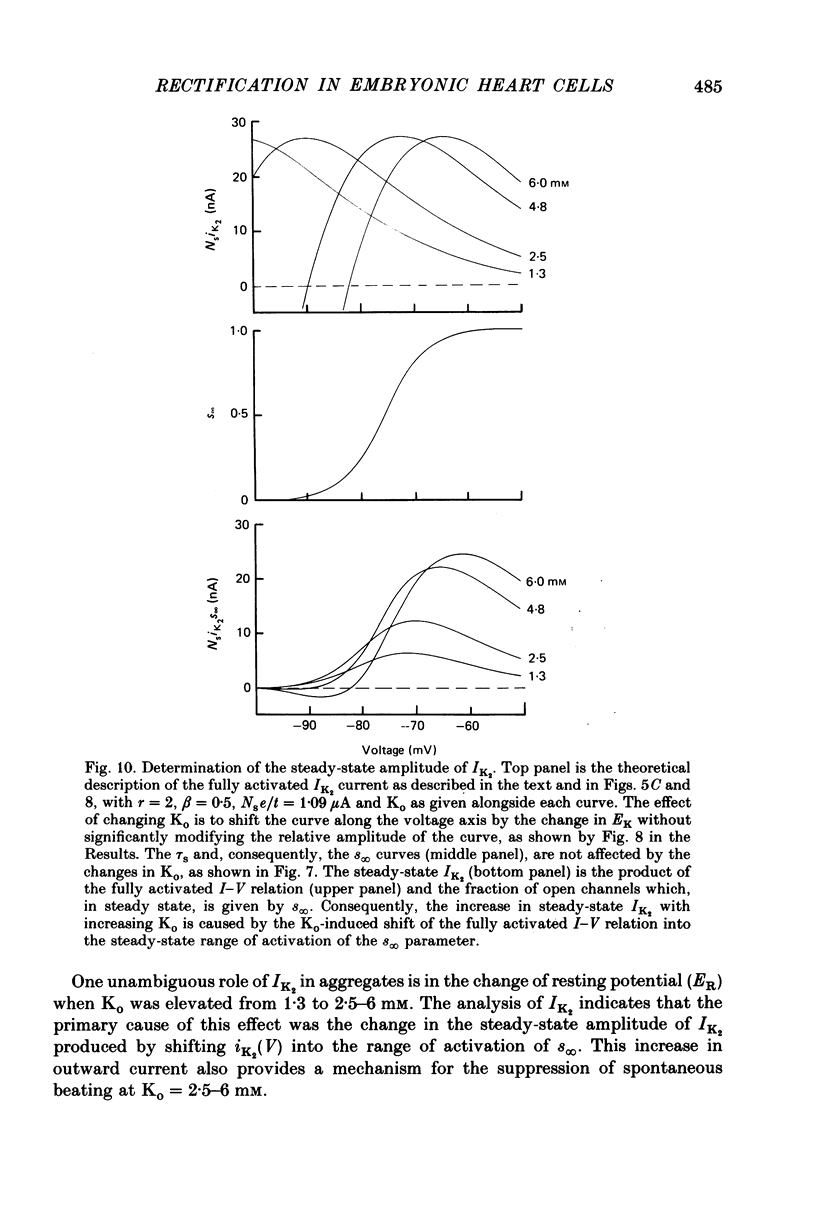
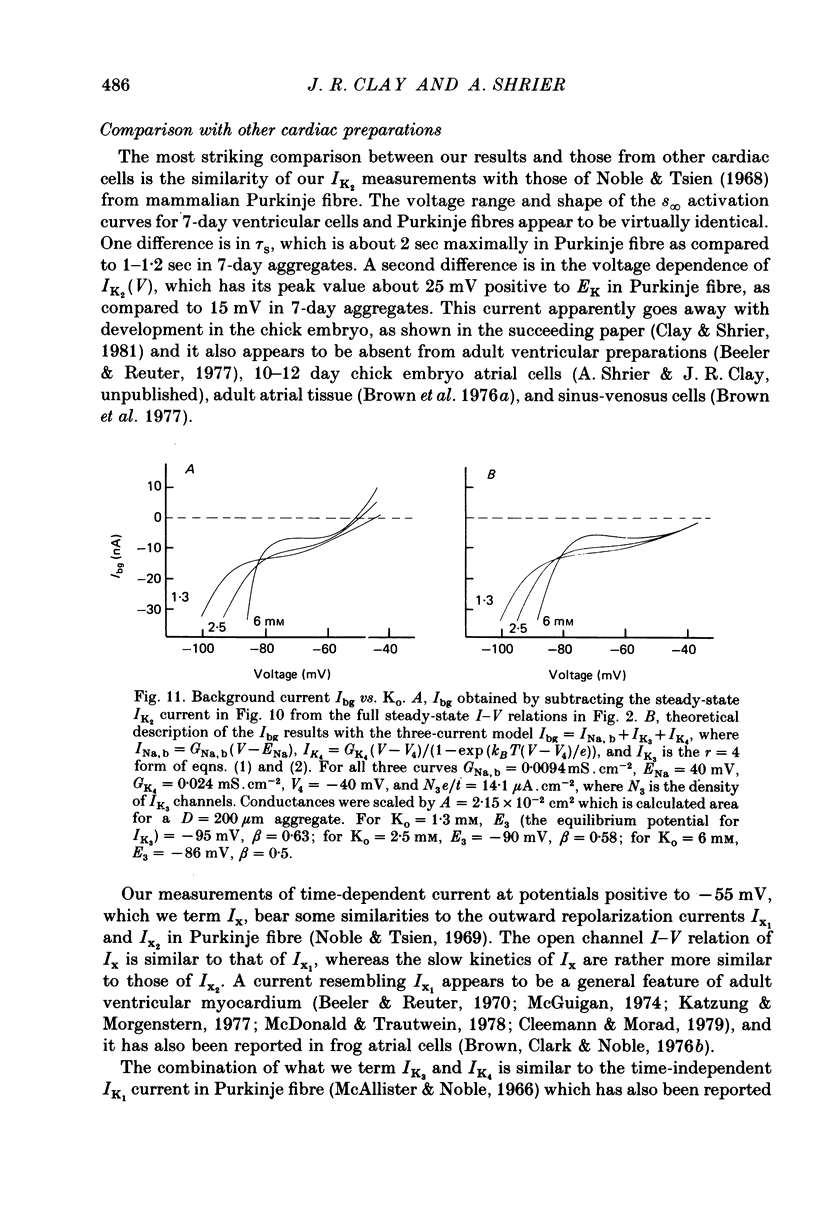
Abstract
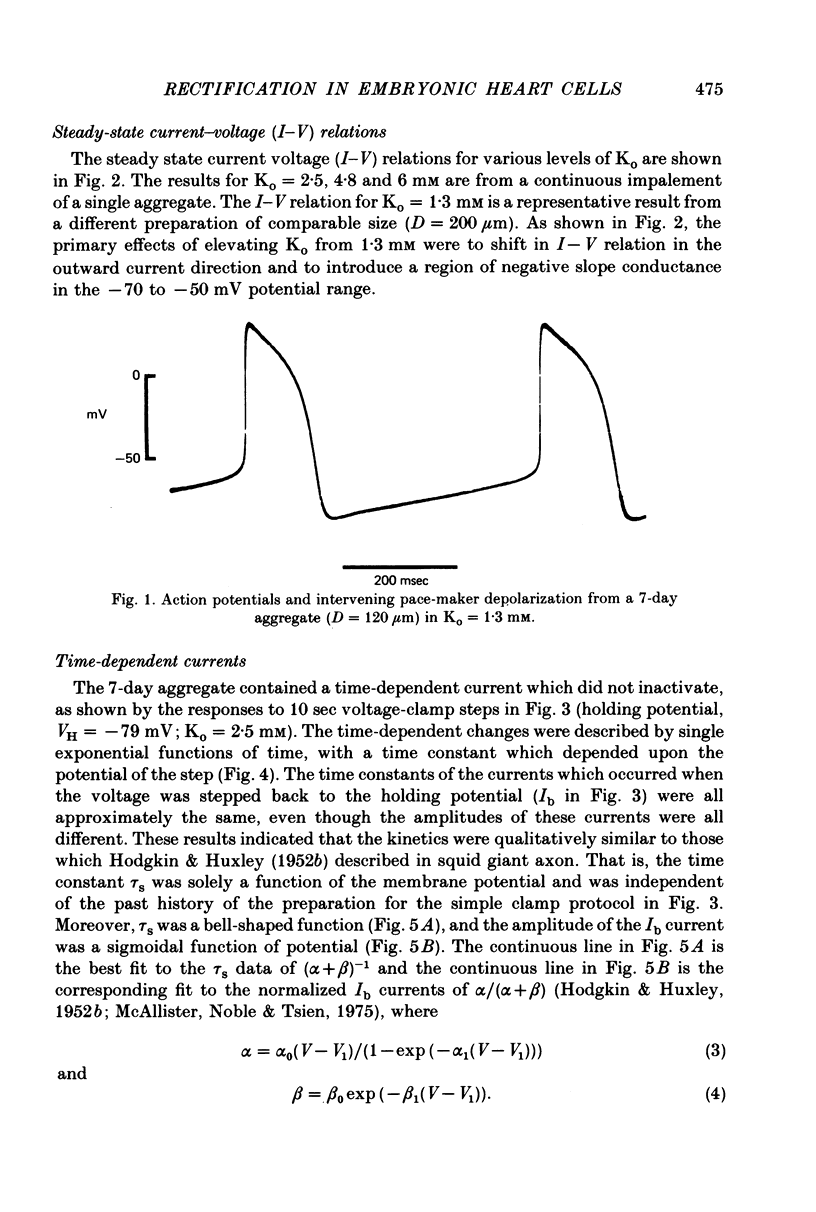
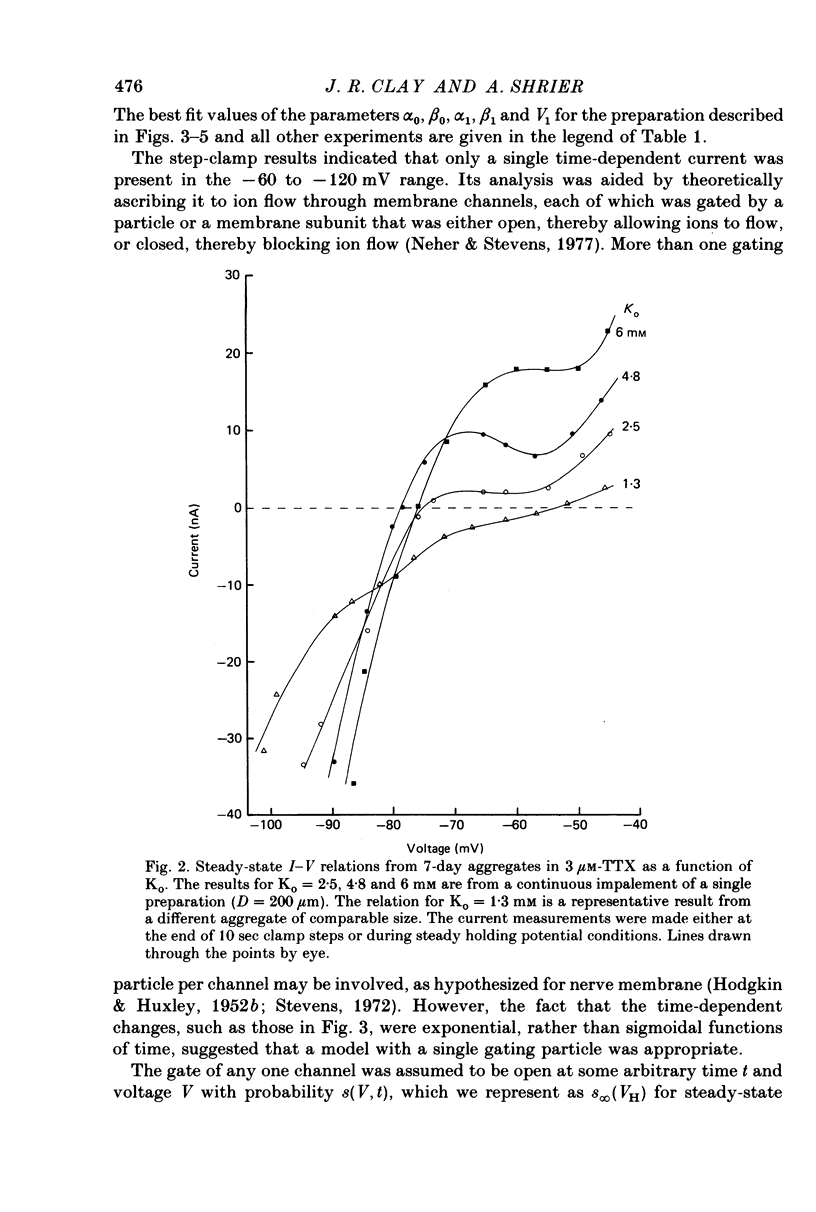
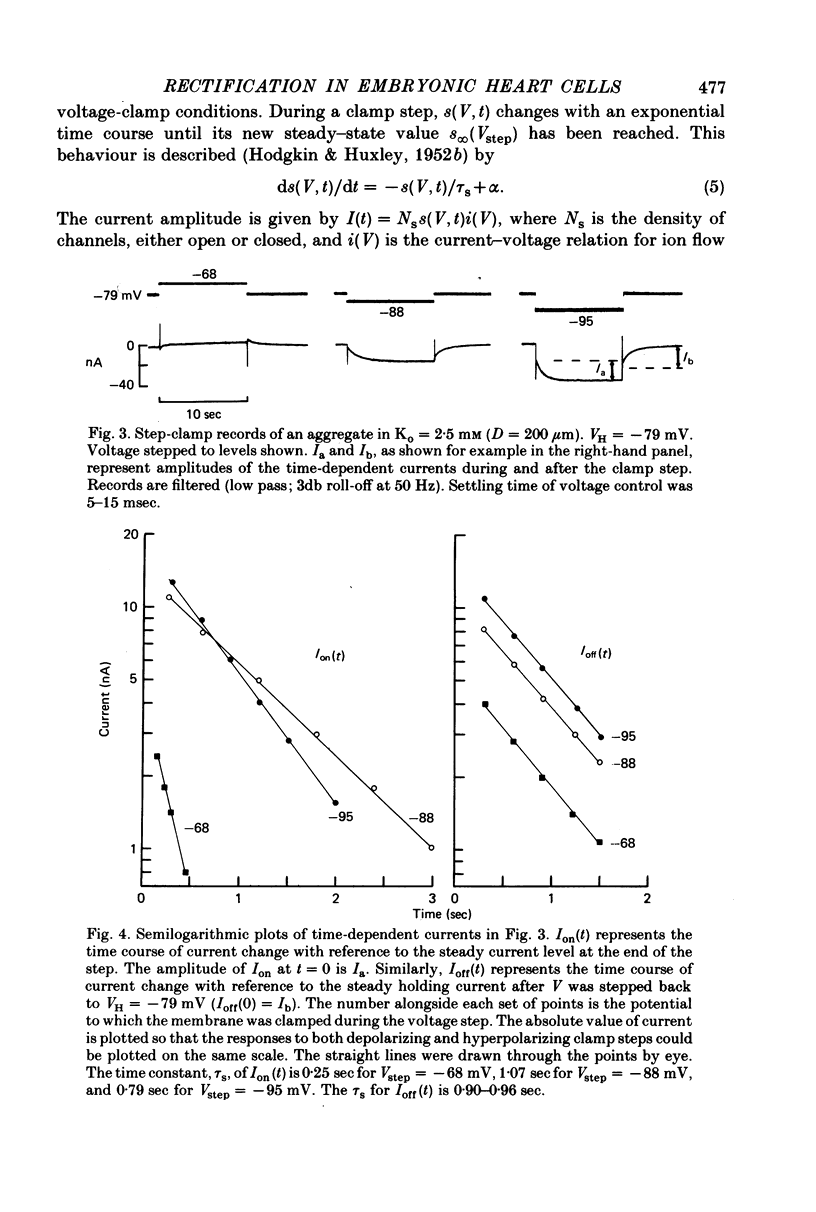
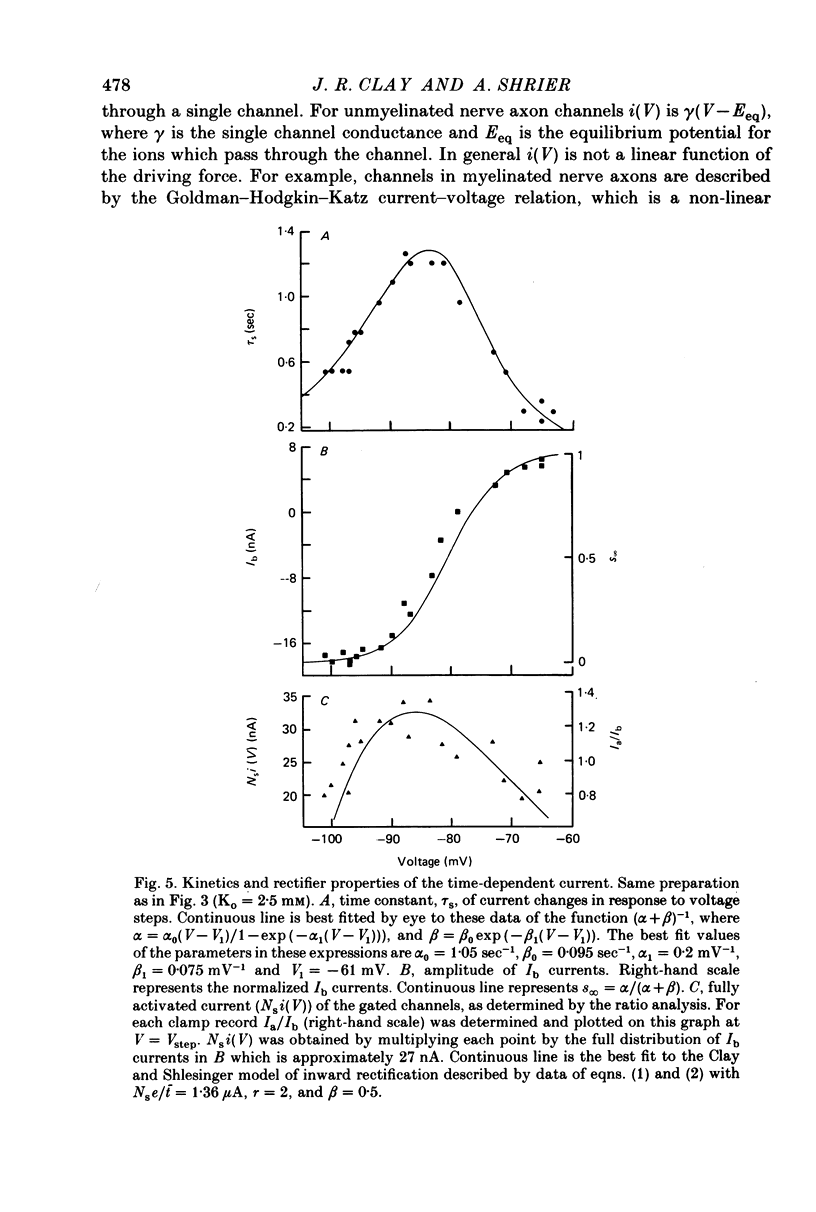
1. Small re-aggregates of cells dissociated from the ventricles of 7-day-old chick embryonic hearts beat spontaneously in low external potassium concentration (Ko = 1.3 mM) tissue culture medium. This activity was blocked by the addition of tetrodotoxin (TTX) or potassium ions to the external medium. 2. A two-micro-electrode voltage-clamp technique was used to analyse the subthreshold currents responsible for the pace-maker depolarization. 3. Voltage-clamp steps 6-10 sec in duration revealed a time-dependent current having first order kinetics. Its membrane potential range of steady-state activation was -90 to -60 mV. 4. The current kinetics were qualitatively similar to those of Hodgkin & Huxley (1952b) with a peak time constant of approximately 1 sec at V = -75 mV. The kinetics were independent of Ko. 5. The time-dependent current was attributed to gated membrane channels. The fully activated current-voltage (I-V) relation