Abstract
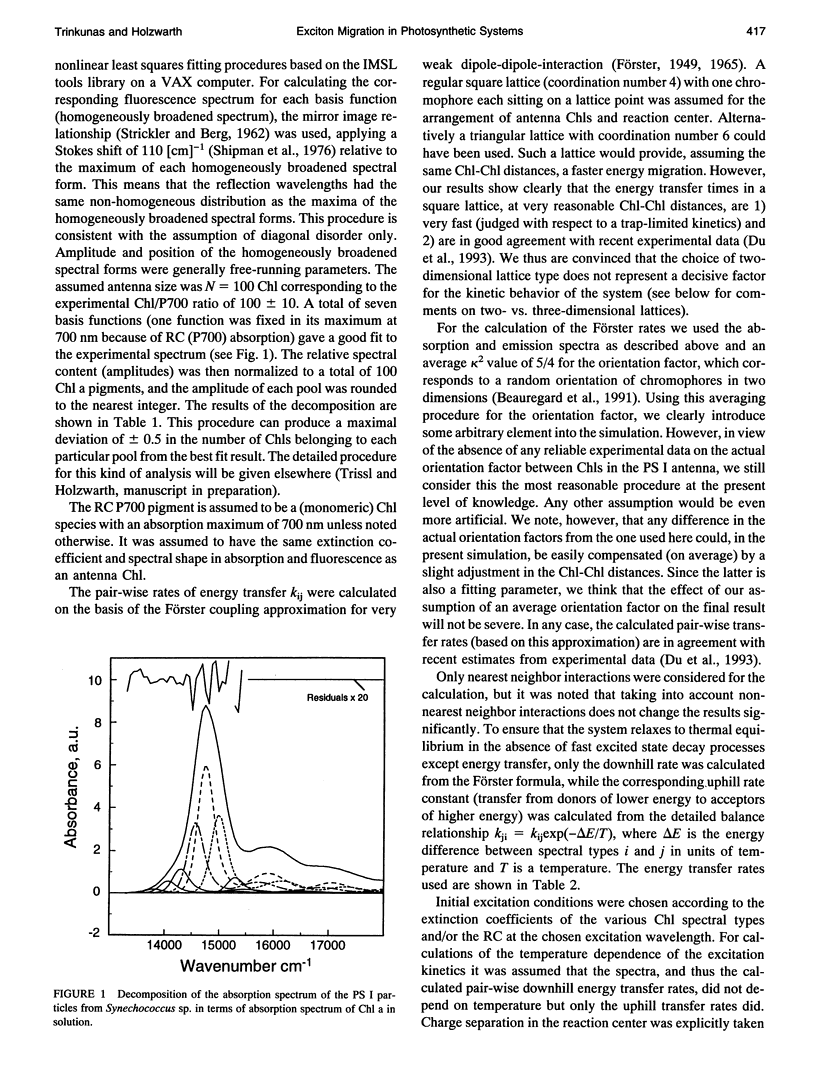
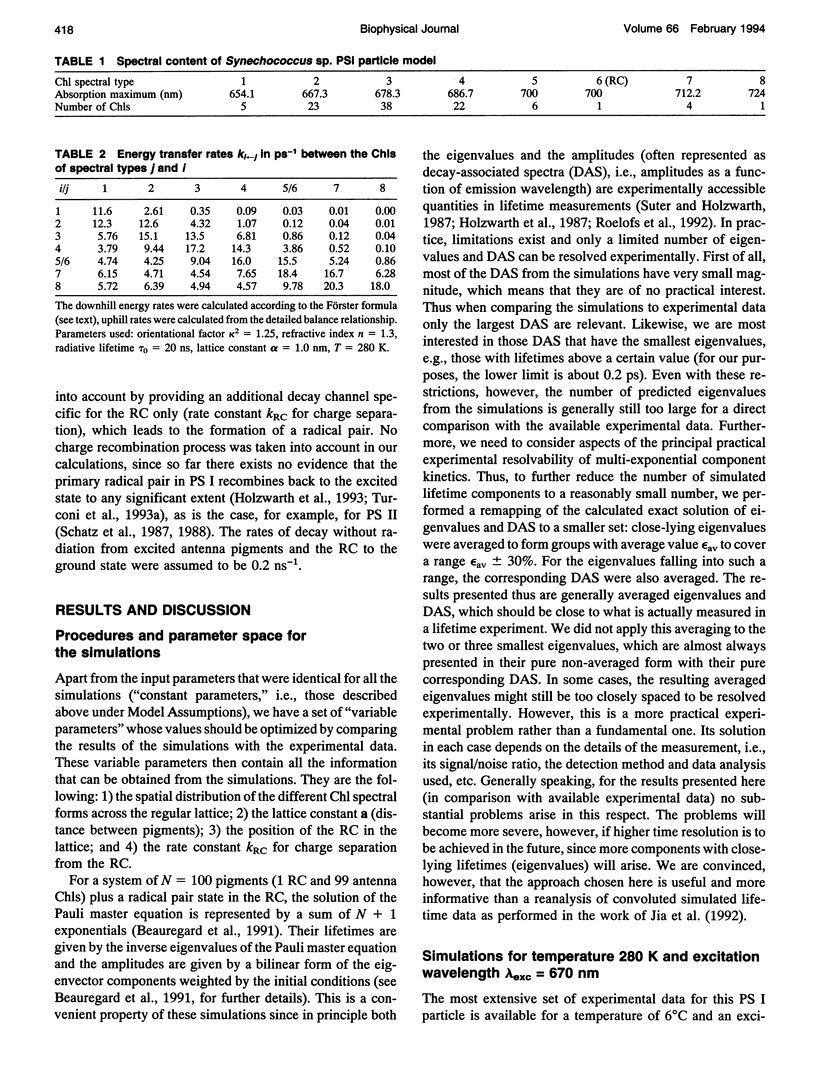
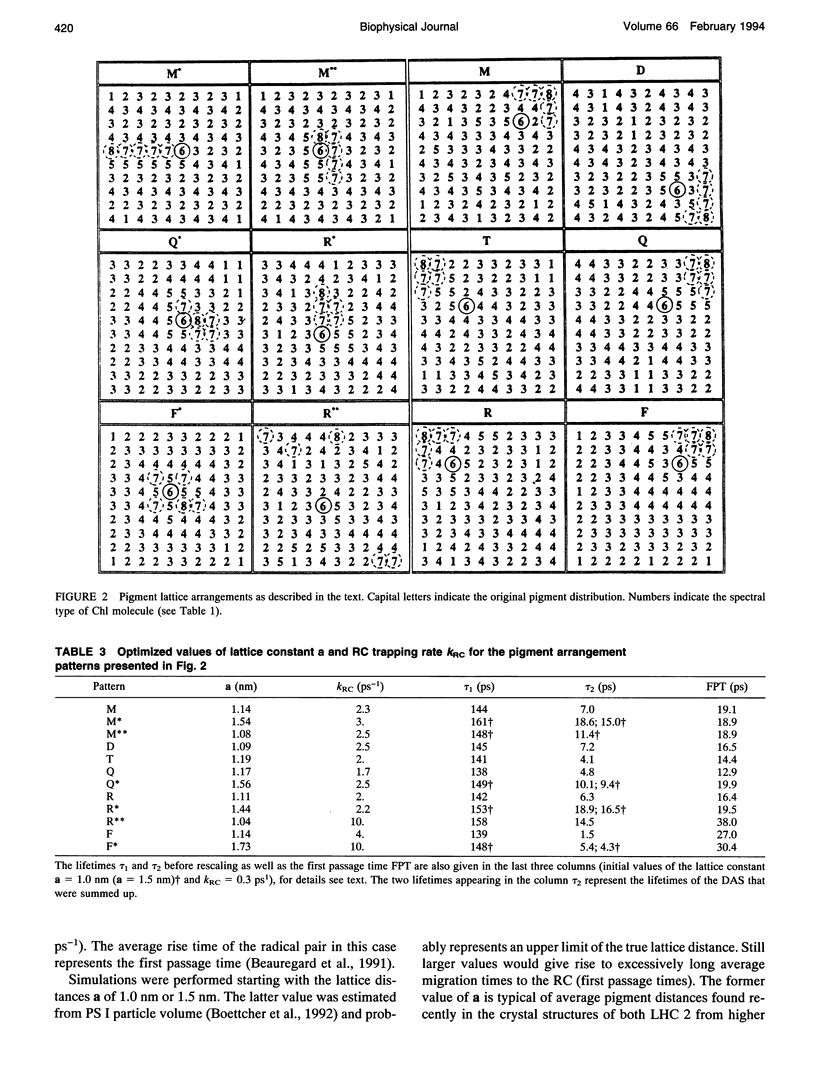
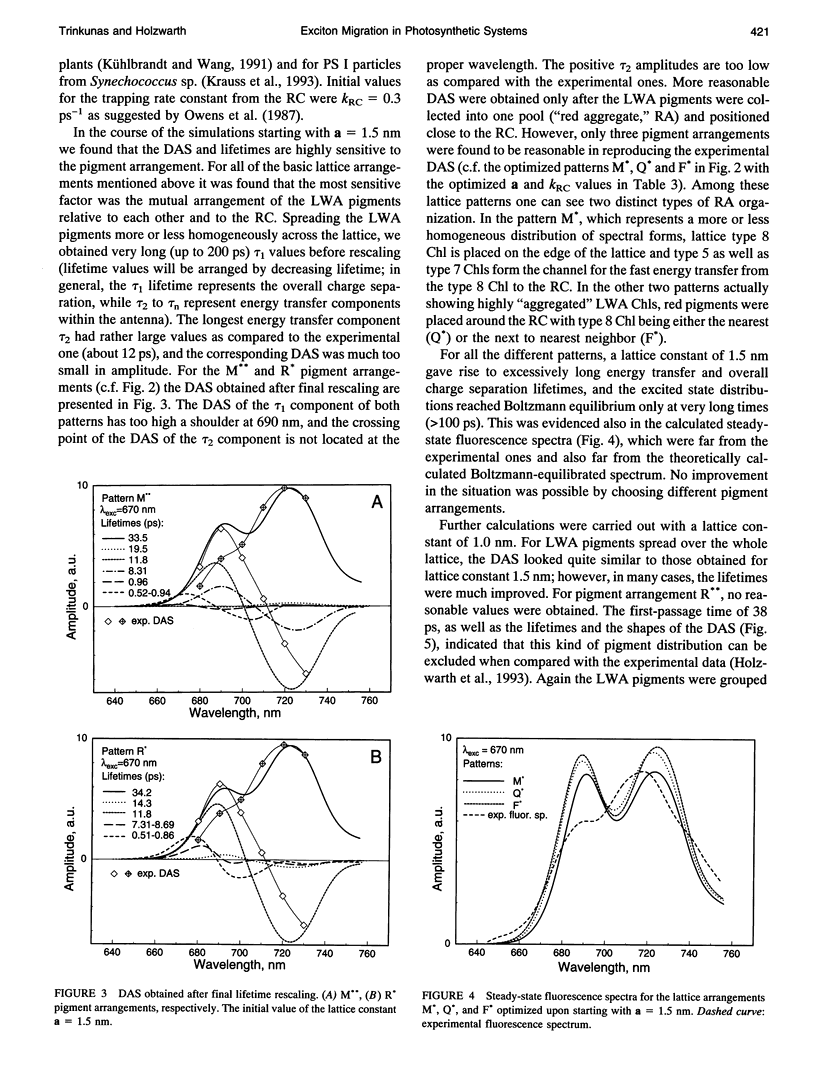
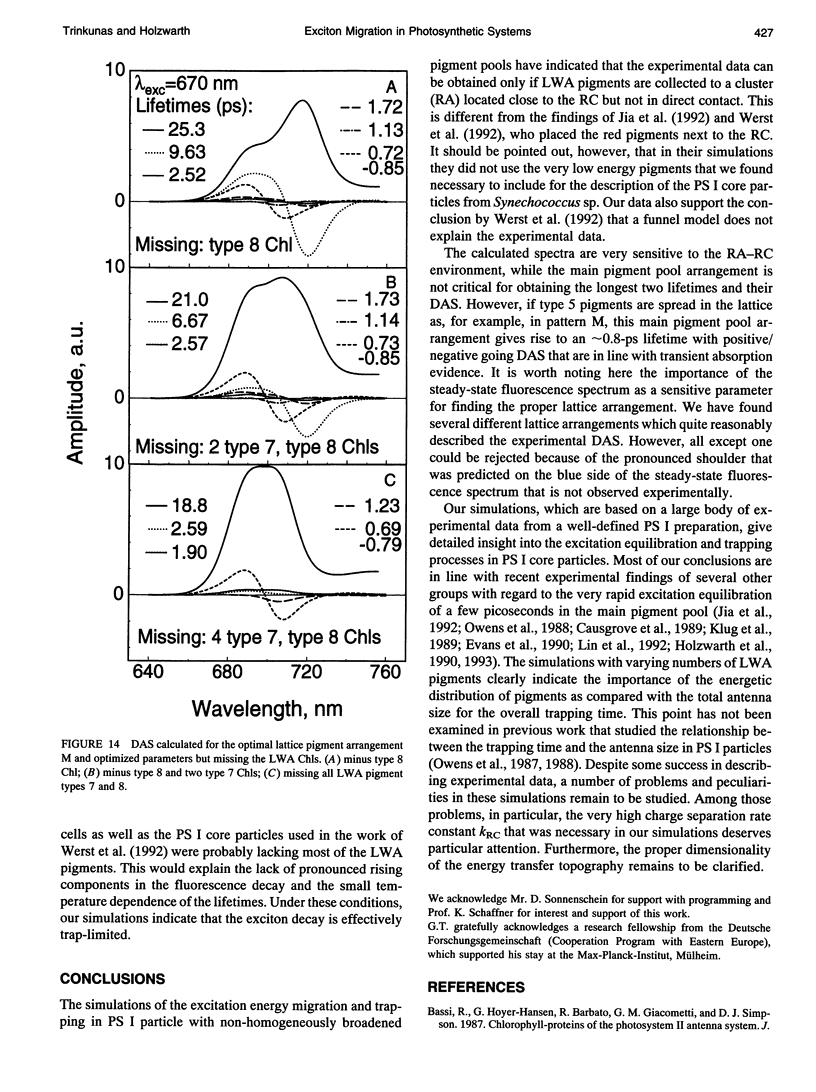
Kinetic modeling of the exciton migration in the cyanobacterial photosystem I core complex from Synechococcus sp. was performed by an exact solution of the Pauli master equation for exciton motion. A square two-dimensional 10 x 10 pigment lattice and a Förster dipole-dipole coupling between chromophores was assumed. We calculated decay-associated spectra and lifetimes and compared them to the corresponding experimental data from picosecond fluorescence and transient absorption obtained by global analysis. Seven spectral chlorophyll(Chl) forms, identical in shape but shifted in their absorption maximums, were used to describe the non-homogeneous broadening of the PS I-100 particle absorption spectrum. The optimized Chl lattice arrangement best reproducing the experimental decay-associated spectra as well as the steady-state fluorescence spectrum indicated the long-wavelength-absorbing Chls forming a cluster in the corner of the lattice with the reaction center (RC) placed apart at a distance of two lattice constants. The variable parameters, i.e., the charge separation rate in the RC and the lattice constant a, were found to be optimal at kRC = 2.3 ps-1 and a = 1.14 nm, respectively. The surprising conclusions of the simulations is that Chls with absorption maxima as long a 724 nm have to be taken into account to describe the time-resolved spectra of this PS I particle properly. The dependencies of the exciton decay in the model PS I particle on the excitation