Abstract
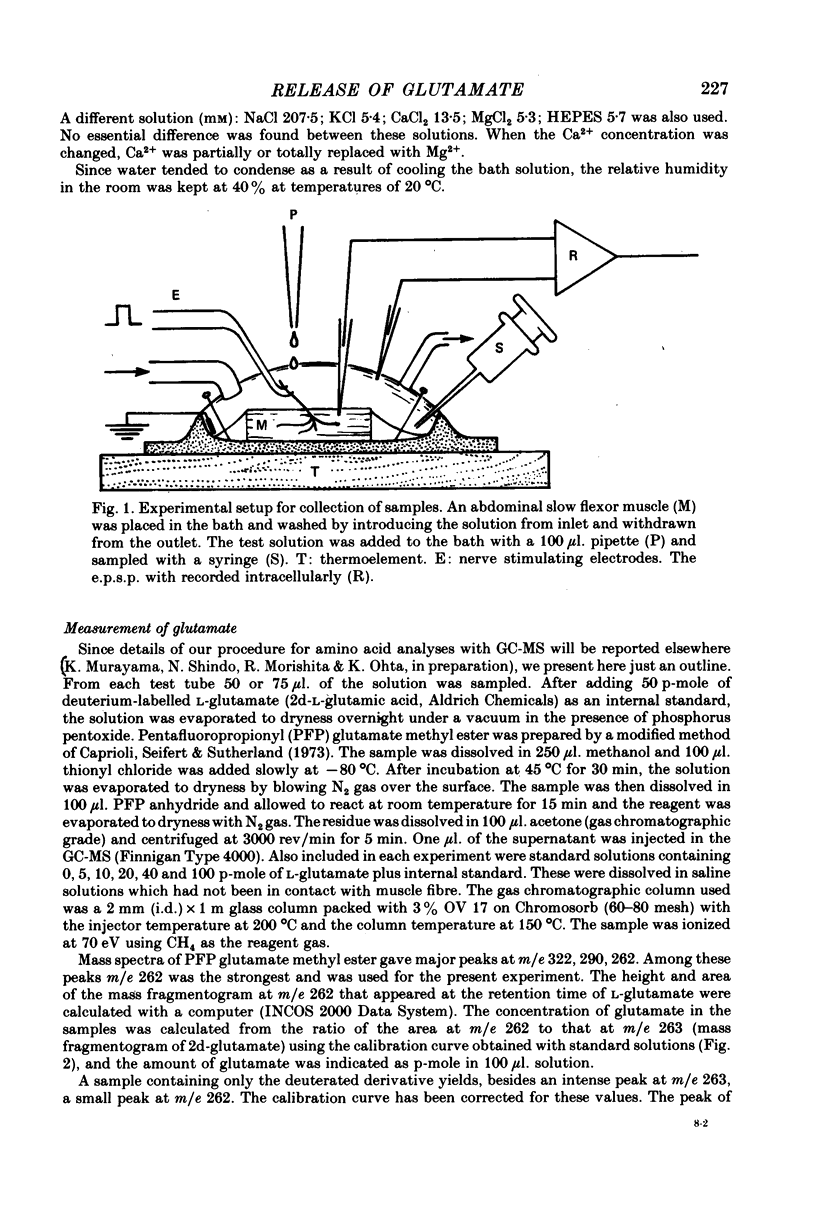
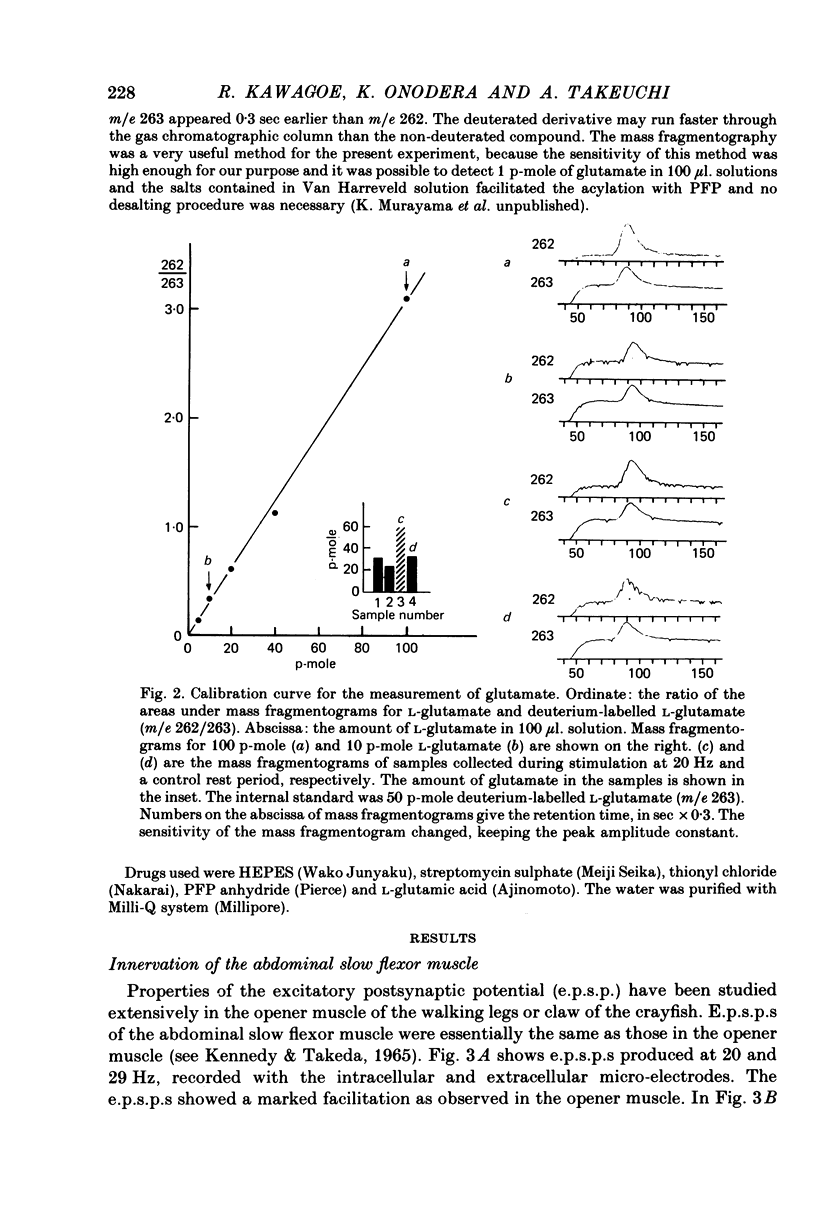
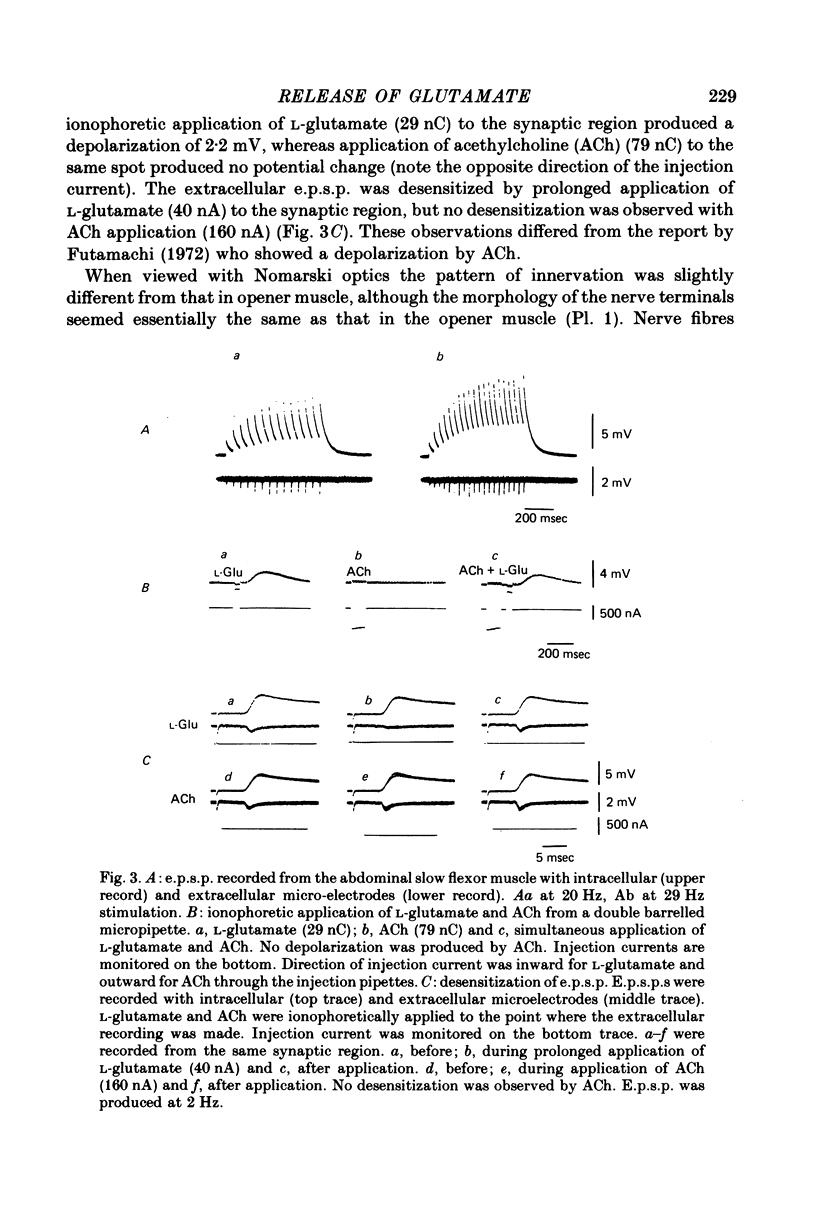
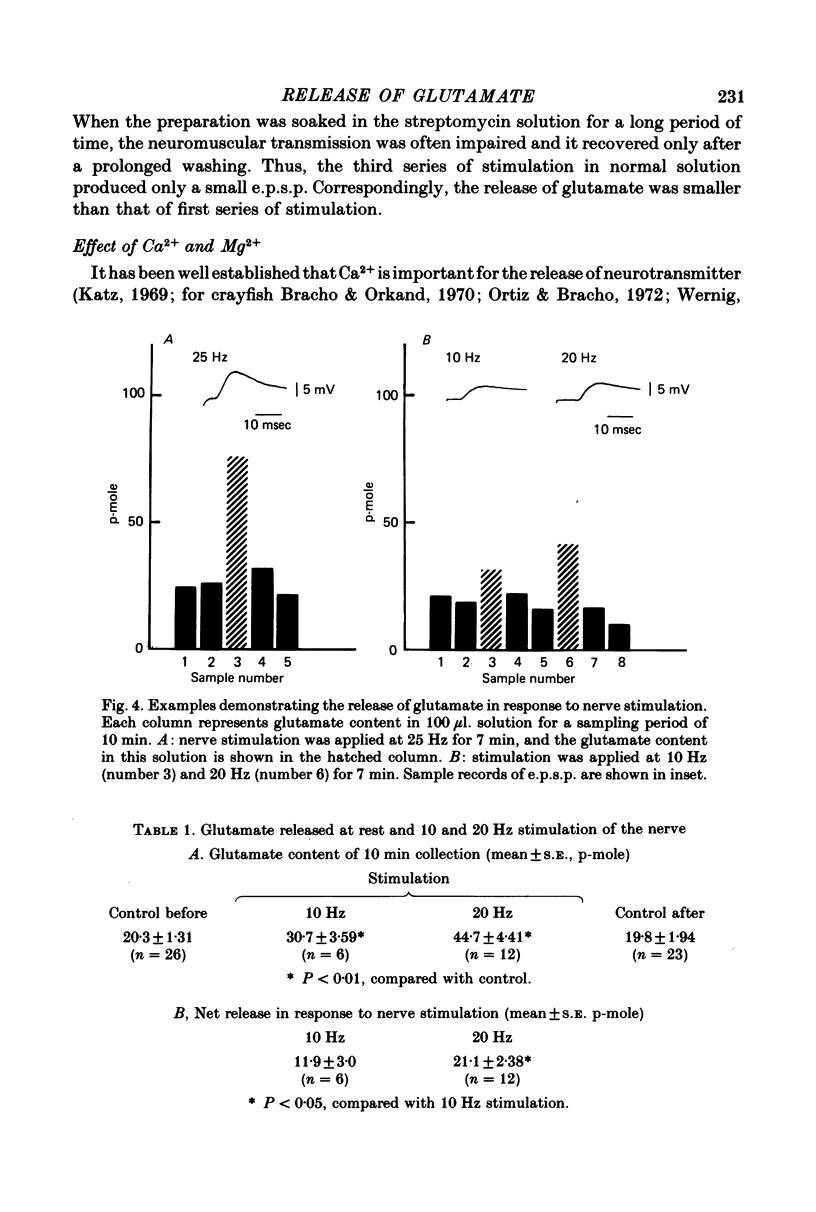
1. The superficial abdominal flow flexor muscle was isolated from the crayfish (Cambarus clarkii) and placed in a bath solution of 100 microliters. The concentration of glutamate in this solution was measured by mass fragmentography using a gas chromatograph-mass spectrometer. 2. The excitatory post-synaptic potential (e.p.s.p.) of the slow flexor muscle and its sensitivity to L-glutamate were similar to those observed in the opener muscle of the dactyl in the walking leg or claw of the crayfish. 3. The background efflux of glutamate during control rest periods was about 20 p-mole/10 min. Nerve stimulation caused a significant increase in the efflux of glutamate. The net release of glutamate above the background was 11.9 p-mole/100 microliters. at 10 Hz stimulation and 21.1 p-mole/100 microliters. at 20 Hz stimulation. 