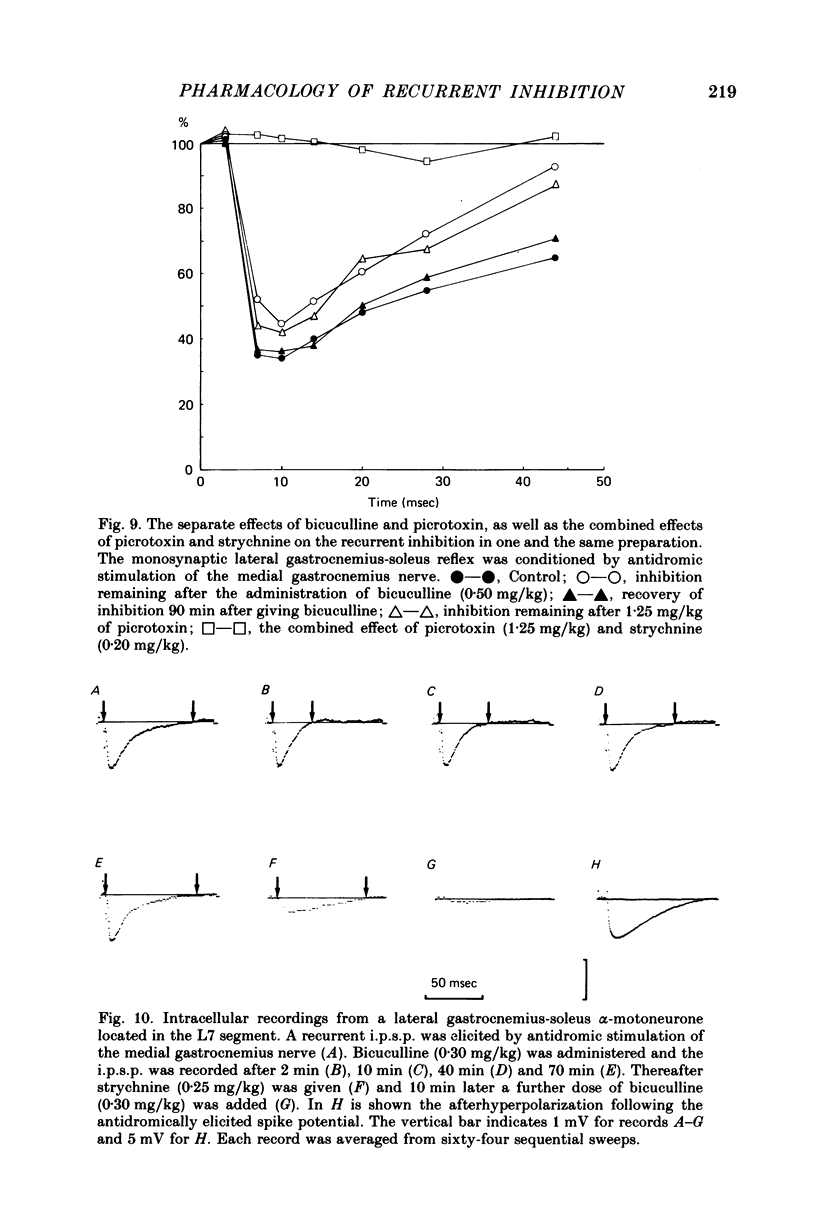
Abstract
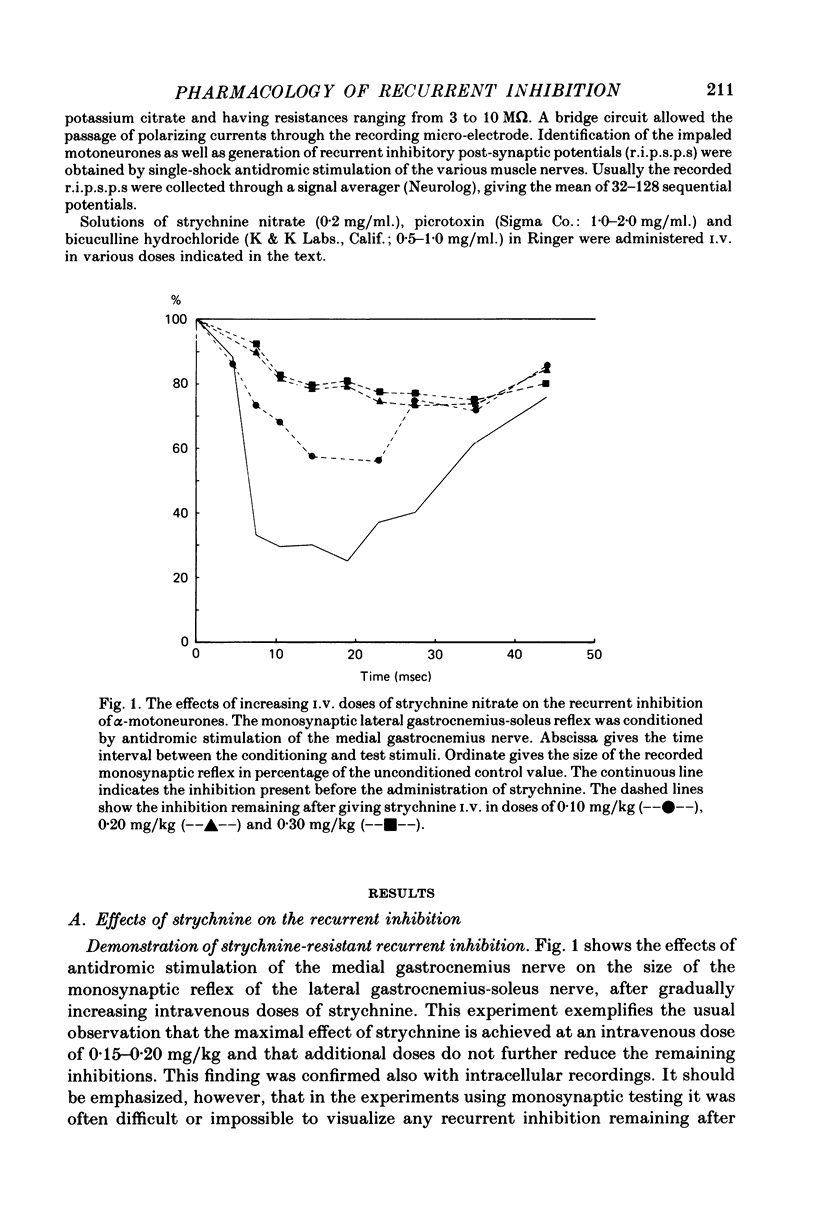
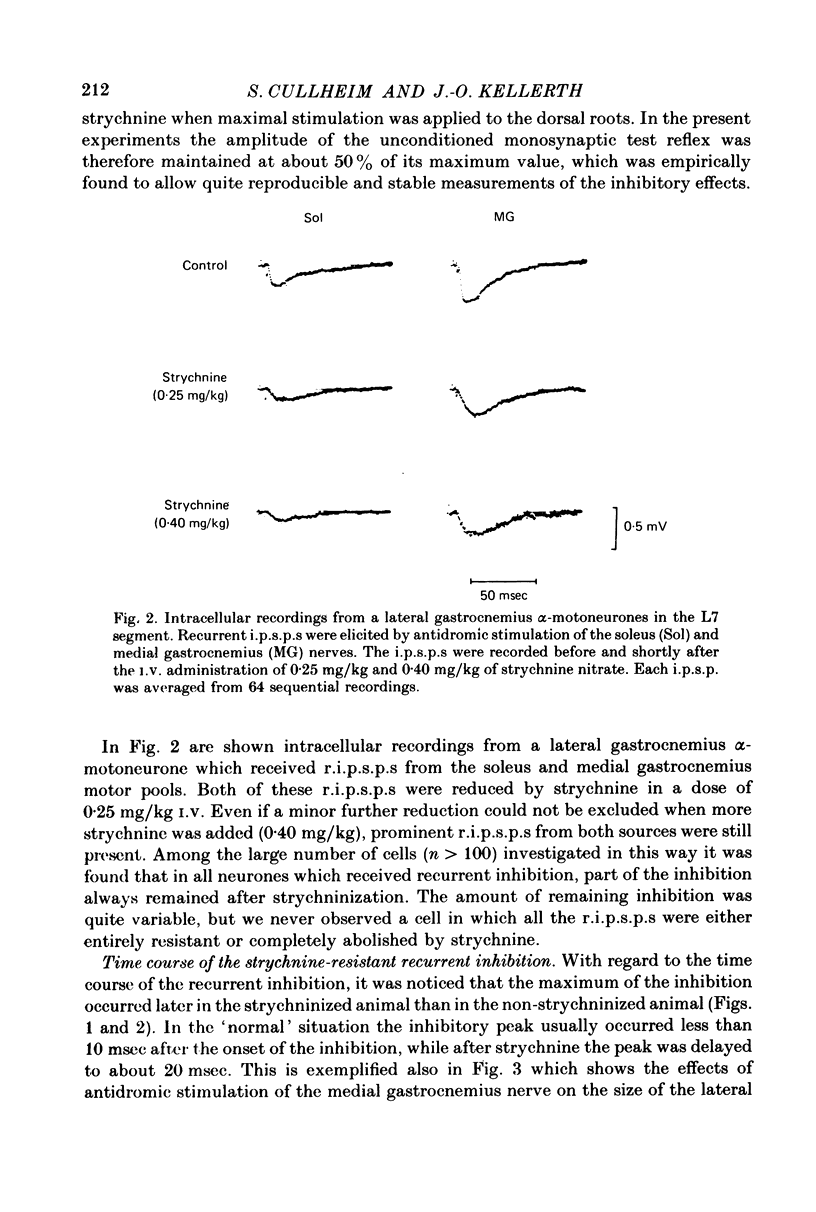
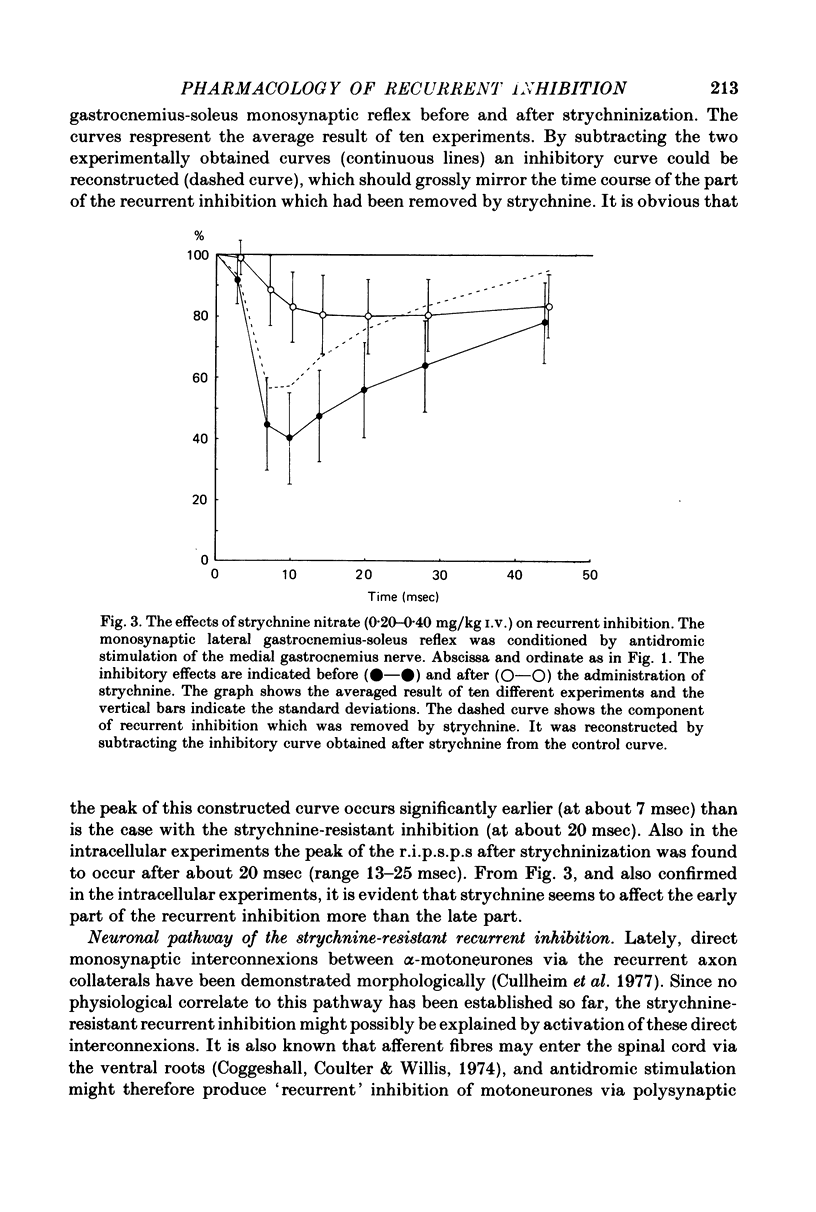
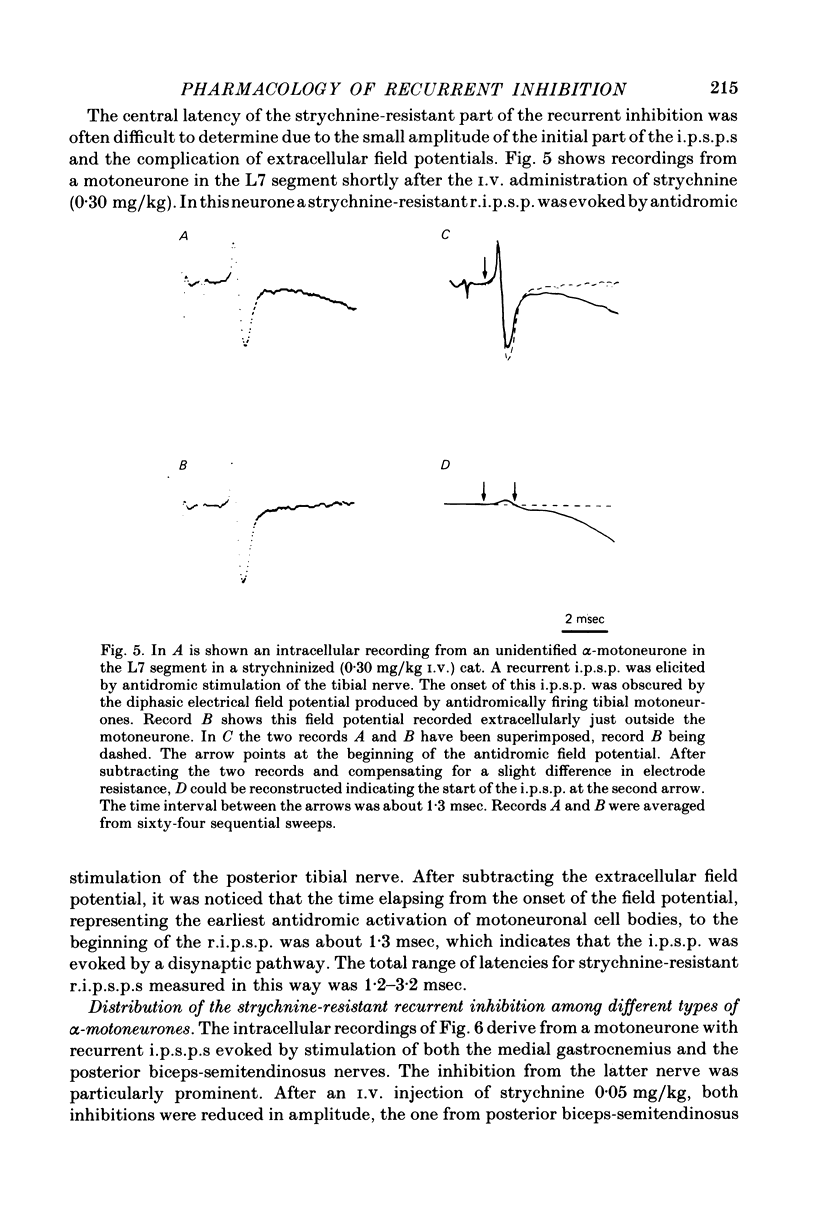
1. The effects of i.v. administration of the glycine-antagonist strychnine nitrate and the GABA-antagonists bicuculline hydrochloride and picrotoxin on the recurrent inhibition of lumbosacral alpha-motoneurones were studied in cats anaesthetized with pentobarbitone sodium. 2. As revealed from both monosynaptic reflex experiments and intracellular recordings, each of the drugs generally reduced, but rarely abolished, the recurrent inhibition. The amount of reduction was more or less identical for bicuculline and picrotoxin. 3. By applying de- and hyperpolarizing currents intracellularly it could be shown that both the strychnine-resistant and bicuculline/picrotoxin-resistant recurrent inhibitory potentials were genuinely post-synaptic in nature. 4. The strychnine-resistant part of the recurrent inhibition had a later maximum and a longer duration than the part which was resistant to bicuculline/picrotoxin. 5. The time course of the strychnine-resistant recurrent inhibition was more or less identical to that of the bicuculline/picrotoxin-sensitive recurrent inhibition. 