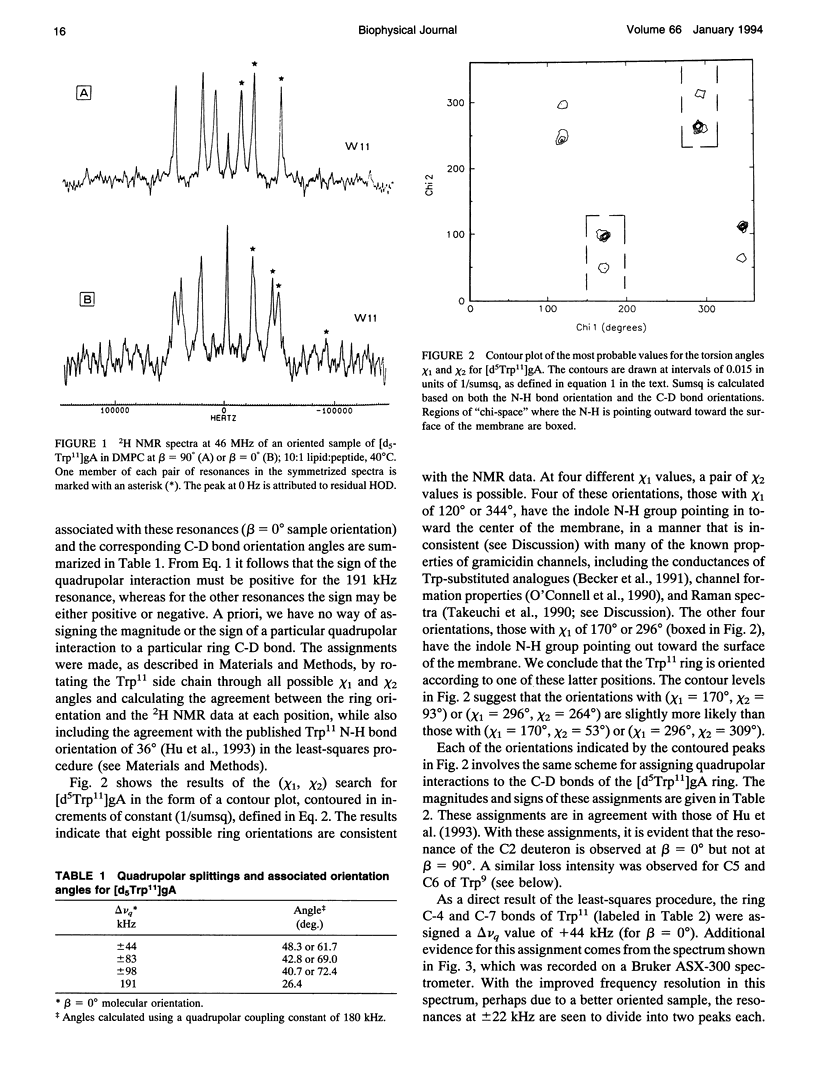
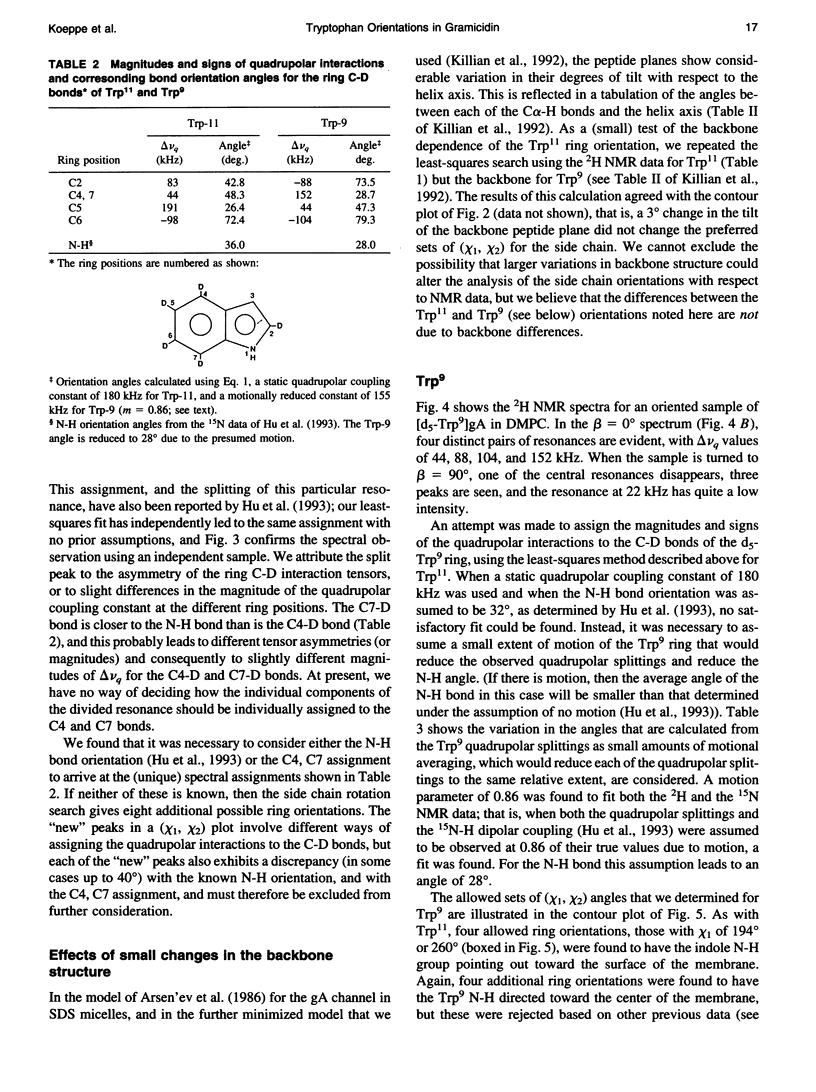
Abstract
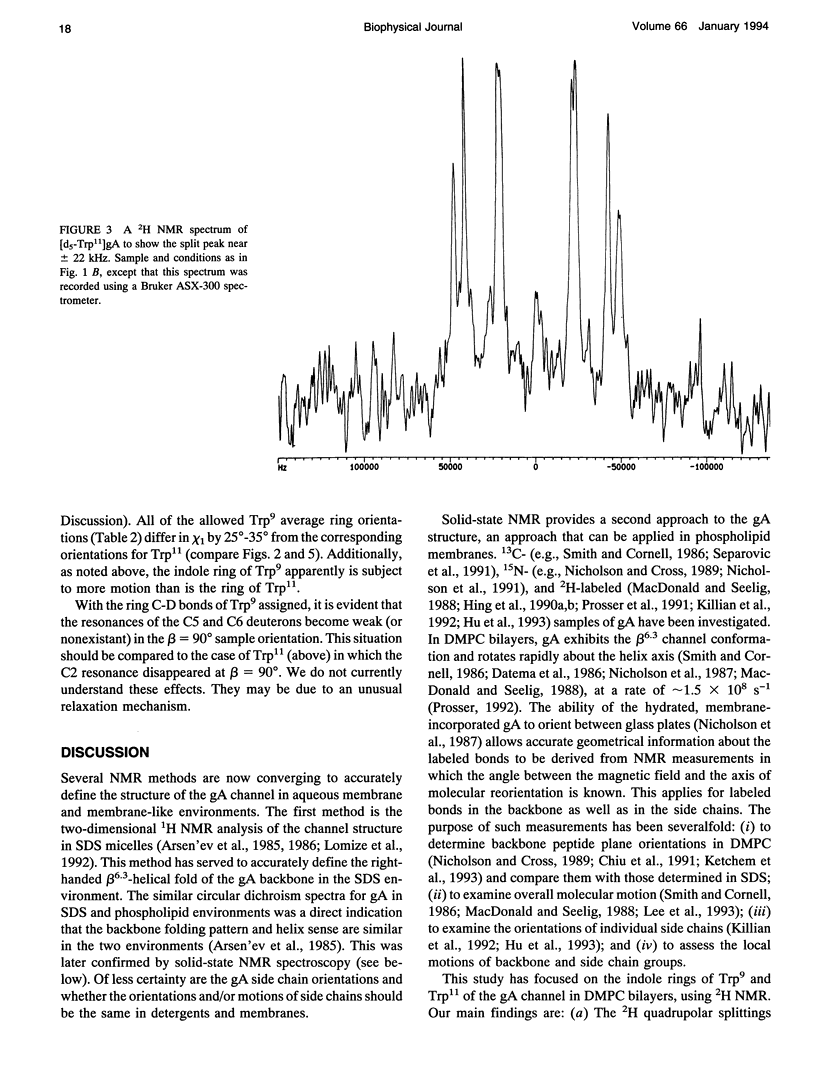
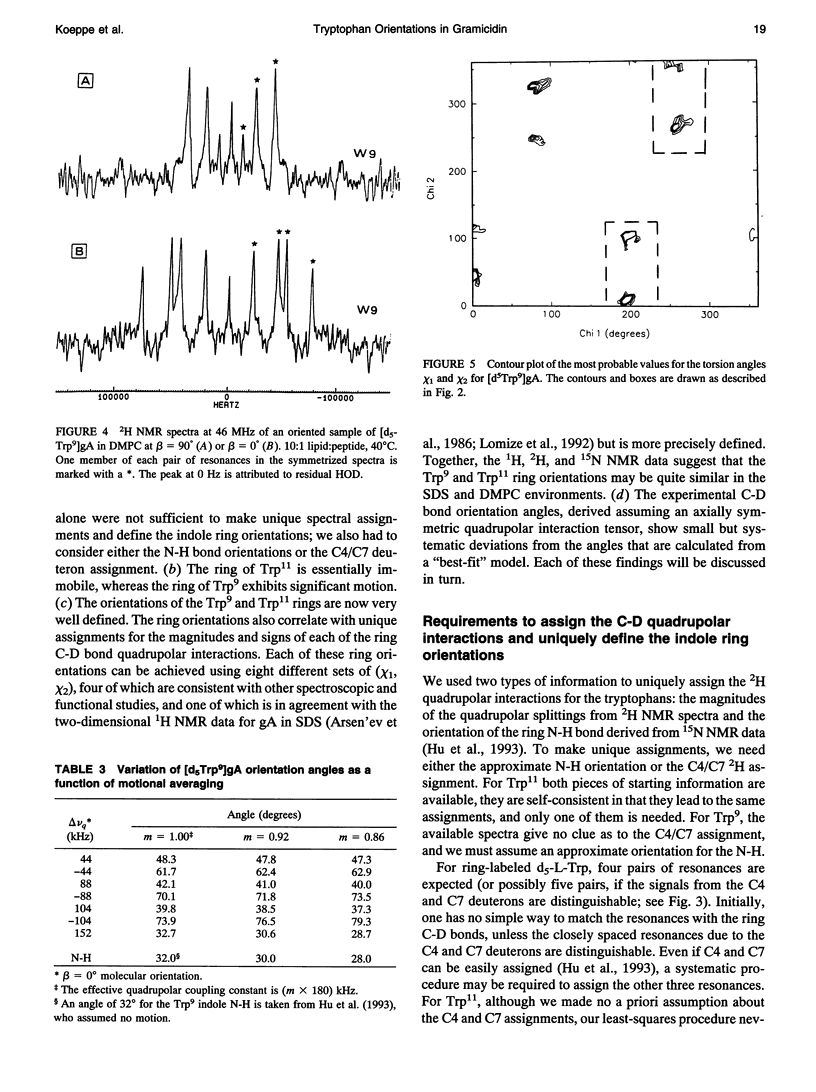
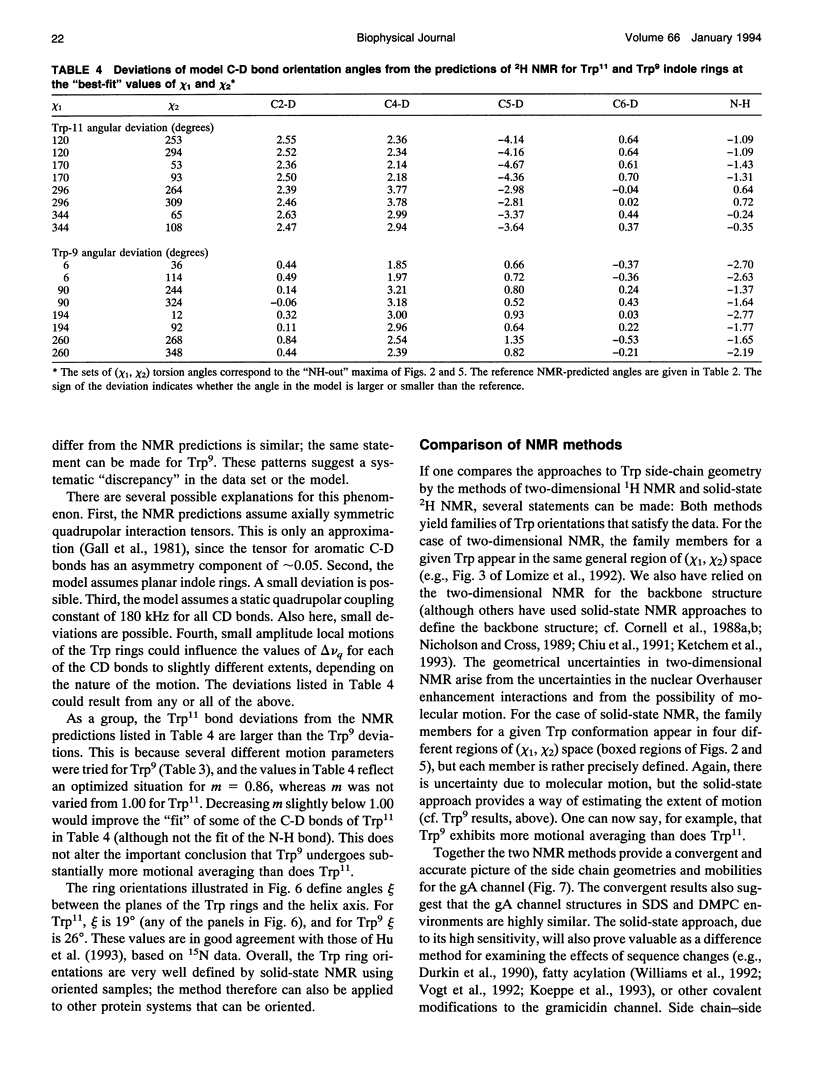
Deuterium nuclear magnetic resonance spectroscopy was used to investigate the orientations of the indole rings of Trp9 and Trp11 in specific indole-d5-labeled samples of gramicidin A incorporated into dimyristoyl phosphatidylcholine bilayers in the beta 6.3 channel conformation. The magnitudes and signs of the deuterium quadrupolar splittings were fit to the rings and assigned to specific ring bonds, using a full rotation search of the chi 1 and chi 2 angles of each Trp and a least-squares method. Unique assignments were obtained. The data and assignments are in close agreement with four sets of (chi 1, chi 2) angles for each Trp in which the indole N-H is oriented toward the membrane's exterior surface. (Four additional sets of (chi 1, chi 2) angles with the N-H's pointing toward the membrane interior are inconsistent with previous observations.) One of the sets of (chi 1, chi 2) angles for each Trp is consistent with the corresponding Trp orientation found by Arsen'ev et al. (1986. Biol. Membr. 3:1077-1104) for gramicidin in sodium dodecyl sulfate micelles. Together, the 1H and 2H nuclear magnetic resonance methods suggest that the Trp9 and Trp11 side chain orientations could be very similar in dimyristoyl phosphatidylcholine membranes and in sodium dodecyl sulfate micelles. The data for Trp11 could be fit using a static quadrupolar coupling constant of 180 kHz under the assumption that the ring is essentially immobile. By contrast, Trp9 could be fit only under the assumption that the quadrupolar splittings for ring 9 are reduced by approximately 14% due to motional averaging. Such a difference in motional averaging between rings 11 and 9 is also consistent with the 15N data of Hu et al. (1993. Biochemistry. 