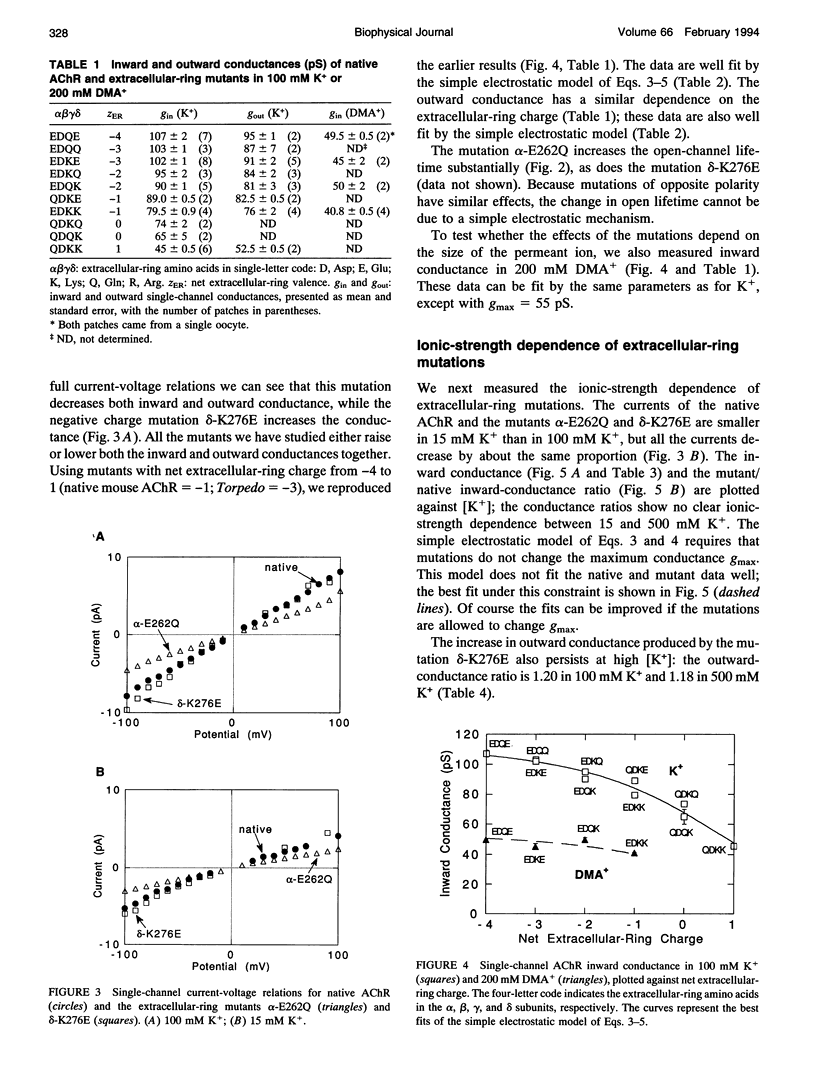
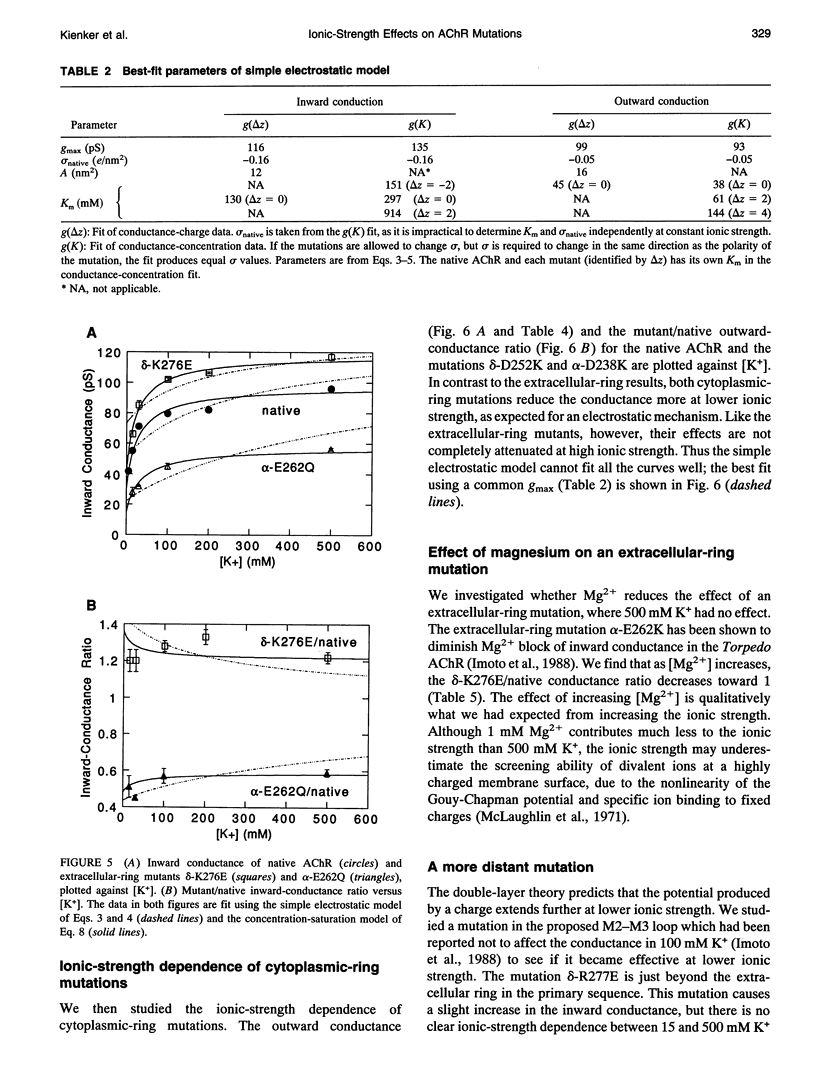
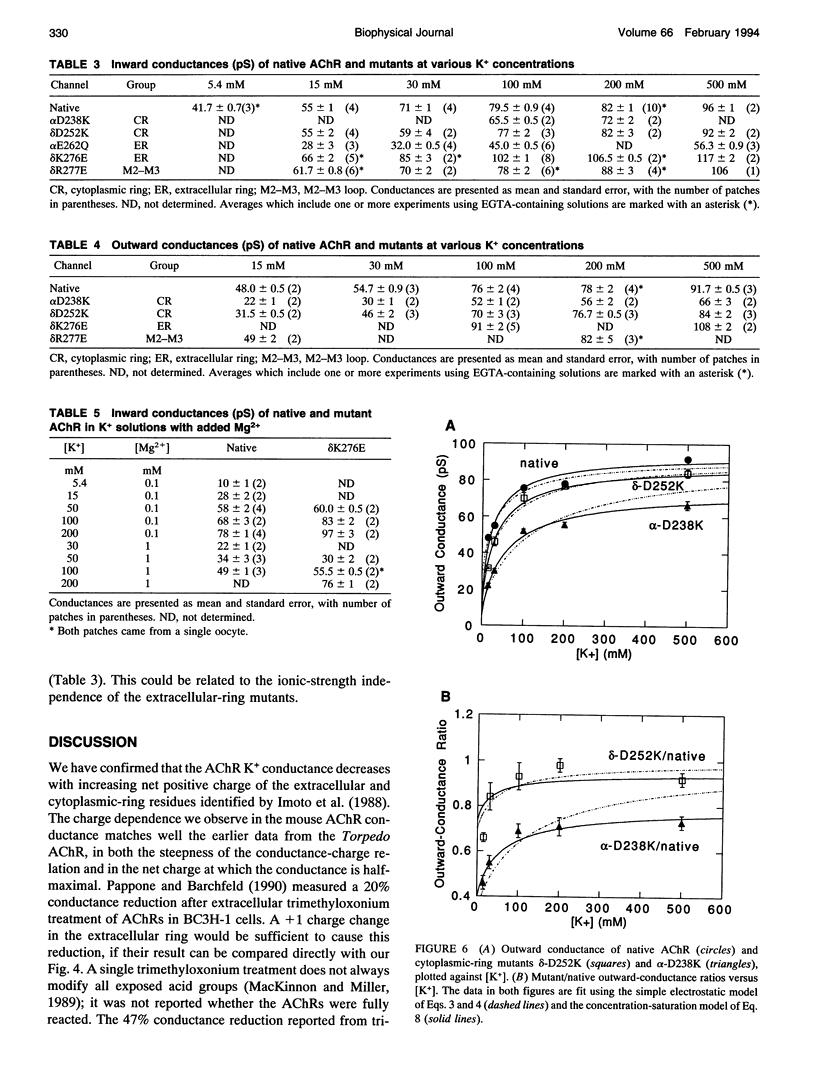
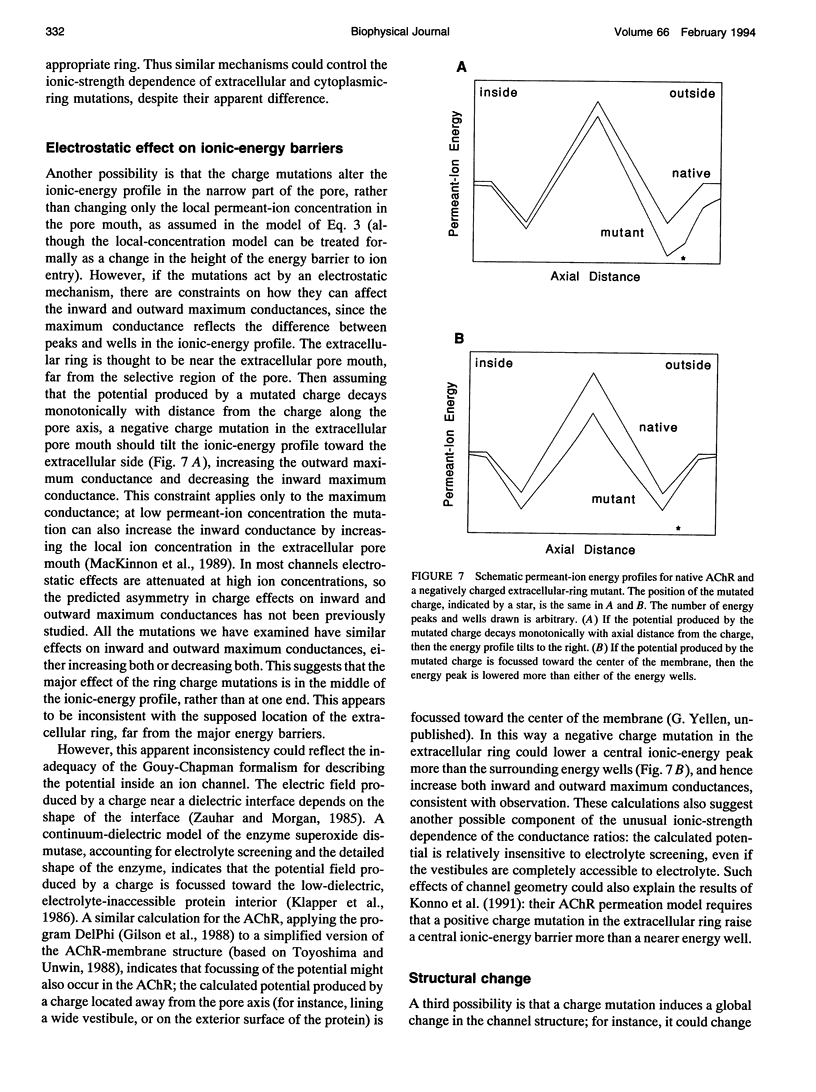
Abstract
Fixed negative charges in many cation channels raise the single-channel conductance, apparently by an electrostatic mechanism: their effects are accentuated in solutions of low ionic strength and attenuated at high ionic strength. The charges of specific amino acids near the ends of the proposed pore-lining M2 segment of the nicotinic acetylcholine receptor, termed the extracellular and cytoplasmic rings, have recently been shown to influence the single-channel K+ conductance (Imoto, K., C. Busch, B. Sakmann, M. Mishina, T. Konno, J. Nakai, H. Bujo, Y. Mori, K. Fukuda and S. Numa. 1988. Nature 335:645-648). We examined whether these charges might act by a direct electrostatic effect on the energy of ions in the pore, rather than indirectly by inducing a structural change. To this end, we measured the conductances of charge mutants over a range of K+ concentrations (ionic strengths). As expected, we found that negative charge mutations raise the conductance, and positive charge mutations lower it. The effects of cytoplasmic-ring mutations are accentuated at low ionic strength, but they are not completely attenuated at high ionic strength. The effects of extracellular-ring mutations are independent of ionic strength. These results are inconsistent with the simplest electrostatic model. We suggest a modified model that qualitatively accounts for the data.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apell H. J., Bamberg E., Alpes H., Läuger P. Formation of ion channels by a negatively charged analog of gramicidin A. J Membr Biol. 1977 Feb 24;31(1-2):171–188. doi: 10.1007/BF01869403. [DOI] [PubMed] [Google Scholar]