4. When the amplitude of e.p.s.p. was decreased by streptomycin, thereby reducing the muscle contraction, the net release of glutamate by nerve stimulation was not changed. Streptomycin depressed the e.p.s.p. by its action on the post-synaptic membrane. 5. When the external concentration of Ca was lowered, the amplitude of e.p.s.p. and the net release of glutamate were decreased. 6. It is concluded that L-glutamate is released from the nerve terminals of the crayfish neuromuscular junction.
Full text
PDF


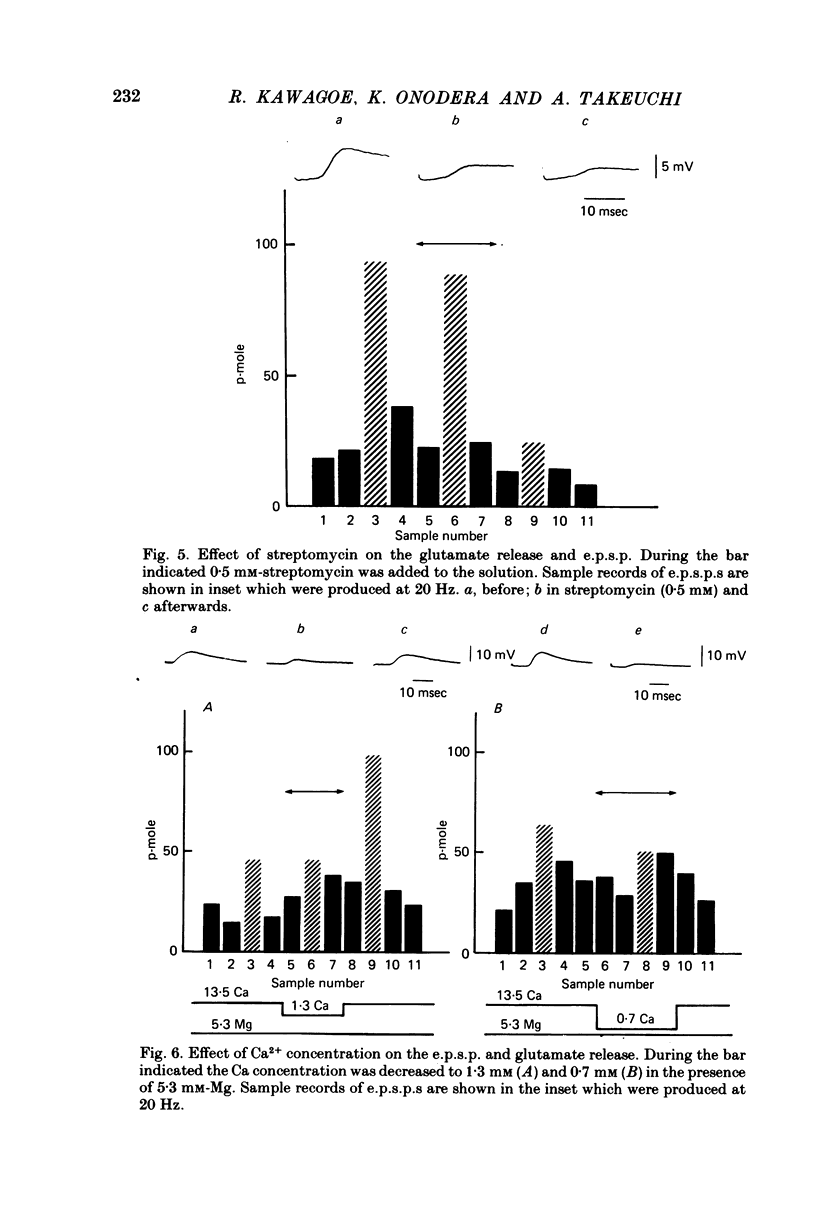




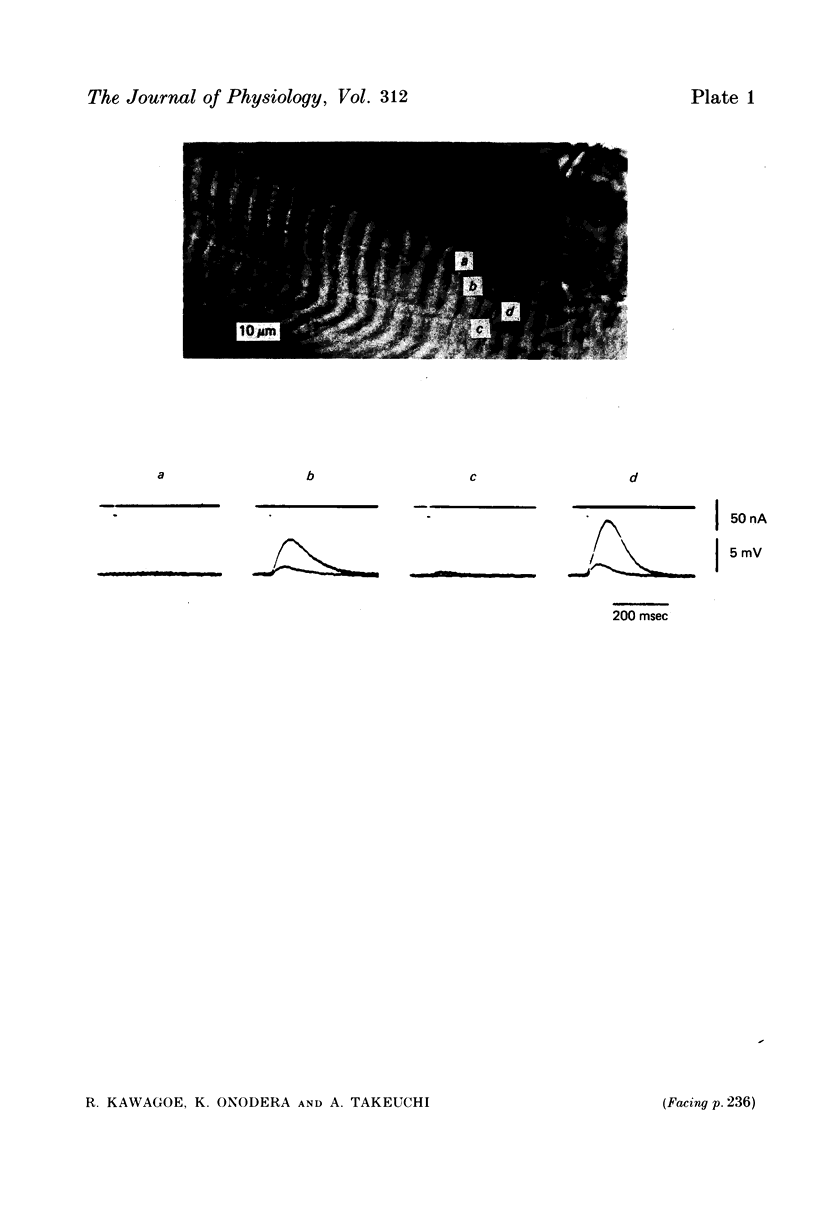
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracho H., Orkand R. K. Effect of calcium on excitatory neuromuscular transmission in the crayfish. J Physiol. 1970 Jan;206(1):61–71. doi: 10.1113/jphysiol.1970.sp008997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli R. M., Seifert W. E., Jr, Sutherland D. E. Polypeptide sequencing: use of dipeptidylaminopeptidase I and gas chromatography-mass spectrometry. Biochem Biophys Res Commun. 1973 Nov 1;55(1):67–75. doi: 10.1016/s0006-291x(73)80060-9. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. On the elementary conductance event produced by L-glutamate and quanta of the natural transmitter at the neuromuscular junctions of Maia squinado. J Physiol. 1976 Jun;258(1):205–225. doi: 10.1113/jphysiol.1976.sp011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Futamachi K. J. Acetylcholine: possible neuromuscular transmitter in Crustacea. Science. 1972 Mar 24;175(4028):1373–1375. doi: 10.1126/science.175.4028.1373. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Hildebrand J. G., Townsel J. G., Kravitz E. A. Distribution of acetylcholine, choline, choline acetyltransferase and acetylcholinesterase in regions and single identified axons of the lobster nervous system. J Neurochem. 1974 Nov;23(5):951–963. doi: 10.1111/j.1471-4159.1974.tb10747.x. [DOI] [PubMed] [Google Scholar]
- Kerkut G. A., Leake L. D., Shapira A., Cowan S., Walker R. J. The presence of glutamate in nerve-muscle perfusates of Helix, Carcinus and Periplaneta. Comp Biochem Physiol. 1965 Aug;15(4):485–502. doi: 10.1016/0010-406x(65)90148-9. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. An analysis of acetylcholine in frog muscle by mass fragmentography. Proc R Soc Lond B Biol Sci. 1977 Jun 15;197(1128):285–297. doi: 10.1098/rspb.1977.0071. [DOI] [PubMed] [Google Scholar]
- Minchin M. C., Iversen L. L. Release of (3H)gamma-aminobutyric acid from glial cells in rat dorsal root ganglia. J Neurochem. 1974 Sep;23(3):533–540. doi: 10.1111/j.1471-4159.1974.tb06056.x. [DOI] [PubMed] [Google Scholar]
- Obata K., Takeda K., Shinozaki H. Electrophoretic release of gamma-aminobutyric acid and glutamic acid from micropipettes. Neuropharmacology. 1970 May;9(3):191–194. doi: 10.1016/0028-3908(70)90066-3. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Inhibitory effect of streptomycin and related antibiotics on the glutamate receptor of the crayfish neuromuscular junction. Neuropharmacology. 1977 Mar;16(3):171–177. doi: 10.1016/0028-3908(77)90092-2. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Ionic mechanism of the excitatory synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1975 Oct;252(1):295–318. doi: 10.1113/jphysiol.1975.sp011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Permeability changes produced by L-glutamate at the excitatory post-synaptic membrane of the crayfish muscle. J Physiol. 1976 Mar;255(3):669–685. doi: 10.1113/jphysiol.1976.sp011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz C. L., Bracho H. Effect of reduced calcium on excitatory transmitter release at the crayfish neuromuscular junction. Comp Biochem Physiol A Comp Physiol. 1972 Apr 1;41(4):805–812. doi: 10.1016/0300-9629(72)90343-x. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Iversen L. L., Hall Z. W., Kravitz E. A. Release of gamma-aminobutyric acid from inhibitory nerves of lobster. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1110–1115. doi: 10.1073/pnas.56.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Actions of transmitter substances on the neuromuscular junctions of vertebrates and invertebrates. Adv Biophys. 1972;3:45–95. [PubMed] [Google Scholar]
- Usherwood P. N., Machili P., Leaf G. L-Glutamate at insect excitatory nerve-muscle synapses. Nature. 1968 Sep 14;219(5159):1169–1172. doi: 10.1038/2191169a0. [DOI] [PubMed] [Google Scholar]
- Wang L. D., Boyarsky L. L. Release of L-glutamate from the excitatory nerve terminals of the crayfish neuromuscular junction. Life Sci. 1979 Mar 12;24(11):1011–1014. doi: 10.1016/0024-3205(79)90320-5. [DOI] [PubMed] [Google Scholar]
- Wernig A. The effects of calcium and magnesium on statistical release parameters at the crayfish neuromuscular junction. J Physiol. 1972 Nov;226(3):761–768. doi: 10.1113/jphysiol.1972.sp010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Washio H. Curare has a voltage-dependent blocking action on the glutamate synapse. Nature. 1979 Oct 4;281(5730):372–373. doi: 10.1038/281372a0. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Characteristics of crayfish neuromuscular facilitation and their calcium dependence. J Physiol. 1974 Aug;241(1):91–110. doi: 10.1113/jphysiol.1974.sp010642. [DOI] [PMC free article] [PubMed] [Google Scholar]