32:7035-7047).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S., Koeppe R. E., 2nd Molecular determinants of channel function. Physiol Rev. 1992 Oct;72(4 Suppl):S89–158. doi: 10.1152/physrev.1992.72.suppl_4.S89. [DOI] [PubMed] [Google Scholar]
- Arseniev A. S., Barsukov I. L., Bystrov V. F., Lomize A. L., Ovchinnikov YuA 1H-NMR study of gramicidin A transmembrane ion channel. Head-to-head right-handed, single-stranded helices. FEBS Lett. 1985 Jul 8;186(2):168–174. doi: 10.1016/0014-5793(85)80702-x. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Noda K., Gross E., Läuger P. Single-channel parameters of gramicidin A,B, and C. Biochim Biophys Acta. 1976 Jan 21;419(2):223–228. doi: 10.1016/0005-2736(76)90348-5. [DOI] [PubMed] [Google Scholar]
- Becker M. D., Greathouse D. V., Koeppe R. E., 2nd, Andersen O. S. Amino acid sequence modulation of gramicidin channel function: effects of tryptophan-to-phenylalanine substitutions on the single-channel conductance and duration. Biochemistry. 1991 Sep 10;30(36):8830–8839. doi: 10.1021/bi00100a015. [DOI] [PubMed] [Google Scholar]
- Becker M. D., Koeppe R. E., 2nd, Andersen O. S. Amino acid substitutions and ion channel function. Model-dependent conclusions. Biophys J. 1992 Apr;62(1):25–27. doi: 10.1016/S0006-3495(92)81767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. W., Nicholson L. K., Brenneman M. T., Subramaniam S., Teng Q., McCammon J. A., Cross T. A., Jakobsson E. Molecular dynamics computations and solid state nuclear magnetic resonance of the gramicidin cation channel. Biophys J. 1991 Oct;60(4):974–978. doi: 