of the channels was determined from the ratio of the amplitudes of the time-dependent currents during and after voltage-clamp steps following the procedure of Noble & Tsien (1968). 6. The fully activated I-V relation displayed inward rectification with negative slope conductance at potentials more than 15 mV positive to its reversal potential. Changes of Ko shifted the I-V curve along the voltage axis like a potassium electrode. 7. The time-independent (background) current was obtained by subtracting the gated channel current from the steady-state I-V curve. This current also rectified in the inward direction. 8. The inwardly rectifying I-V relations were theoretically described by a channel having a row of ion-selective sites along which ions move in a single file (Hodgkin & Keynes, 1955), and a membrane-bound particle which blocked the channel in a voltage-dependent manner. 9. The relationship of the voltage-clamp results to spontaneous activity is discussed and comparisons are made with measurements from whole embryonic heart and other cardiac tissues.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H., FREYGANG W. H. Potassium conductance of frog muscle membrane under controlled voltage. J Physiol. 1962 Aug;163:104–114. doi: 10.1113/jphysiol.1962.sp006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten C. M., Isenberg G. Depletion and accumulation of potassium in the extracellular clefts of cardiac Purkinje fibers during voltage clamp hyperpolarization and depolarization. Pflugers Arch. 1977 Mar 11;368(1-2):19–31. doi: 10.1007/BF01063450. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Voltage clamp experiments on ventricular myocarial fibres. J Physiol. 1970 Mar;207(1):165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Clark A., Noble S. J. Analysis of pace-maker and repolarization currents in frog atrial muscle. J Physiol. 1976 Jul;258(3):547–577. doi: 10.1113/jphysiol.1976.sp011435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Clark A., Noble S. J. Identification of the pace-maker current in frog atrium. J Physiol. 1976 Jul;258(3):521–545. doi: 10.1113/jphysiol.1976.sp011434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Giles W., Noble S. J. Membrane currents underlying activity in frog sinus venosus. J Physiol. 