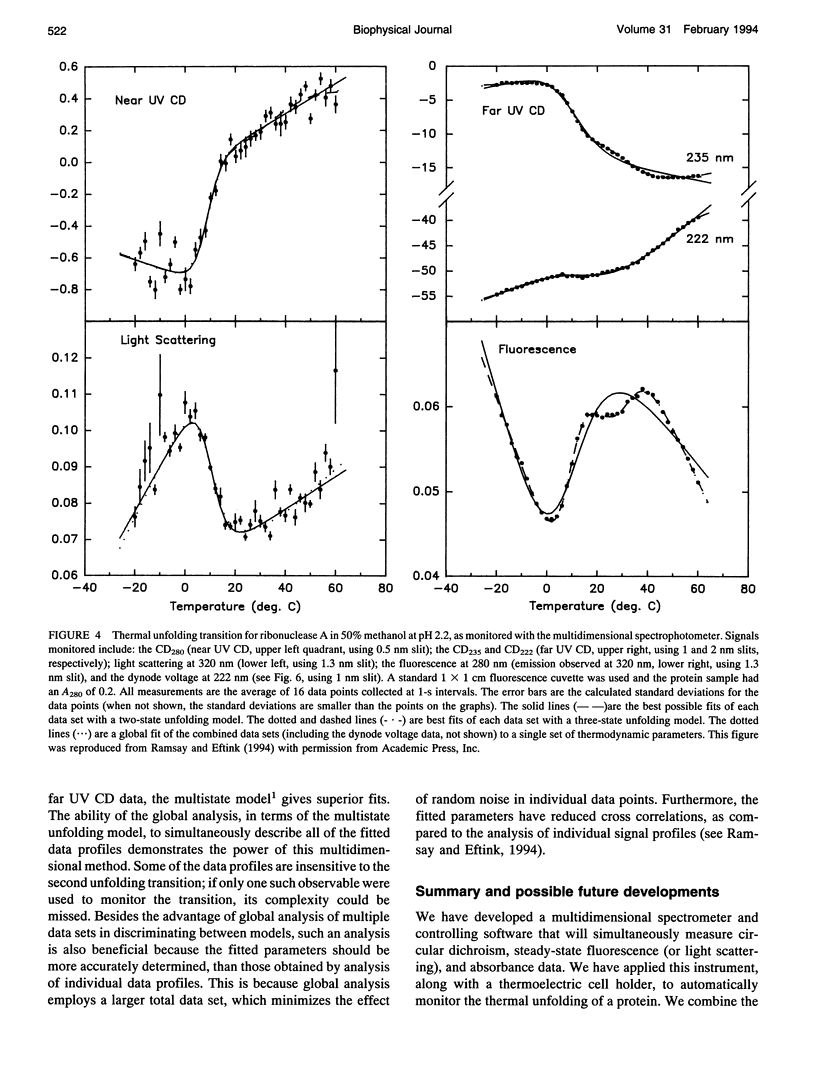
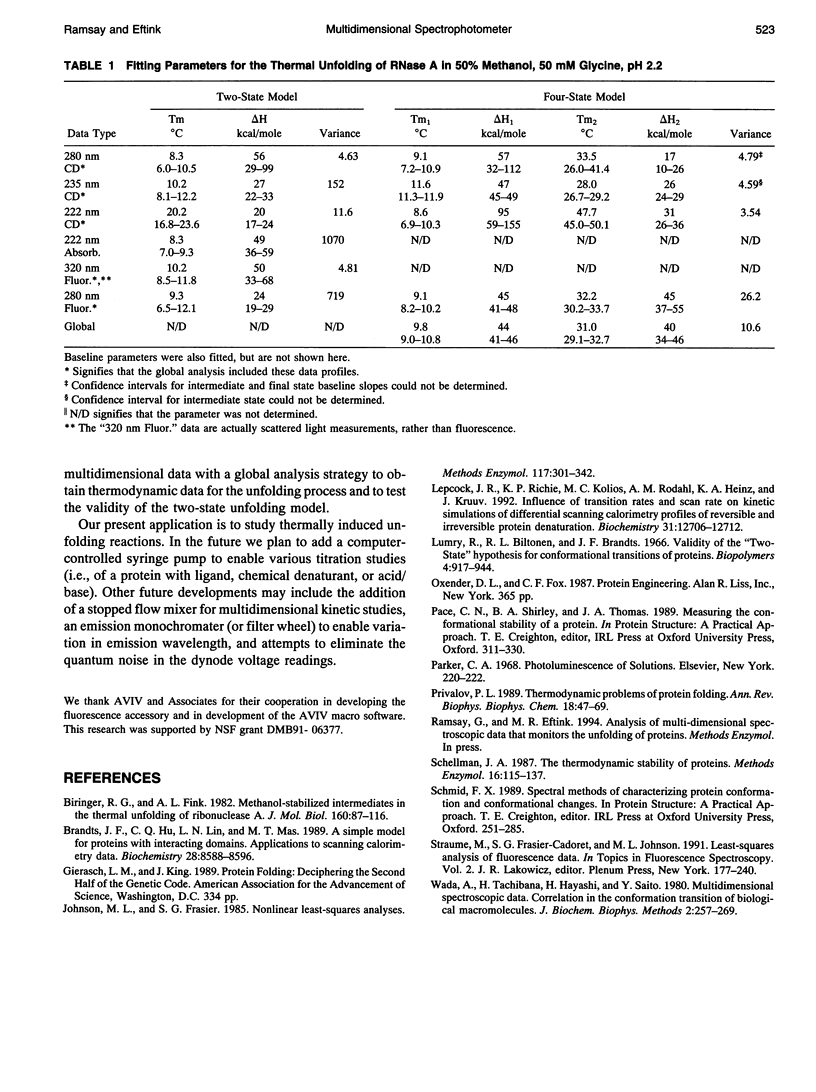
Abstract
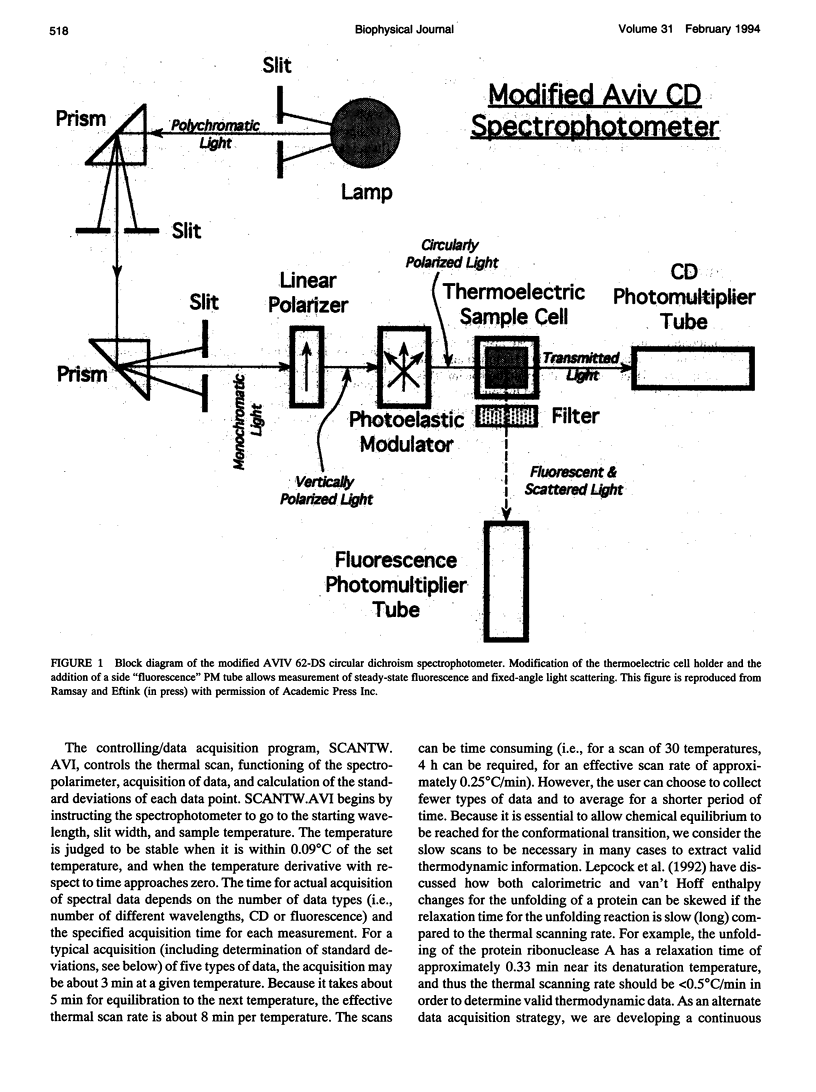
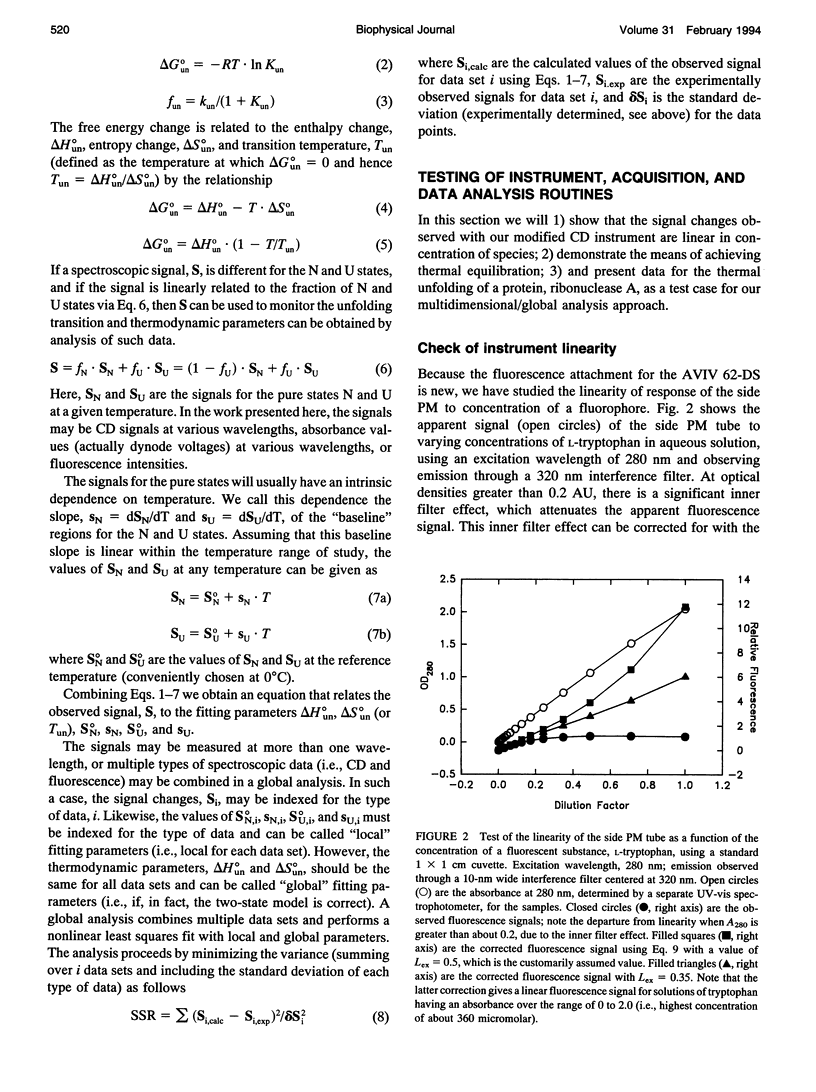
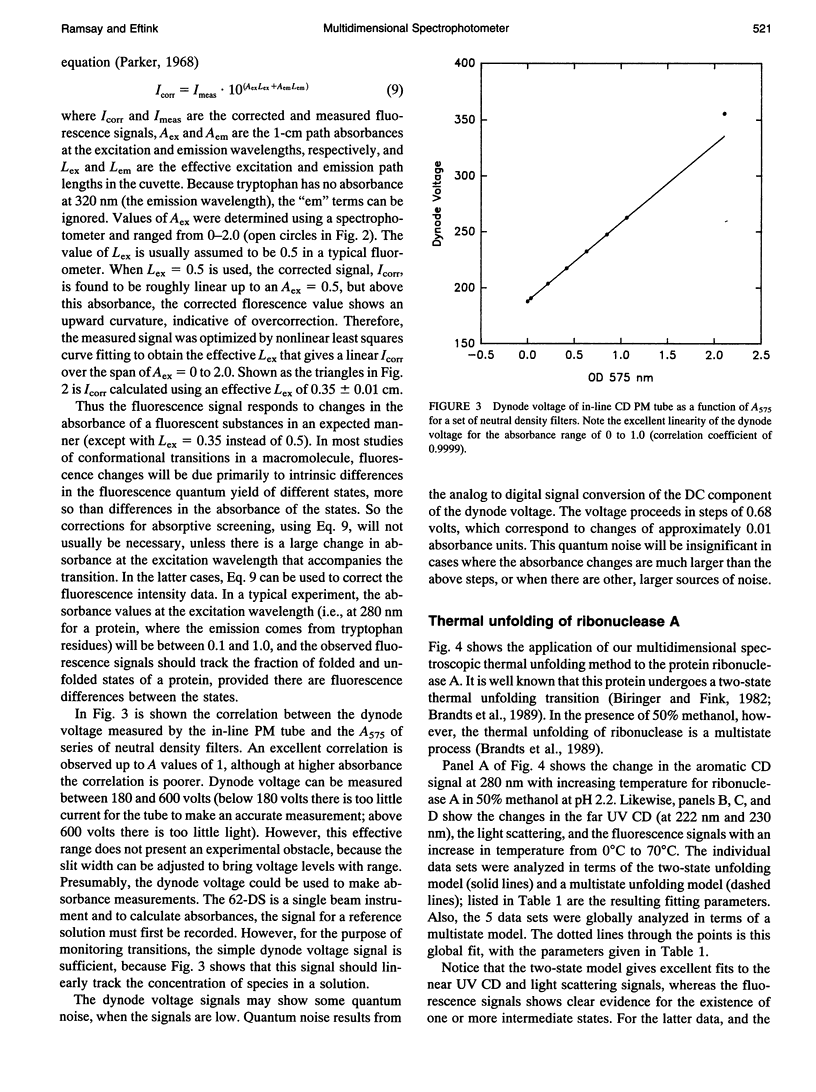
We describe a multidimensional spectrometer that is capable of (nearly) simultaneous measurement of circular dichroism, steady-state fluorescence, and absorbance values on the same sample in a standard 1 x 1 cm cuvette. With a computer controlled thermoelectric cell holder, this instrument is capable of measuring the above types of spectral data at various wavelengths as a function of temperature. We have developed software to control the various acquisition functions and to convert the data