wavelength and on the temperature are discussed. We also show that the excited state decay of similar PS I particles that lack the long-wavelength absorbing Chls is nearly mono-exponential. Various critical factors that limit the general reliability of the conclusions of such simulations are discussed in detail.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. L. A far-red absorbing form of chlorophyll. in vivo. Arch Biochem Biophys. 1961 May;93:413–422. doi: 10.1016/0003-9861(61)90287-9. [DOI] [PubMed] [Google Scholar]
- Bassi R., Høyer-Hansen G., Barbato R., Giacometti G. M., Simpson D. J. Chlorophyll-proteins of the photosystem II antenna system. J Biol Chem. 1987 Sep 25;262(27):13333–13341. [PubMed] [Google Scholar]
- Brown J. S., Schoch S. Spectral analysis of chlorophyll-protein complexes from higher plant chloroplasts. Biochim Biophys Acta. 1981 Jul;636(2):201–209. doi: 10.1016/0005-2728(81)90094-3. [DOI] [PubMed] [Google Scholar]
- Böttcher B., Gräber P., Boekema E. J. The structure of Photosystem I from the thermophilic cyanobacterium Synechococcus sp. determined by electron microscopy of two-dimensional crystals. Biochim Biophys Acta. 1992 May 20;1100(2):125–136. doi: 10.1016/0005-2728(92)90073-b. [DOI] [PubMed] [Google Scholar]
- Holzwarth A. R., Schatz G., Brock H., Bittersmann E. Energy transfer and charge separation kinetics in photosystem I: Part 1: Picosecond transient absorption and fluorescence study of cyanobacterial photosystem I particles. Biophys J. 1993 Jun;64(6):1813–1826. doi: 10.1016/S0006-3495(93)81552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzwarth A. R., Wendler J., Suter G. W. Studies on Chromophore Coupling in