1977 Oct;271(3):783–816. doi: 10.1113/jphysiol.1977.sp012026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. E., Horres C. R., Lieberman M., Vereecke J. S. Developmental aspects of potassium flux and permeability of the embryonic chick heart. J Physiol. 1976 Jan;254(3):673–692. doi: 10.1113/jphysiol.1976.sp011252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., DeFelice L. J., DeHaan R. L. Current noise parameters derived from voltage noise and impedance in embryonic heart cell aggregates. Biophys J. 1979 Nov;28(2):169–184. doi: 10.1016/S0006-3495(79)85169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., DeHaan R. L. Fluctuations in interbeat interval in rhythmic heart-cell clusters. Role of membrane voltage noise. Biophys J. 1979 Dec;28(3):377–389. doi: 10.1016/S0006-3495(79)85187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., Shlesinger M. F. Random walk analysis of potassium fluxes associated with nerve impulses. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5543–5546. doi: 10.1073/pnas.74.12.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., Shrier A. Developmental changes in subthreshold pace-maker currents in chick embryonic heart cells. J Physiol. 1981 Mar;312:491–504. doi: 10.1113/jphysiol.1981.sp013640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleemann L., Morad M. Potassium currents in frog ventricular muscle: evidence from voltage clamp currents and extracellular K accumulation. J Physiol. 1979 Jan;286:113–143. doi: 10.1113/jphysiol.1979.sp012609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Inward and delayed outward membrane currents in isolated neural somata under voltage clamp. J Physiol. 1971 Feb;213(1):1–19. doi: 10.1113/jphysiol.1971.sp009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaan R. L. The potassium-sensitivity of isolated embryonic heart cells increases with development. Dev Biol. 1970 Oct;23(2):226–240. doi: 10.1016/0012-1606(70)90096-5. [DOI] [PubMed] [Google Scholar]
- Dehaan R. L., Fozzard H. A. Membrane response to current pulses in spheroidal aggregates of embryonic heart cells. J Gen Physiol. 1975 Feb;65(2):207–222. doi: 10.1085/jgp.65.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellam D. C., Studt J. W. A core-conductor model of the cardiac Purkinje fibre based on structural analysis. J Physiol. 