- Apell H. J., Bamberg E., Läuger P. Effects of surface charge on the conductance of the gramicidin channel. Biochim Biophys Acta. 1979 Apr 19;552(3):369–378. doi: 10.1016/0005-2736(79)90181-0. [DOI] [PubMed] [Google Scholar]
- Bell J. E., Miller C. Effects of phospholipid surface charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophys J. 1984 Jan;45(1):279–287. doi: 10.1016/S0006-3495(84)84154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Luyten W., Evans K., Mason P., Ballivet M., Goldman D., Stengelin S., Martin G., Heinemann S., Patrick J. Isolation of a clone coding for the alpha-subunit of a mouse acetylcholine receptor. J Neurosci. 1985 Sep;5(9):2545–2552. doi: 10.1523/JNEUROSCI.05-09-02545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A., Mudd J., Shah V., Merlie J. P. A universal oligonucleotide probe for acetylcholine receptor genes. Selection and sequencing of cDNA clones for the mouse muscle beta subunit. J Biol Chem. 1986 Dec 15;261(35):16451–16458. [PubMed] [Google Scholar]
- Cecchi X., Alvarez O., Latorre R. A three-barrier model for the hemocyanin channel. J Gen Physiol. 1981 Dec;78(6):657–681. doi: 10.1085/jgp.78.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnet P., Labarca C., Leonard R. J., Vogelaar N. J., Czyzyk L., Gouin A., Davidson N., Lester H. A. An open-channel blocker interacts with adjacent turns of alpha-helices in the nicotinic acetylcholine receptor. Neuron. 1990 Jan;4(1):87–95. doi: 10.1016/0896-6273(90)90445-l. [DOI] [PubMed] [Google Scholar]
- Coronado R., Affolter H. Insulation of the conduction pathway of muscle transverse tubule calcium channels from the surface charge of bilayer phospholipid. J Gen Physiol. 1986 Jun;87(6):933–953. doi: 10.1085/jgp.87.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A., Eisenman G. Monovalent and divalent cation permeation in acetylcholine receptor channels. Ion transport related to structure. J Gen Physiol. 1987 Jun;89(6):959–983. doi: 10.1085/jgp.89.