Abstract
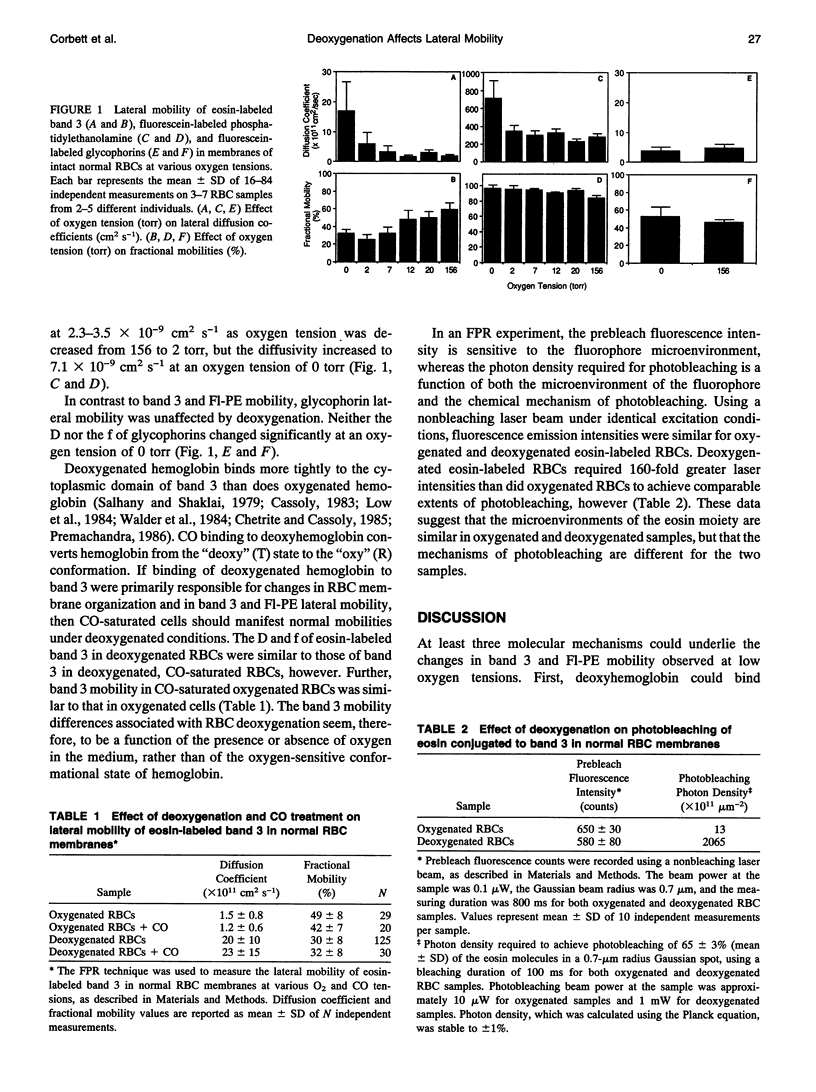
We have used the fluorescence photobleaching recovery technique to study the dependence on oxygen tension of the lateral mobility of fluorescently labeled band 3, the phospholipid analogue fluorescein phosphatidylethanolamine, and glycophorins in normal red blood cell membranes. Band 3 protein and sialic acid moieties on glycophorins were labeled specifically with eosin maleimide and fluorescein thiosemicarbazide, respectively. The band 3 diffusion rate increased from 1.7 x 10(-11) cm2 s-1 to 6.0 x 10(-11) cm2 s-1 as oxygen tension was decreased from 156 to 2 torr, and a further increase to 17 x 10(-11) cm2 s-1 occurred as oxygen tension was decreased from 2 to 0 torr. The fractional mobility of band 3 decreased from 58 to 32% as oxygen tension was decreased from 156 to 0 torr. The phospholipid diffusion coefficient remained constant as oxygen tension was decreased from 156 to 20 torr, but increased from 2.3 x 10(-9) cm2 s-1 to 7.1 x 10(-9) cm2 s-1 as oxygen tension was decreased from 20 to 0 torr. Neither the diffusion coefficient nor the fractional mobility of glycophorins changed significantly at low oxygen tension. Under non-bleaching excitation conditions, intensities of fluorescence emission were identical for oxygenated and deoxygenated eosin-labeled RBCs. Deoxygenated eosin-labeled RBCs required 160-fold greater laser intensities than did oxygenated RBCs to achieve comparable extents of photobleaching, however. Oxygen seems to act as a facilitator of fluorophore photobleaching and may thereby protect the fluorescently labeled red cell membrane from photodamage. Removal of oxygen may allow excited state fluorophores in close proximity to the plasma membrane to react with neighboring proteins or lipids during photobleaching. This effect has important implications for the ability of the fluorescence photobleaching recovery technique to report accurate lateral mobilities of cell membrane molecules under hypoxic conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bierer B. E., Herrmann S. H., Brown C. S., Burakoff S. J., Golan D. E. Lateral mobility of class I histocompatibility antigens in B lymphoblastoid cell membranes: modulation by cross-linking and effect of cell density. J Cell Biol. 1987 Sep;105(3):1147–1152. doi: 10.1083/jcb.105.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarneson D. W., Petersen N. O. Effects of second order photobleaching on recovered diffusion parameters from fluorescence photobleaching recovery. Biophys J. 1991 Nov;60(5):1128–1131. doi: 10.1016/S0006-3495(91)82148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassoly R. Quantitative analysis of the association of human hemoglobin with the cytoplasmic fragment of band 3 protein. J Biol Chem. 1983 Mar 25;258(6):3859–3864. [PubMed] [Google Scholar]