1974 Dec;243(3):637–660. doi: 10.1113/jphysiol.1974.sp010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzung B. G., Morgenstern J. A. Effects of extracellular potassium on ventricular automaticity and evidence for a pacemaker current in mammalian ventricular myocardium. Circ Res. 1977 Jan;40(1):105–111. doi: 10.1161/01.res.40.1.105. [DOI] [PubMed] [Google Scholar]
- McAllister R. E., Noble D. The time and voltage dependence of the slow outward current in cardiac Purkinje fibres. J Physiol. 1966 Oct;186(3):632–662. doi: 10.1113/jphysiol.1966.sp008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., DeHaan R. L. Ion levels and membrane potential in chick heart tissue and cultured cells. J Gen Physiol. 1973 Jan;61(1):89–109. doi: 10.1085/jgp.61.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. The potassium current underlying delayed rectification in cat ventricular muscle. J Physiol. 1978 Jan;274:217–246. doi: 10.1113/jphysiol.1978.sp012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan J. A. Some limitations of the double sucrose gap, and its use in a study of the slow outward current in mammalian ventricular muscle. J Physiol. 1974 Aug;240(3):775–806. doi: 10.1113/jphysiol.1974.sp010634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R. D., DeHaan R. L. Voltage clamp analysis of embryonic heart cell aggregates. J Gen Physiol. 