Abstract
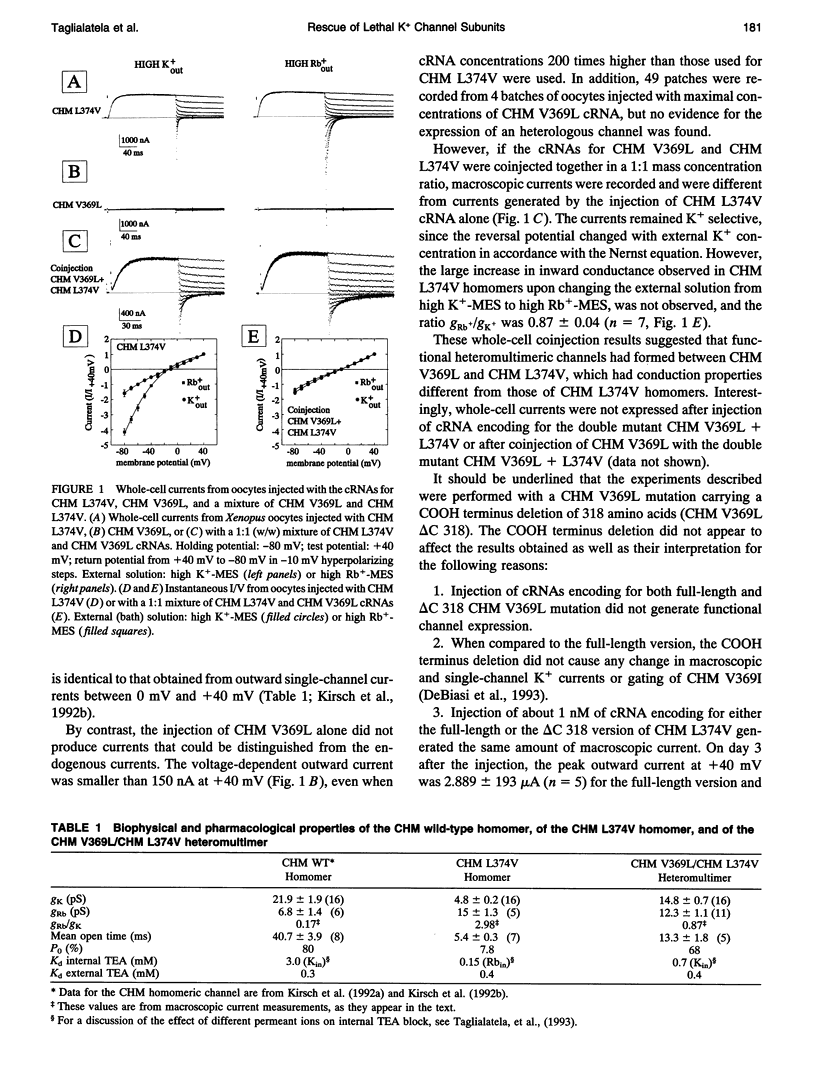
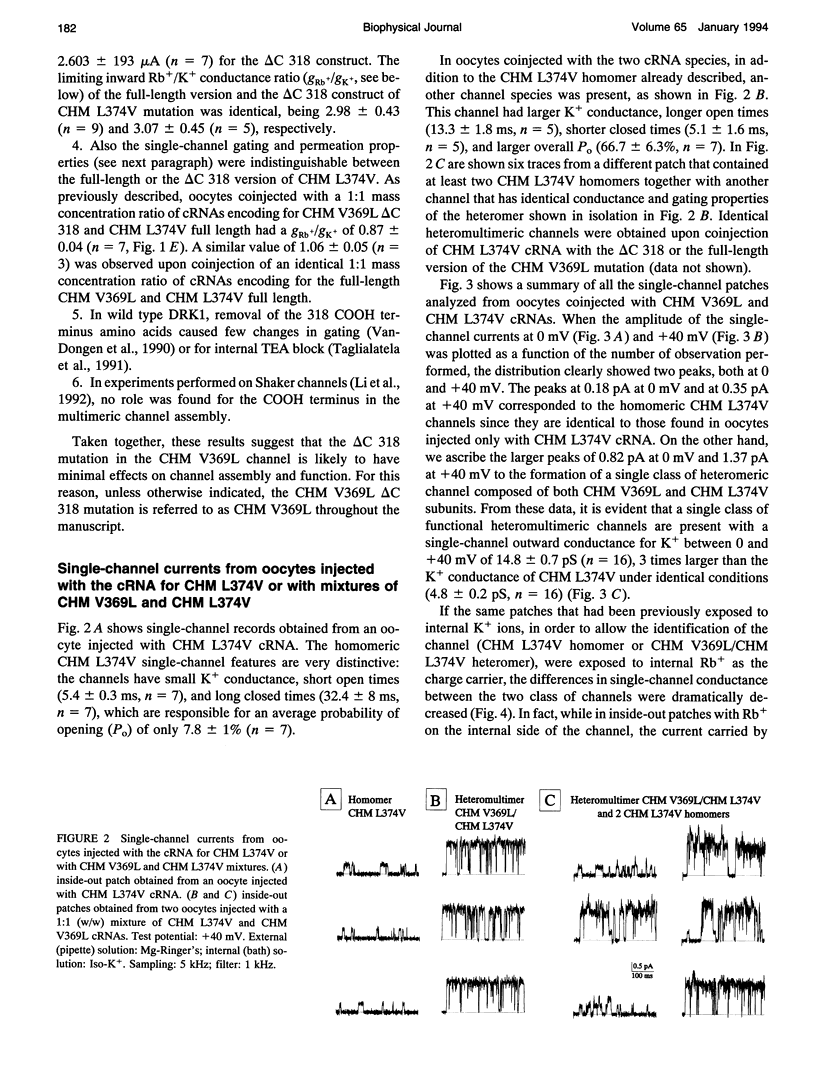
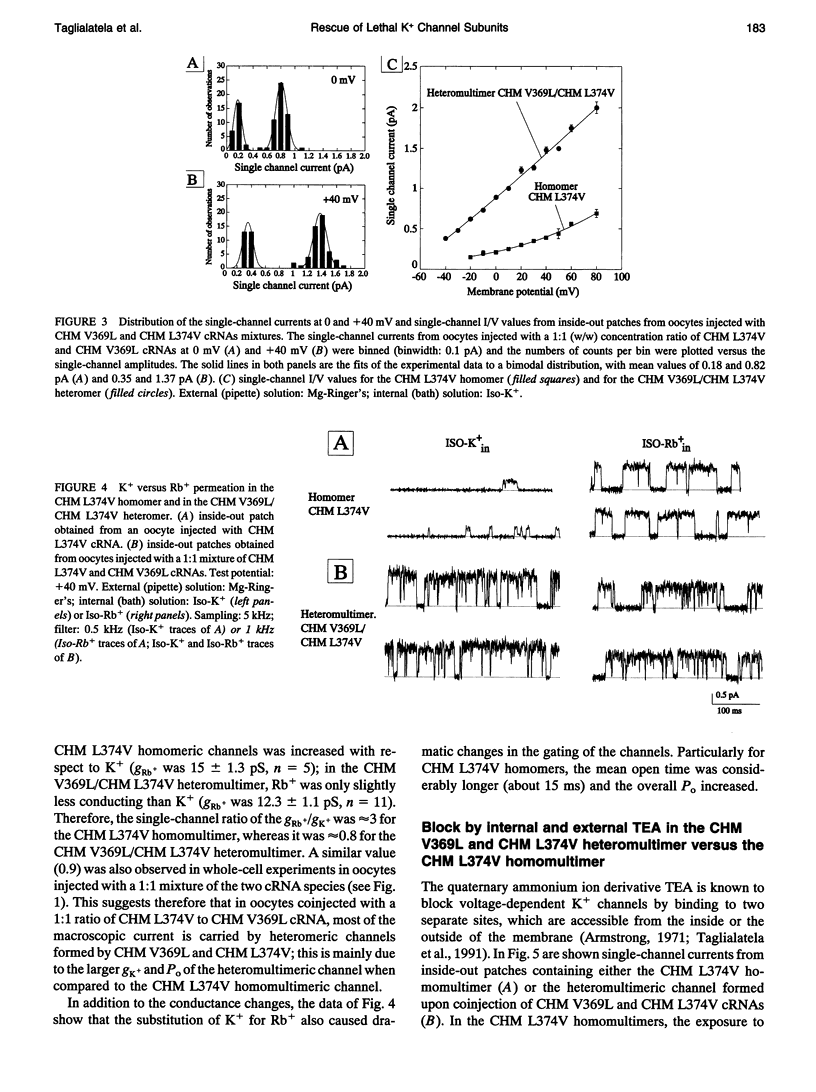
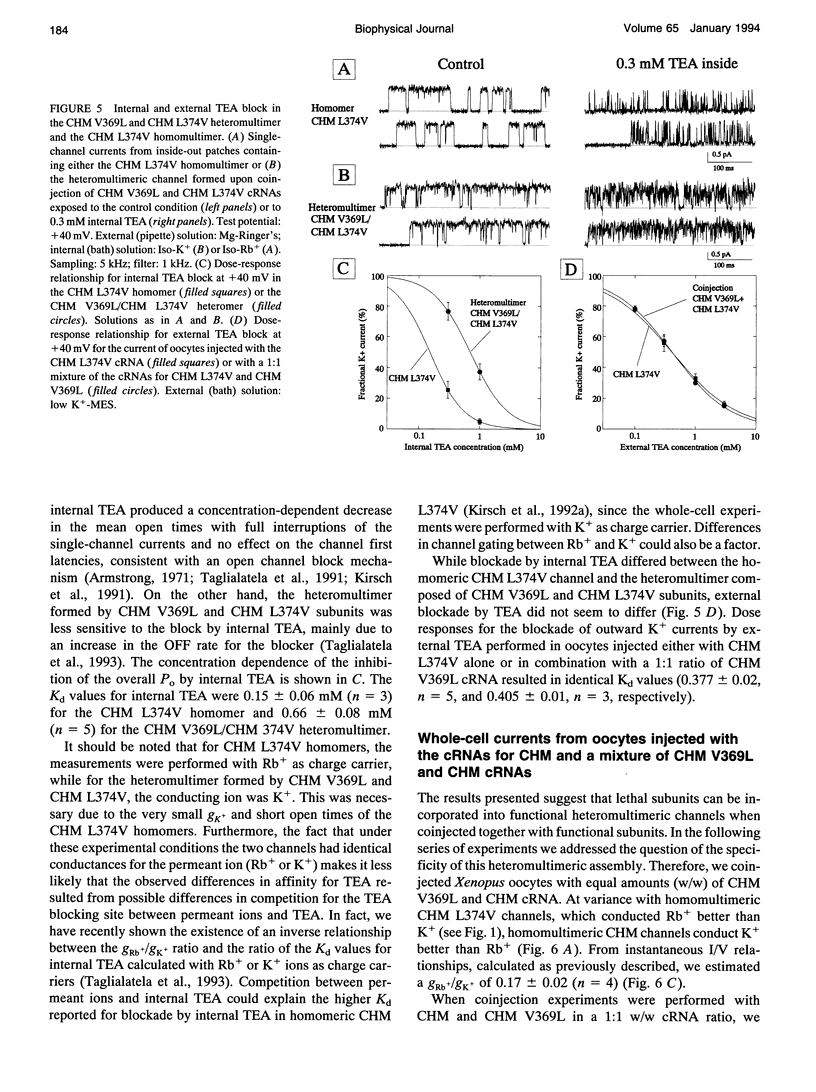
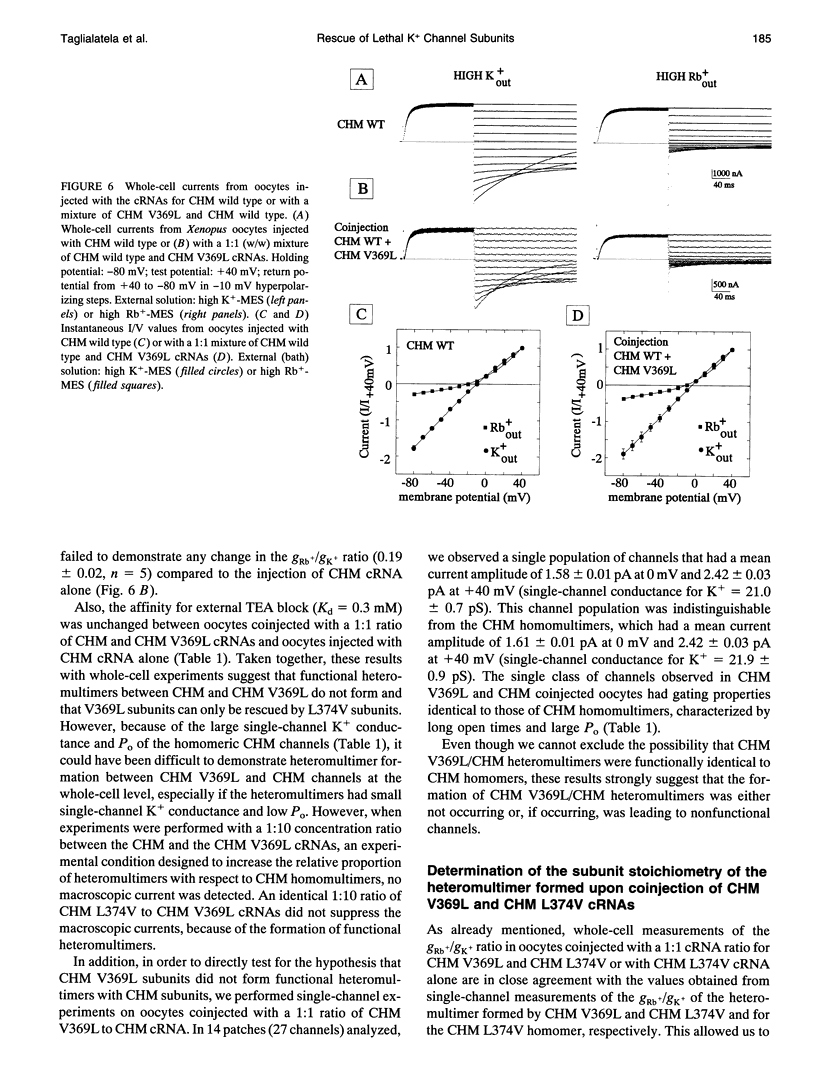
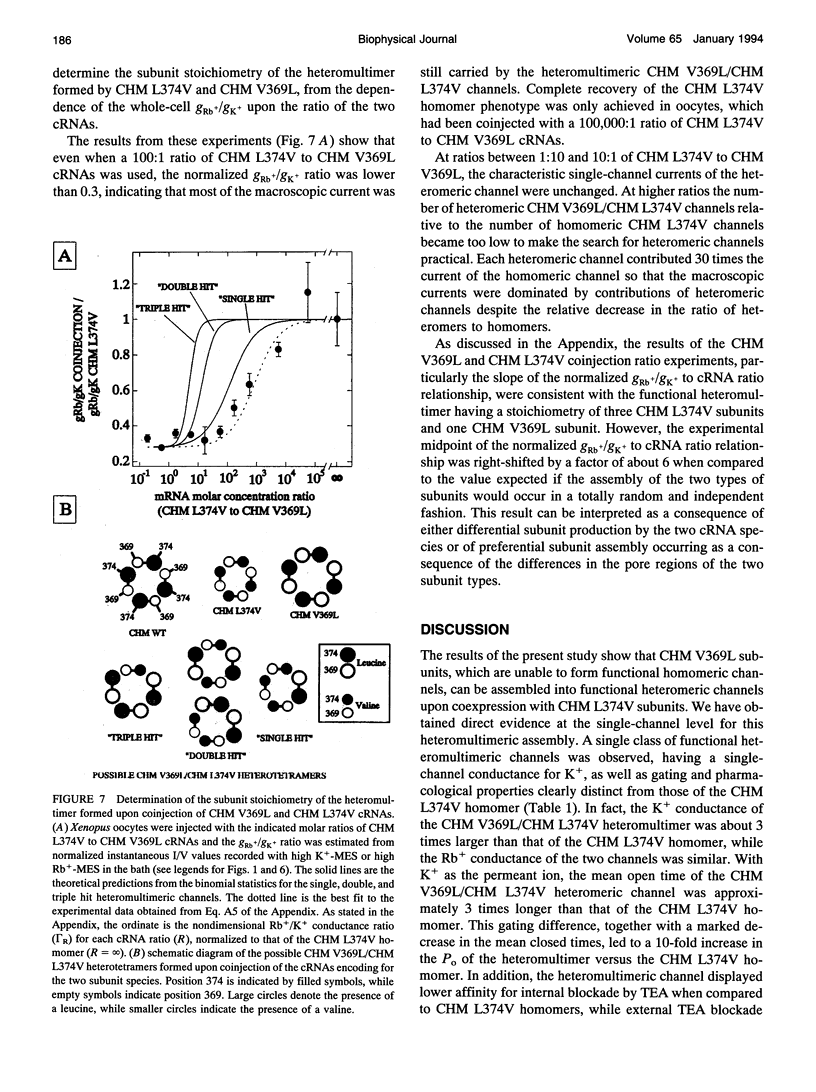
In a chimeric, voltage-dependent K+ channel (CHM), the valine at position 369 and the leucine at position 374 interact within the pore or P-region to regulate ion permeation and block. Here we show that the point mutation, CHM V369L, abolished channel function whereas previous experiments showed that CHM V369 and CHM V369I are functional. Coinjection of "lethal" CHM V369L cRNA with CHM L374V cRNA but not CHM cRNA generated functional heteromultimers. The whole-cell Rb+/K+ conductance ratio was 2.98 +/- 0.43 for CHM L374V and was reduced to 0.87 +/- 0.04 for the coexpressed CHM V369L and CHM L374V subunits. When single-channel currents were recorded, a single class of CHM V369L/CHM L374V heteromultimers was identified. This class was readily distinguishable from CHM L374V homomultimers by K+ conductance, gating, and blockade by internal tetraethylammonium. Coinjection experiments at various RNA ratios suggest that the CHM V369L/CHM L374V heteromultime, assuming it to be a tetramer, was composed of three CHM L374V subunits and one CHM V369L subunit. It appears that in the critical P-region of CHM position 369 may tolerate only one leucine.