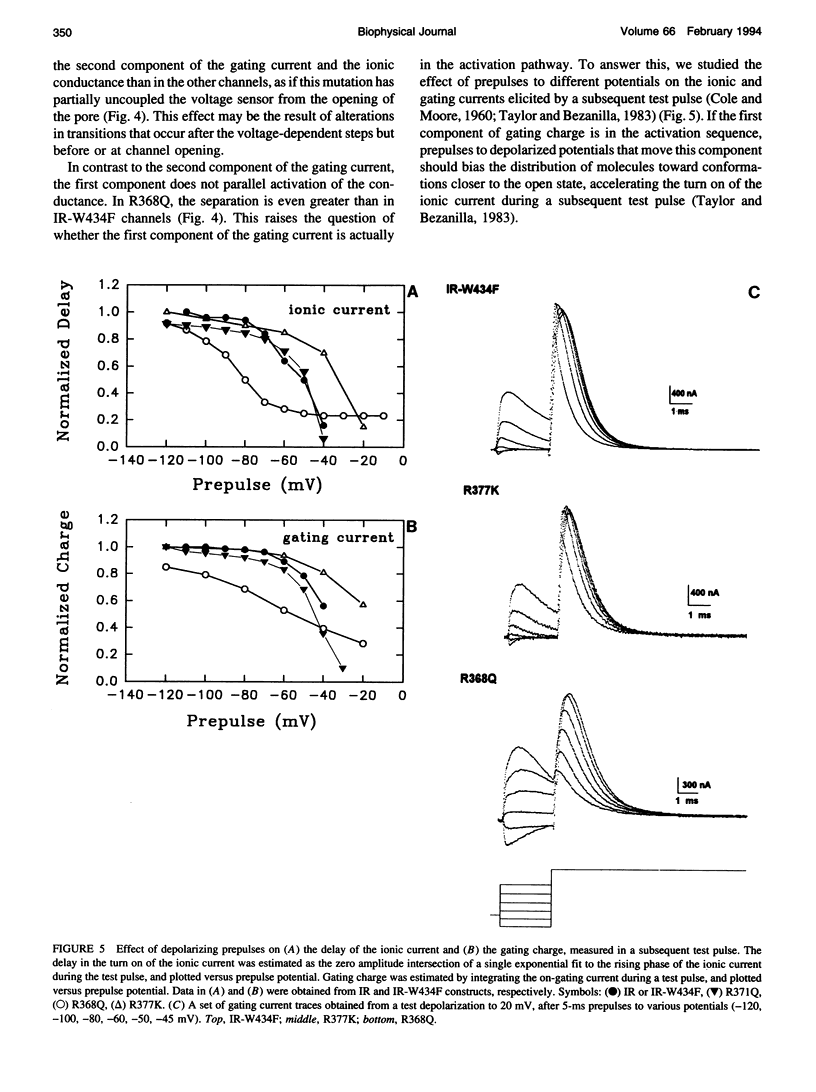
Abstract
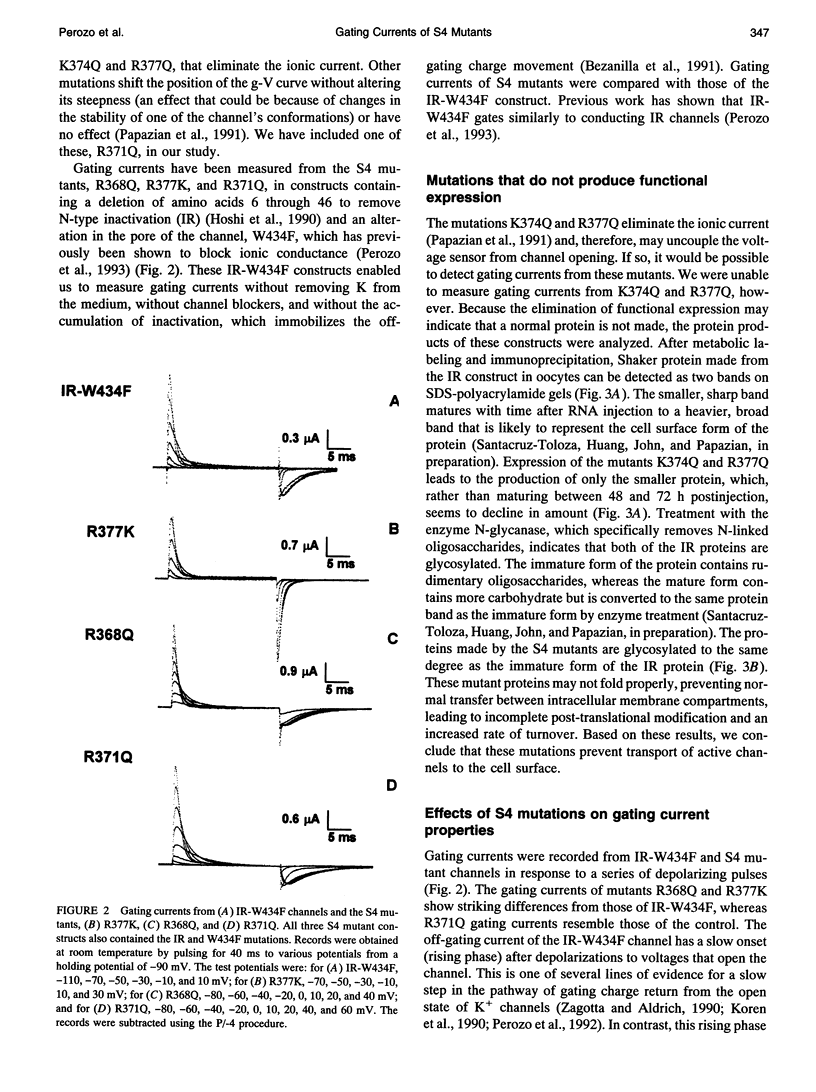
Activation of voltage-dependent channels involves charge-moving conformational changes of the voltage sensor that can be detected as gating currents. In Shaker K channels, the S4 sequence comprises at least part of the voltage sensor. We have measured gating currents in three S4 mutants: R368Q, R377K, and R371Q. R368Q enhances the separation of two components of charge movement and greatly reduces the valence of one component. R377K partially uncouples charge movement from channel opening. In contrast, the gating currents of R371Q resemble those of the control. Two other S4 mutations, R377Q and K374Q, make proteins that are not properly processed and transported to the cell surface and thereby eliminate the gating current. To explain the effects of R368Q, we hypothesize that R368 is part of a salt bridge that is broken early in activation. Subsequently, the S4 segment undergoes a conformational change, and, after a final, relatively voltage-independent step, the channel opens.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973 Apr 13;242(5398):459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- Atkinson N. S., Robertson G. A., Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991 Aug 2;253(5019):551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Perozo E., Papazian D. M., Stefani E. Molecular basis of gating charge immobilization in Shaker potassium channels. Science. 1991 Nov 1;254(5032):679–683. doi: 10.1126/science.1948047. [DOI] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- Dougherty D. A., Stauffer D. A. Acetylcholine binding by a synthetic receptor: implications for biological recognition. Science. 1990 Dec 14;250(4987):1558–1560. doi: 10.1126/science.2274786. [DOI] [PubMed] [Google Scholar]
- Greenblatt R. E., Blatt Y., Montal M. The structure of the voltage-sensitive sodium channel. Inferences derived from computer-aided analysis of the Electrophorus electricus channel primary structure. FEBS Lett. 1985 Dec 2;193(2):125–134. doi: 10.1016/0014-5793(85)80136-8. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Seetharamulu P. Molecular model of the action potential sodium channel. Proc Natl Acad Sci U S A. 1986 Jan;83(2):508–512. doi: 10.1073/pnas.83.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992 Mar;8(3):483–491. doi: 10.1016/0896-6273(92)90276-j. [DOI] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L., Flewelling R. F. Electrostatic interactions in membranes and proteins. Annu Rev Biophys Biophys Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L. Stability of "salt bridges" in membrane proteins. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5412–5416. doi: 10.1073/pnas.81.17.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. A superfamily of ion channels. Nature. 1990 Jun 21;345(6277):672–672. doi: 10.1038/345672a0. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N. J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989 Dec 14;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Koren G., Liman E. R., Logothetis D. E., Nadal-Ginard B., Hess P. Gating mechanism of a cloned potassium channel expressed in frog oocytes and mammalian cells. Neuron. 1990 Jan;4(1):39–51. doi: 10.1016/0896-6273(90)90442-i. [DOI] [PubMed] [Google Scholar]
- Lee J. I., Hwang P. P., Hansen C., Wilson T. H. Possible salt bridges between transmembrane alpha-helices of the lactose carrier of Escherichia coli. J Biol Chem. 1992 Oct 15;267(29):20758–20764. [PubMed] [Google Scholar]
- Liman E. R., Hess P., Weaver F., Koren G. Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature. 1991 Oct 24;353(6346):752–756. doi: 10.1038/353752a0. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Movahedi S., Satler C., Lindpaintner K., Nadal-Ginard B. Incremental reductions of positive charge within the S4 region of a voltage-gated K+ channel result in corresponding decreases in gating charge. Neuron. 1992 Mar;8(3):531–540. doi: 10.1016/0896-6273(92)90281-h. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Papazian D. M., Timpe L. C., Jan Y. N., Jan L. Y. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 1991 Jan 24;349(6307):305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- Parsegian A. Energy of an ion crossing a low dielectric membrane: solutions to four relevant electrostatic problems. Nature. 1969 Mar 1;221(5183):844–846. doi: 10.1038/221844a0. [DOI] [PubMed] [Google Scholar]
- Perozo E., MacKinnon R., Bezanilla F., Stefani E. Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron. 1993 Aug;11(2):353–358. doi: 10.1016/0896-6273(93)90190-3. [DOI] [PubMed] [Google Scholar]
- Perozo E., Papazian D. M., Stefani E., Bezanilla F. Gating currents in Shaker K+ channels. Implications for activation and inactivation models. Biophys J. 1992 Apr;62(1):160–171. doi: 10.1016/S0006-3495(92)81802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa N. E., McCormack K., Tanouye M. A., Sigworth F. J. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 1992 Mar 27;255(5052):1712–1715. doi: 10.1126/science.1553560. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Papazian D. M., Carretto R. C., Jan Y. N., Jan L. Y. Immunological characterization of K+ channel components from the Shaker locus and differential distribution of splicing variants in Drosophila. Neuron. 1990 Jan;4(1):119–127. doi: 10.1016/0896-6273(90)90448-o. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988 Jan 14;331(6152):137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- Shao X. M., Papazian D. M. S4 mutations alter the single-channel gating kinetics of Shaker K+ channels. Neuron. 1993 Aug;11(2):343–352. doi: 10.1016/0896-6273(93)90189-x. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Khorana H. G. Structure-function studies on bacteriorhodopsin. X. Individual substitutions of arginine residues by glutamine affect chromophore formation, photocycle, and proton translocation. J Biol Chem. 1989 Aug 25;264(24):14202–14208. [PubMed] [Google Scholar]
- Stühmer W., Conti F., Stocker M., Pongs O., Heinemann S. H. Gating currents of inactivating and non-inactivating potassium channels expressed in Xenopus oocytes. Pflugers Arch. 1991 May;418(4):423–429. doi: 10.1007/BF00550881. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Conti F., Suzuki H., Wang X. D., Noda M., Yahagi N., Kubo H., Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989 Jun 22;339(6226):597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Taglialatela M., Toro L., Stefani E. Novel voltage clamp to record small, fast currents from ion channels expressed in Xenopus oocytes. Biophys J. 1992 Jan;61(1):78–82. doi: 10.1016/S0006-3495(92)81817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Taylor R. E., Bezanilla F. Sodium and gating current time shifts resulting from changes in initial conditions. J Gen Physiol. 1983 Jun;81(6):773–784. doi: 10.1085/jgp.81.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel B. L., Papazian D. M., Schwarz T. L., Jan Y. N., Jan L. Y. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987 Aug 14;237(4816):770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- Timpe L. C., Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Expression of functional potassium channels from Shaker cDNA in Xenopus oocytes. Nature. 1988 Jan 14;331(6152):143–145. doi: 10.1038/331143a0. [DOI] [PubMed] [Google Scholar]
- Tytgat J., Hess P. Evidence for cooperative interactions in potassium channel gating. Nature. 1992 Oct 1;359(6394):420–423. doi: 10.1038/359420a0. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Aldrich R. W. Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J Gen Physiol. 1990 Jan;95(1):29–60. doi: 10.1085/jgp.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]