files to a format appropriate for use with the nonlinear least squares program, NONLIN (Johnson and Frasier, 1985). We have tested various features of this instrument and we have applied this instrument and data analysis procedure to study the thermal unfolding of ribonuclease A, under conditions were the unfolding of this protein involves an intermediate state.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biringer R. G., Fink A. L. Methanol-stabilized intermediates in the thermal unfolding of ribonuclease A. Characterization by 1H nuclear magnetic resonance. J Mol Biol. 1982 Sep;160(1):87–116. doi: 10.1016/0022-2836(82)90133-4. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Hu C. Q., Lin L. N., Mos M. T. A simple model for proteins with interacting domains. Applications to scanning calorimetry data. Biochemistry. 1989 Oct 17;28(21):8588–8596. doi: 10.1021/bi00447a048. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Ritchie K. P., Kolios M. C., Rodahl A. M., Heinz K. A., Kruuv J. Influence of transition rates and scan rate on kinetic simulations of differential scanning calorimetry profiles of reversible and irreversible protein denaturation. Biochemistry. 1992 Dec 22;31(50):12706–12712. doi: 10.1021/bi00165a023. [DOI] [PubMed] [Google Scholar]
- Lumry R., Biltonen R. Validity of the "two-state" hypothesis for conformational transitions of proteins. Biopolymers. 1966 Sep;4(8):917–944. doi: 10.1002/bip.1966.360040808. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Thermodynamic problems of protein structure. Annu Rev Biophys Biophys Chem. 1989;18:47–69. doi: 10.1146/annurev.bb.18.060189.000403. [DOI] [PubMed] [Google Scholar]
- Schellman J. A. The thermodynamic stability of proteins. Annu Rev Biophys Biophys Chem. 1987;16:115–137. doi: 10.1146/annurev.bb.16.060187.000555. [DOI] [PubMed] [Google Scholar]
- Wada A., Tachibana H., Hayashi H., Saito Y. Multidimensional spectroscopic data correlation in the conformation transition of biological macromolecules. J Biochem Biophys Methods. 1980 May;2(5):257–269. doi: 10.1016/0165-022x(80)90050-0. [DOI] [PubMed] [Google Scholar]