10.1016/S0006-3495(91)82131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell B. A., Separovic F., Baldassi A. J., Smith R. Conformation and orientation of gramicidin a in oriented phospholipid bilayers measured by solid state carbon-13 NMR. Biophys J. 1988 Jan;53(1):67–76. doi: 10.1016/S0006-3495(88)83066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema K. P., Pauls K. P., Bloom M. Deuterium nuclear magnetic resonance investigation of the exchangeable sites on gramicidin A and gramicidin S in multilamellar vesicles of dipalmitoylphosphatidylcholine. Biochemistry. 1986 Jul 1;25(13):3796–3803. doi: 10.1021/bi00361a010. [DOI] [PubMed] [Google Scholar]
- Durkin J. T., Koeppe R. E., 2nd, Andersen O. S. Energetics of gramicidin hybrid channel formation as a test for structural equivalence. Side-chain substitutions in the native sequence. J Mol Biol. 1990 Jan 5;211(1):221–234. doi: 10.1016/0022-2836(90)90022-E. [DOI] [PubMed] [Google Scholar]
- Durkin J. T., Providence L. L., Koeppe R. E., 2nd, Andersen O. S. Formation of non-beta 6.3-helical gramicidin channels between sequence-substituted gramicidin analogues. Biophys J. 1992 Apr;62(1):145–159. doi: 10.1016/S0006-3495(92)81801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields C. G., Fields G. B., Noble R. L., Cross T. A. Solid phase peptide synthesis of 15N-gramicidins A, B, and C and high performance liquid chromatographic purification. Int J Pept Protein Res. 1989 Apr;33(4):298–303. doi: 10.1111/j.1399-3011.1989.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Griffin R. G. Solid state nuclear magnetic resonance of lipid bilayers. Methods Enzymol. 1981;72:108–174. doi: 10.1016/s0076-6879(81)72010-x. [DOI] [PubMed] [Google Scholar]