Isolated Phycobiliproteins: II. Picosecond Energy Transfer Kinetics and Time-Resolved Fluorescence Spectra of C-Phycocyanin from Synechococcus 6301 as a Function of the Aggregation State. Biophys J. 1987 Jan;51(1):1–12. doi: 10.1016/S0006-3495(87)83306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean J. M., Chan C. K., Fleming G. R., Owens T. G. Excitation transport and trapping on spectrally disordered lattices. Biophys J. 1989 Dec;56(6):1203–1215. doi: 10.1016/S0006-3495(89)82767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Jean J. M., Werst M. M., Chan C. K., Fleming G. R. Simulations of the temperature dependence of energy transfer in the PSI core antenna. Biophys J. 1992 Jul;63(1):259–273. doi: 10.1016/S0006-3495(92)81589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R. S. On the theory of trapping of excitation in the photosynthetic unit. J Theor Biol. 1968 Nov;21(2):244–259. doi: 10.1016/0022-5193(68)90073-8. [DOI] [PubMed] [Google Scholar]
- Kuang T. Y., Argyroudi-Akoyunoglou J. H., Nakatani H. Y., Watson J., Arntzen C. J. The origin of the long-wavelength fluorescence emission band (77 degrees K) from photosystem I. Arch Biochem Biophys. 1984 Dec;235(2):618–627. doi: 10.1016/0003-9861(84)90236-4. [DOI] [PubMed] [Google Scholar]
- Kudzmauskas S., Valkunas L., Borisov A. Y. A theory of excitation transfer in photosynthetic units. J Theor Biol. 1983 Nov 7;105(1):13–23. doi: 10.1016/0022-5193(83)90421-6. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Downing K. H. Two-dimensional structure of plant light-harvesting complex at 3.7 A [corrected] resolution by electron crystallography. J Mol Biol. 1989 Jun 20;207(4):823–828. doi: 10.1016/0022-2836(89)90247-7. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N. Three-dimensional structure of plant light-harvesting complex determined by electron crystallography. Nature. 1991 Mar 14;350(6314):130–134. doi: 10.1038/350130a0. [DOI] [PubMed] [Google Scholar]
- Owens T. G., Webb S. P., Alberte R. S., Mets L., Fleming G. R. Antenna structure and excitation dynamics in photosystem I. I. Studies of detergent-isolated photosystem I preparations using time-resolved fluorescence analysis. Biophys J. 1988 May;53(5):733–745. doi: 10.1016/S0006-3495(88)83154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. G., Webb S. P., Mets L., Alberte R. S., Fleming G. R. Antenna size dependence of fluorescence decay in the core antenna of photosystem I: estimates of charge separation and energy transfer rates. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1532–1536. doi: 10.1073/pnas.84.6.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs T. A., Lee C. H., Holzwarth A. R. Global target analysis of picosecond chlorophyll fluorescence kinetics from pea chloroplasts: A new approach to the characterization of the primary processes in photosystem II alpha- and beta-units. Biophys J. 1992 May;61(5):1147–1163. doi: 10.1016/s0006-3495(92)81924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. H., Brock H., Holzwarth A. R. Kinetic and Energetic Model for the Primary Processes in Photosystem II. Biophys J. 1988 Sep;54(3):397–405. doi: 10.1016/S0006-3495(88)82973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. H., Brock H., Holzwarth A. R. Picosecond kinetics of fluorescence and absorbance changes in photosystem II particles excited at low photon density. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8414–8418. doi: 10.1073/pnas.84.23.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa J. A., Alberte R. S., Thornber J. P. The P700-chlorophyll a-protein. Isolation and some characteristics of the complex in higher plants. Arch Biochem Biophys. 1974 Nov;165(1):388–397. doi: 10.1016/0003-9861(74)90177-5. [DOI] [PubMed] [Google Scholar]
- Shipman L. L., Cotton T. M., Norris J. R., Katz J. J. An analysis of the visible absorption spectrum of chlorophyll a monomer, dimer,and oligomers in solution. J Am Chem Soc. 1976 Dec 8;98(25):8222–8230. doi: 10.1021/ja00441a056. [DOI] [PubMed] [Google Scholar]
- Strasser R. J., Butler W. L. Fluorescence emission spectra of photosystem I, photosystem II and the light-harvesting chlorophyll a/b complex of higher plants. Biochim Biophys Acta. 1977 Nov 17;462(2):307–313. doi: 10.1016/0005-2728(77)90129-3. [DOI] [PubMed] [Google Scholar]
- Suter G. W., Holzwarth A. R. A kinetic model for the energy transfer in phycobilisomes. Biophys J. 1987 Nov;52(5):673–683. doi: 10.1016/S0006-3495(87)83262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasielewski M. R., Johnson D. G., Seibert M., Govindjee Determination of the primary charge separation rate in isolated photosystem II reaction centers with 500-fs time resolution. Proc Natl Acad Sci U S A. 1989 Jan;86(2):524–528. doi: 10.1073/pnas.86.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werst M., Jia Y., Mets L., Fleming G. R. Energy transfer and trapping in the photosystem I core antenna. A temperature study. Biophys J. 1992 Apr;61(4):868–878. doi: 10.1016/S0006-3495(92)81894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