- Chan P. Y., Lawrence M. B., Dustin M. L., Ferguson L. M., Golan D. E., Springer T. A. Influence of receptor lateral mobility on adhesion strengthening between membranes containing LFA-3 and CD2. J Cell Biol. 1991 Oct;115(1):245–255. doi: 10.1083/jcb.115.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chétrite G., Cassoly R. Affinity of hemoglobin for the cytoplasmic fragment of human erythrocyte membrane band 3. Equilibrium measurements at physiological pH using matrix-bound proteins: the effects of ionic strength, deoxygenation and of 2,3-diphosphoglycerate. J Mol Biol. 1985 Oct 5;185(3):639–644. doi: 10.1016/0022-2836(85)90076-2. [DOI] [PubMed] [Google Scholar]
- Corbett J. D., Golan D. E. Band 3 and glycophorin are progressively aggregated in density-fractionated sickle and normal red blood cells. Evidence from rotational and lateral mobility studies. J Clin Invest. 1993 Jan;91(1):208–217. doi: 10.1172/JCI116172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Zuniga M. Lateral diffusion of wild-type and mutant Ld antigens in L cells. J Cell Biol. 1984 Dec;99(6):2333–2335. doi: 10.1083/jcb.99.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger J., Halperin B. I. Effects of spatial variation in membrane diffusibility and solubility on the lateral transport of membrane components. Biophys J. 1986 Sep;50(3):513–521. doi: 10.1016/S0006-3495(86)83489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan D. E., Alecio M. R., Veatch W. R., Rando R. R. Lateral mobility of phospholipid and cholesterol in the human erythrocyte membrane: effects of protein-lipid interactions. Biochemistry. 1984 Jan 17;23(2):332–339. doi: 10.1021/bi00297a024. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Brown C. S., Cianci C. M., Furlong S. T., Caulfield J. P. Schistosomula of Schistosoma mansoni use lysophosphatidylcholine to lyse adherent human red blood cells and immobilize red cell membrane components. J Cell Biol. 1986 Sep;103(3):819–828. doi: 10.1083/jcb.103.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Hou Y., Wojcieszyn J. Evidence for lack of damage during photobleaching measurements of the lateral mobility of cell surface components. Exp Cell Res. 1978 Oct 1;116(1):179–189. doi: 10.1016/0014-4827(78)90074-5. [DOI] [PubMed] [Google Scholar]
- Johnson P., Garland P. B. Depolarization of fluorescence depletion. A microscopic method for measuring rotational diffusion of membrane proteins on the surface of a single cell. FEBS Lett. 1981 Sep 28;132(2):252–256. doi: 10.1016/0014-5793(81)81172-6. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P. Fluorescence photobleaching does not alter the lateral mobility of erythrocyte membrane glycoproteins. Nature. 1981 Sep 10;293(5828):159–161. doi: 10.1038/293159a0. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Thompson J. E., Kruuv J. Photoinduced crosslinking of membrane proteins by fluorescein isothiocyanate. Biochem Biophys Res Commun. 1978 Nov 14;85(1):344–350. doi: 10.1016/s0006-291x(78)80048-5. [DOI] [PubMed] [Google Scholar]