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A. Ion-channel entrances influence permeation. Net charge, size, shape, and binding considerations. Biophys J. 1986 Mar;49(3):607–618. doi: 10.1016/S0006-3495(86)83688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A. Open channel structure and ion binding sites of the nicotinic acetylcholine receptor channel. J Neurosci. 1989 Mar;9(3):884–892. doi: 10.1523/JNEUROSCI.09-03-00884.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W. N., Andersen O. S. Surface charges and ion channel function. Annu Rev Physiol. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Imoto K., Methfessel C., Sakmann B., Mishina M., Mori Y., Konno T., Fukuda K., Kurasaki M., Bujo H., Fujita Y. Location of a delta-subunit region determining ion transport through the acetylcholine receptor channel. Nature. 1986 Dec 18;324(6098):670–674. doi: 10.1038/324670a0. [DOI] [PubMed] [Google Scholar]
- Klapper I., Hagstrom R., Fine R., Sharp K., Honig B. Focusing of electric fields in the active site of Cu-Zn superoxide dismutase: effects of ionic strength and amino-acid modification. Proteins. 1986 Sep;1(1):47–59. doi: 10.1002/prot.340010109. [DOI] [PubMed] [Google Scholar]
- Konno T., Busch C., Von Kitzing E., Imoto K., Wang F., Nakai J., Mishina M., Numa S., Sakmann B. Rings of anionic amino acids as structural determinants of ion selectivity in the acetylcholine receptor channel. Proc Biol Sci. 1991 May 22;244(1310):69–79. doi: 10.1098/rspb.1991.0053. [DOI] [PubMed] [Google Scholar]
- LaPolla R. J., Mayne K. M., Davidson N. Isolation and characterization of a cDNA clone for the complete protein coding region of the delta subunit of the mouse acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7970–7974. doi: 10.1073/pnas.81.24.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Ion transport through pores: a rate-theory analysis. Biochim Biophys Acta. 1973 Jul 6;311(3):423–441. doi: 10.1016/0005-2736(73)90323-4. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Latorre R., Miller C. Role of surface electrostatics in the operation of a high-conductance Ca2+-activated K+ channel. Biochemistry. 1989 Oct 3;28(20):8092–8099. doi: 10.1021/bi00446a020. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Miller C. Functional modification of a Ca2+-activated K+ channel by trimethyloxonium. Biochemistry. 1989 Oct 3;28(20):8087–8092. doi: 10.1021/bi00446a019. [DOI] [PubMed] [Google Scholar]
- Matthew J. B. Electrostatic effects in proteins. Annu Rev Biophys Biophys Chem. 1985;14:387–417. doi: 10.1146/annurev.bb.14.060185.002131. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Menestrina G., Forti S., Gambale F. Interaction of tetanus toxin with lipid vesicles. Effects of pH, surface charge, and transmembrane potential on the kinetics of channel formation. Biophys J. 1989 Mar;55(3):393–405. doi: 10.1016/S0006-3495(89)82833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Alvarez O., Vergara C., Latorre R. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers. J Membr Biol. 1985;83(3):273–282. doi: 10.1007/BF01868701. [DOI] [PubMed] [Google Scholar]
- Pappone P. A., Barchfeld G. L. Modifications of single acetylcholine-activated channels in BC3H-1 cells. Effects of trimethyloxonium and pH. J Gen Physiol. 1990 Jul;96(1):1–22. doi: 10.1085/jgp.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeske R. W., Hrinyo-Pavlina T. P., Pottorf R. S., Bridal T., Jin X. Z., Busath D. Synthesis and channel properties of [Tau 16]gramicidin A. Biochim Biophys Acta. 1989 Jul 10;982(2):223–227. doi: 10.1016/0005-2736(89)90058-8. [DOI] [PubMed] [Google Scholar]
- Sanchez J. A., Dani J. A., Siemen D., Hille B. Slow permeation of organic cations in acetylcholine receptor channels. J Gen Physiol. 1986 Jun;87(6):985–1001. doi: 10.1085/jgp.87.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Spalding B. C. Chemical modification reduces the conductance of sodium channels in nerve. Nature. 1980 Jan 17;283(5744):293–295. doi: 10.1038/283293a0. [DOI] [PubMed] [Google Scholar]