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M. J., North R. A., Osborne P. B., Douglass J., Adelman J. P. Heteropolymeric potassium channels expressed in Xenopus oocytes from cloned subunits. Neuron. 1990 Mar;4(3):405–411. doi: 10.1016/0896-6273(90)90052-h. [DOI] [PubMed] [Google Scholar]
- De Biasi M., Hartmann H. A., Drewe J. A., Taglialatela M., Brown A. M., Kirsch G. E. Inactivation determined by a single site in K+ pores. Pflugers Arch. 1993 Jan;422(4):354–363. doi: 10.1007/BF00374291. [DOI] [PubMed] [Google Scholar]
- Frech G. C., VanDongen A. M., Schuster G., Brown A. M., Joho R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989 Aug 24;340(6235):642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- Goldstein R. F., Stryer L. Cooperative polymerization reactions. Analytical approximations, numerical examples, and experimental strategy. Biophys J. 1986 Oct;50(4):583–599. doi: 10.1016/S0006-3495(86)83498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann H. A., Kirsch G. E., Drewe J. A., Taglialatela M., Joho R. H., Brown A. M. Exchange of conduction pathways between two related K+ channels. Science. 1991 Feb 22;251(4996):942–944. doi: 10.1126/science.2000495. [DOI] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990 Jun 7;345(6275):530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Joho R. H., Moorman J. R., VanDongen A. M., Kirsch G. E., Silberberg H., Schuster G., Brown A. M. Toxin and kinetic profile of rat brain type III sodium channels expressed in Xenopus oocytes. Brain Res Mol Brain Res. 1990 Feb;7(2):105–113. doi: 10.1016/0169-328x(90)90087-t. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Hurst R. S., Yakel J., Varnum M. D., Adelman J. P., North R. A. Multiple subunits of a voltage-dependent potassium channel contribute to the binding site for tetraethylammonium. Neuron. 1992 Mar;8(3):493–497. doi: 10.1016/0896-6273(92)90277-k. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Drewe J. A., Hartmann H. A., Taglialatela M., de Biasi M., Brown A. M., Joho R. H. Differences between the deep pores of K+ channels determined by an interacting pair of nonpolar amino acids. Neuron. 1992 Mar;8(3):499–505. doi: 10.1016/0896-6273(92)90278-l. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Drewe J. A., Taglialatela M., Joho R. H., DeBiasi M., Hartmann H. A., Brown A. M. A single nonpolar residue in the deep pore of related K+ channels acts as a K+:Rb+ conductance switch. Biophys J. 1992 Apr;62(1):136–144. doi: 10.1016/S0006-3495(92)81800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Taglialatela M., Brown A. M. Internal and external TEA block in single cloned K+ channels. Am J Physiol. 1991 Oct;261(4 Pt 1):C583–C590. doi: 10.1152/ajpcell.1991.261.4.C583. [DOI] [PubMed] [Google Scholar]