- Hing A. W., Adams S. P., Silbert D. F., Norberg R. E. Deuterium NMR of 2HCO-Val1...gramicidin A and 2HCO-Val1-D-Leu2...gramicidin A in oriented DMPC bilayers. Biochemistry. 1990 May 1;29(17):4156–4166. doi: 10.1021/bi00469a019. [DOI] [PubMed] [Google Scholar]
- Hing A. W., Adams S. P., Silbert D. F., Norberg R. E. Deuterium NMR of Val1...(2-2H)Ala3...gramicidin A in oriented DMPC bilayers. Biochemistry. 1990 May 1;29(17):4144–4156. doi: 10.1021/bi00469a018. [DOI] [PubMed] [Google Scholar]
- Hu W., Lee K. C., Cross T. A. Tryptophans in membrane proteins: indole ring orientations and functional implications in the gramicidin channel. Biochemistry. 1993 Jul 13;32(27):7035–7047. doi: 10.1021/bi00078a032. [DOI] [PubMed] [Google Scholar]
- Ketchem R. R., Hu W., Cross T. A. High-resolution conformation of gramicidin A in a lipid bilayer by solid-state NMR. Science. 1993 Sep 10;261(5127):1457–1460. doi: 10.1126/science.7690158. [DOI] [PubMed] [Google Scholar]
- Killian J. A. Gramicidin and gramicidin-lipid interactions. Biochim Biophys Acta. 1992 Dec 11;1113(3-4):391–425. doi: 10.1016/0304-4157(92)90008-x. [DOI] [PubMed] [Google Scholar]
- Killian J. A., Taylor M. J., Koeppe R. E., 2nd Orientation of the valine-1 side chain of the gramicidin transmembrane channel and implications for channel functioning. A 2H NMR study. Biochemistry. 1992 Nov 24;31(46):11283–11290. doi: 10.1021/bi00161a004. [DOI] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Providence L. L., Greathouse D. V., Heitz F., Trudelle Y., Purdie N., Andersen O. S. On the helix sense of gramicidin A single channels. Proteins. 1992 Jan;12(1):49–62. doi: 10.1002/prot.340120107. [DOI] [PubMed] [Google Scholar]