- Tomaselli G. F., McLaughlin J. T., Jurman M. E., Hawrot E., Yellen G. Mutations affecting agonist sensitivity of the nicotinic acetylcholine receptor. Biophys J. 1991 Sep;60(3):721–727. doi: 10.1016/S0006-3495(91)82102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima C., Unwin N. Ion channel of acetylcholine receptor reconstructed from images of postsynaptic membranes. Nature. 1988 Nov 17;336(6196):247–250. doi: 10.1038/336247a0. [DOI] [PubMed] [Google Scholar]
- Villarroel A., Sakmann B. Threonine in the selectivity filter of the acetylcholine receptor channel. Biophys J. 1992 Apr;62(1):196–208. doi: 10.1016/S0006-3495(92)81805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley J. F., 3rd, French R. J., Krueger B. K. Trimethyloxonium modification of single batrachotoxin-activated sodium channels in planar bilayers. Changes in unit conductance and in block by saxitoxin and calcium. J Gen Physiol. 1986 Feb;87(2):327–349. doi: 10.1085/jgp.87.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., LaPolla R. J., Davidson N. Mouse muscle nicotinic acetylcholine receptor gamma subunit: cDNA sequence and gene expression. Nucleic Acids Res. 1986 Apr 25;14(8):3539–3555. doi: 10.1093/nar/14.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauhar R. J., Morgan R. S. A new method for computing the macromolecular electric potential. J Mol Biol. 1985 Dec 20;186(4):815–820. doi: 10.1016/0022-2836(85)90399-7. [DOI] [PubMed] [Google Scholar]
- van der Goot F. G., González-Mañas J. M., Lakey J. H., Pattus F. A 'molten-globule' membrane-insertion intermediate of the pore-forming domain of colicin A. Nature. 1991 Dec 5;354(6352):408–410. doi: 10.1038/354408a0. [DOI] [PubMed] [Google Scholar]