6. The bicuculline/picrotoxin-resistant recurrent inhibition was blocked by strychnine and, vice versa, the strychnine-resistant recurrent inhibition was blocked by bicuculline/picrotoxin. The combined administration of strychnine and bicuculline/picrotoxin always resulted in a virtual abolition of the recurrent inhibitory effects. 7. The values for central delay suggested that both the strychnine-resistant and bicuculline/picrotoxin-resistant inhibitions were mediated via disynaptic pathways. 8. The results suggest that both glycine and GABA act as transmitter substances of Renshaw cells in mediating recurrent inhibition to alpha-motoneurones. 9. No organizational pattern of the two types of recurrent inhibition based on motor pool category or motor unit type could be detected.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS V. B., WILSON V. J. Recurrent inhibition in the cat's spinal cord. J Physiol. 1959 May 19;146(2):380–391. doi: 10.1113/jphysiol.1959.sp006199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Lawrence D. G., Matthews P. B. Antidromic inhibition of presumed fusimotor neurones by repetitive stimulation of the ventral root in the decerebrate cat. Experientia. 1968 Dec 15;24(12):1210–1212. doi: 10.1007/BF02146625. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Salcman M., Tsairis P. Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. J Physiol. 1974 May;238(3):503–514. doi: 10.1113/jphysiol.1974.sp010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Zajac F. E., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971 Nov 12;174(4010):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E., Coulter J. D., Willis W. D., Jr Unmyelinated axons in the ventral roots of the cat lumbosacral enlargement. J Comp Neurol. 1974 Jan 1;153(1):39–58. doi: 10.1002/cne.901530105. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E., Ito H. Sensory fibres in ventral roots L7 and Si in the cat. J Physiol. 1977 May;267(1):215–235. doi: 10.1113/jphysiol.1977.sp011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different functional types of muscle unit. J Physiol. 1978 Aug;281:301–313. doi: 10.1113/jphysiol.1978.sp012423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different hind-limb muscles. J Physiol. 1978 Aug;281:285–299. doi: 10.1113/jphysiol.1978.sp012422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O., Conradi S. Evidence for direct synaptic interconnections between cat spinal alpha-motoneurons via the recurrent axon collaterals: a morphological study using intracellular injection of horseradish peroxidase. Brain Res. 1977 Aug 19;132(1):1–10. doi: 10.1016/0006-8993(77)90702-8. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Game C. J., Lodge D., McCulloch R. M. A pharmacological study of Renshaw cell inhibition. J Physiol. 1976 Jun;258(1):227–242. doi: 10.1113/jphysiol.1976.sp011416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Biophysical aspects of neuro-muscular transmission. Prog Biophys Biophys Chem. 1956;6:121–170. [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol. 1971 Jul;216(2):419–439. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg C., Kellerth J. O. Studies of some twitch and fatigue properties of different motor unit types in the ankle muscles of the adult cat. Acta Physiol Scand. 1975 Nov;95(3):231–242. doi: 10.1111/j.1748-1716.1975.tb10047.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition from motor axon collatersls in interneurones monosynaptically activated rom la afferents. Brain Res. 1968 Jul;9(2):367–369. doi: 10.1016/0006-8993(68)90240-0. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. J Physiol. 1971 Jul;215(3):613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. A. Neuropharmacology of amino acid inhibitory transmitters. Annu Rev Pharmacol Toxicol. 1978;18:269–289. doi: 10.1146/annurev.pa.18.040178.001413. [DOI] [PubMed] [Google Scholar]
- Lagerbäck P. A., Ronnevi L. O., Cullheim S., Kellerth J. O. Ultrastructural characteristics of a central cholinergic synapse in the cat. Brain Res. 1978 Jun 9;148(1):197–201. doi: 10.1016/0006-8993(78)90389-x. [DOI] [PubMed] [Google Scholar]
- Larson M. D. An analysis of the action of strychnine on the recurrent IPSP and amino acid induced inhibitions in the cat spinal cord. Brain Res. 1969 Sep;15(1):185–200. doi: 10.1016/0006-8993(69)90318-7. [DOI] [PubMed] [Google Scholar]
- Lindström S., Schomburg E. D. Recurrent inhibition from motor axon collaterals of ventral spinocerebellar tract neurones. Acta Physiol Scand. 1973 Aug;88(4):505–515. doi: 10.1111/j.1748-1716.1973.tb05479.x. [DOI] [PubMed] [Google Scholar]
- Loeb G. E. Ventral root projections of myelinated dorsal root ganglion cells in the cat. Brain Res. 1976 Apr 16;106(1):159–165. doi: 10.1016/0006-8993(76)90081-0. [DOI] [PubMed] [Google Scholar]
- Piercey M. F., Goldfarb J., Ryall R. W. Effects of picrotoxin and bicuculline on the excitation and inhibition of Renshaw cells. Neuropharmacology. 1973 Oct;12(10):975–982. doi: 10.1016/0028-3908(73)90029-4. [DOI] [PubMed] [Google Scholar]
- Ryall R. W. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970 Mar;33(2):257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]