- Lee K. C., Hu W., Cross T. A. 2H NMR determination of the global correlation time of the gramicidin channel in a lipid bilayer. Biophys J. 1993 Sep;65(3):1162–1167. doi: 10.1016/S0006-3495(93)81150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald P. M., Seelig J. Dynamic properties of gramicidin A in phospholipid membranes. Biochemistry. 1988 Apr 5;27(7):2357–2364. doi: 10.1021/bi00407a017. [DOI] [PubMed] [Google Scholar]
- Mazet J. L., Andersen O. S., Koeppe R. E., 2nd Single-channel studies on linear gramicidins with altered amino acid sequences. A comparison of phenylalanine, tryptophane, and tyrosine substitutions at positions 1 and 11. Biophys J. 1984 Jan;45(1):263–276. doi: 10.1016/S0006-3495(84)84153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson L. K., Cross T. A. Gramicidin cation channel: an experimental determination of the right-handed helix sense and verification of beta-type hydrogen bonding. Biochemistry. 1989 Nov 28;28(24):9379–9385. doi: 10.1021/bi00450a019. [DOI] [PubMed] [Google Scholar]
- Nicholson L. K., Moll F., Mixon T. E., LoGrasso P. V., Lay J. C., Cross T. A. Solid-state 15N NMR of oriented lipid bilayer bound gramicidin A'. Biochemistry. 1987 Oct 20;26(21):6621–6626. doi: 10.1021/bi00395a009. [DOI] [PubMed] [Google Scholar]
- Nicholson L. K., Teng Q., Cross T. A. Solid-state nuclear magnetic resonance derived model for dynamics in the polypeptide backbone of the gramicidin A channel. J Mol Biol. 1991 Apr 5;218(3):621–637. doi: 10.1016/0022-2836(91)90706-c. [DOI] [PubMed] [Google Scholar]
- O'Connell A. M., Koeppe R. E., 2nd, Andersen O. S. Kinetics of gramicidin channel formation in lipid bilayers: transmembrane monomer association. Science. 1990 Nov 30;250(4985):1256–1259. doi: 10.1126/science.1700867. [DOI] [PubMed] [Google Scholar]
- Prosser R. S., Davis J. H., Dahlquist F. W., Lindorfer M. A. 2H nuclear magnetic resonance of the gramicidin A backbone in a phospholipid bilayer. Biochemistry. 1991 May 14;30(19):4687–4696. doi: 10.1021/bi00233a008. [DOI] [PubMed] [Google Scholar]