- Low P. S., Westfall M. A., Allen D. P., Appell K. C. Characterization of the reversible conformational equilibrium of the cytoplasmic domain of erythrocyte membrane band 3. J Biol Chem. 1984 Nov 10;259(21):13070–13076. [PubMed] [Google Scholar]
- Nigg E., Kessler M., Cherry R. J. Labeling of human erythrocyte membranes with eosin probes used for protein diffusion measurements: inhibition of anion transport and photo-oxidative inactivation of acetylcholinesterase. Biochim Biophys Acta. 1979 Jan 19;550(2):328–340. doi: 10.1016/0005-2736(79)90219-0. [DOI] [PubMed] [Google Scholar]
- Premachandra B. R. Interaction of hemoglobin and its component alpha and beta chains with band 3 protein. Biochemistry. 1986 Jun 3;25(11):3455–3462. doi: 10.1021/bi00359a054. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Shaklai N. Functional properties of human hemoglobin bound to the erythrocyte membrane. Biochemistry. 1979 Mar 6;18(5):893–899. doi: 10.1021/bi00572a025. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Distance dependence of the diffusion coefficient. Biophys J. 1989 Sep;56(3):615–622. doi: 10.1016/S0006-3495(89)82708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Effects of impermeable patches on diffusion in a cell membrane. Biophys J. 1982 Aug;39(2):165–173. doi: 10.1016/S0006-3495(82)84504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. The effect of mobile obstacles. Biophys J. 1987 Dec;52(6):989–997. doi: 10.1016/S0006-3495(87)83291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Koppel D. E. Membrane damage caused by irradiation of fluorescent concanavalin A. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3314–3317. doi: 10.1073/pnas.76.7.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Ohnishi S. Restriction of the lateral motion of band 3 in the erythrocyte membrane by the cytoskeletal network: dependence on spectrin association state. Biochemistry. 1986 Oct 7;25(20):6133–6139. doi: 10.1021/bi00368a045. [DOI] [PubMed] [Google Scholar]
- Walder J. A., Chatterjee R., Steck T. L., Low P. S., Musso G. F., Kaiser E. T., Rogers P. H., Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984 Aug 25;259(16):10238–10246. [PubMed] [Google Scholar]
- Weaver F. E., Polster H., Febboriello P., Sheetz M. P., Schmid-Schonbein H., Koppel D. E. Normal band 3-cytoskeletal interactions are maintained on tanktreading erythrocytes. Biophys J. 1990 Dec;58(6):1427–1436. doi: 10.1016/S0006-3495(90)82488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. E., Edidin M., Dragsten P. R. Effect of bleaching light on measurements of lateral diffusion in cell membranes by the fluorescence photobleaching recovery method. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2043–2045. doi: 10.1073/pnas.77.4.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. M., Barisas B. G. Protein rotational motion in solution measured by polarized fluorescence depletion. Biophys J. 1986 Jul;50(1):41–53. doi: 10.1016/S0006-3495(86)83437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