- Li M., Jan Y. N., Jan L. Y. Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel. Science. 1992 Aug 28;257(5074):1225–1230. doi: 10.1126/science.1519059. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- McCormack K., Tanouye M. A., Iverson L. E., Lin J. W., Ramaswami M., McCormack T., Campanelli J. T., Mathew M. K., Rudy B. A role for hydrophobic residues in the voltage-dependent gating of Shaker K+ channels. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2931–2935. doi: 10.1073/pnas.88.7.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Perozo E., MacKinnon R., Bezanilla F., Stefani E. Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron. 1993 Aug;11(2):353–358. doi: 10.1016/0896-6273(93)90190-3. [DOI] [PubMed] [Google Scholar]
- Po S., Roberds S., Snyders D. J., Tamkun M. M., Bennett P. B. Heteromultimeric assembly of human potassium channels. Molecular basis of a transient outward current? Circ Res. 1993 Jun;72(6):1326–1336. doi: 10.1161/01.res.72.6.1326. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Sewing S., Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990 Jun 7;345(6275):535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Sala S., Matteson D. R. Voltage-dependent slowing of K channel closing kinetics by Rb+. J Gen Physiol. 1991 Sep;98(3):535–554. doi: 10.1085/jgp.98.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L., Baker K., Butler A., Covarrubias M., Pak M. D., Wei A. An essential 'set' of K+ channels conserved in flies, mice and humans. Trends Neurosci. 1992 May;15(5):161–166. doi: 10.1016/0166-2236(92)90165-5. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988 Jan 14;331(6152):137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- Shapiro M. S., DeCoursey T. E. Permeant ion effects on the gating kinetics of the type L potassium channel in mouse lymphocytes. J Gen Physiol. 1991 Jun;97(6):1251–1278. doi: 10.1085/jgp.97.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N. V., Chen X., Boyer M. M., Pfaffinger P. J. Deletion analysis of K+ channel assembly. Neuron. 1993 Jul;11(1):67–76. doi: 10.1016/0896-6273(93)90271-r. [DOI] [PubMed] [Google Scholar]
- Shen N. V., Chen X., Boyer M. M., Pfaffinger P. J. Deletion analysis of K+ channel assembly. Neuron. 1993 Jul;11(1):67–76. doi: 10.1016/0896-6273(93)90271-r. [DOI] [PubMed] [Google Scholar]
- Taglialatela M., Drewe J. A., Kirsch G. E., De Biasi M., Hartmann H. A., Brown A. M. Regulation of K+/Rb+ selectivity and internal TEA blockade by mutations at a single site in K+ pores. Pflugers Arch. 1993 Apr;423(1-2):104–112. doi: 10.1007/BF00374967. [DOI] [PubMed] [Google Scholar]