- SARGES R., WITKOP B. GRAMICIDIN A. V. THE STRUCTURE OF VALINE- AND ISOLEUCINE-GRAMICIDIN A. J Am Chem Soc. 1965 May 5;87:2011–2020. doi: 10.1021/ja01087a027. [DOI] [PubMed] [Google Scholar]
- Sawyer D. B., Williams L. P., Whaley W. L., Koeppe R. E., 2nd, Andersen O. S. Gramicidins A, B, and C form structurally equivalent ion channels. Biophys J. 1990 Nov;58(5):1207–1212. doi: 10.1016/S0006-3495(90)82461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Cornell B. A. The dynamics of the intrinsic membrane polypeptide gramicidin a in phospholipid bilayers: a solid state carbon-13 NMR study. Biophys J. 1986 Jan;49(1):117–118. doi: 10.1016/S0006-3495(86)83617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Nemoto Y., Harada I. Environments and conformations of tryptophan side chains of gramicidin A in phospholipid bilayers studied by Raman spectroscopy. Biochemistry. 1990 Feb 13;29(6):1572–1579. doi: 10.1021/bi00458a031. [DOI] [PubMed] [Google Scholar]
- Urry D. W. The gramicidin A transmembrane channel: a proposed pi(L,D) helix. Proc Natl Acad Sci U S A. 1971 Mar;68(3):672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt T. C., Killian J. A., De Kruijff B., Andersen O. S. Influence of acylation on the channel characteristics of gramicidin A. Biochemistry. 1992 Aug 18;31(32):7320–7324. doi: 10.1021/bi00147a016. [DOI] [PubMed] [Google Scholar]
- Williams L. P., Narcessian E. J., Andersen O. S., Waller G. R., Taylor M. J., Lazenby J. P., Hinton J. F., Koeppe R. E., 2nd Molecular and channel-forming characteristics of gramicidin K's: a family of naturally occurring acylated gramicidins. Biochemistry. 1992 Aug 18;31(32):7311–7319. doi: 10.1021/bi00147a015. [DOI] [PubMed] [Google Scholar]