1979 Feb;73(2):175–198. doi: 10.1085/jgp.73.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S. J. Potassium accumulation and depletion in frog atrial muscle. J Physiol. 1976 Jul;258(3):579–613. doi: 10.1113/jphysiol.1976.sp011436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs H. G., DeHaan R. L. Embryonic myocardial cell aggregates: volume and pulsation rate. Dev Biol. 1973 Jan;30(1):233–240. doi: 10.1016/0012-1606(73)90064-x. [DOI] [PubMed] [Google Scholar]
- Shrier A., Clay J. R. Pacemaker currents in chick embryonic heart cells change with development. Nature. 1980 Feb 14;283(5748):670–671. doi: 10.1038/283670a0. [DOI] [PubMed] [Google Scholar]
- TASAKI I. New measurements of the capacity and the resistance of the myelin sheath and the nodal membrane of the isolated frog nerve fiber. Am J Physiol. 1955 Jun;181(3):639–650. doi: 10.1152/ajplegacy.1955.181.3.639. [DOI] [PubMed] [Google Scholar]
- Van Mierop L. H. Location of pacemaker in chick embryo heart at the time of initiation of heartbeat. Am J Physiol. 1967 Feb;212(2):407–415. doi: 10.1152/ajplegacy.1967.212.2.407. [DOI] [PubMed] [Google Scholar]