- Taglialatela M., Vandongen A. M., Drewe J. A., Joho R. H., Brown A. M., Kirsch G. E. Patterns of internal and external tetraethylammonium block in four homologous K+ channels. Mol Pharmacol. 1991 Aug;40(2):299–307. [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- VanDongen A. M., Frech G. C., Drewe J. A., Joho R. H., Brown A. M. Alteration and restoration of K+ channel function by deletions at the N- and C-termini. Neuron. 1990 Oct;5(4):433–443. doi: 10.1016/0896-6273(90)90082-q. [DOI] [PubMed] [Google Scholar]
- Yellen G., Jurman M. E., Abramson T., MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991 Feb 22;251(4996):939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Imoto K., Kawamura T., Higashida H., Iwabe N., Miyata T., Numa S. Potassium channels from NG108-15 neuroblastoma-glioma hybrid cells. Primary structure and functional expression from cDNAs. FEBS Lett. 1989 Dec 18;259(1):37–42. doi: 10.1016/0014-5793(89)81488-7. [DOI] [PubMed] [Google Scholar]
- Yool A. J., Schwarz T. L. Alteration of ionic selectivity of a K+ channel by mutation of the H5 region. Nature. 1991 Feb 21;349(6311):700–704. doi: 10.1038/349700a0. [DOI] [PubMed] [Google Scholar]



