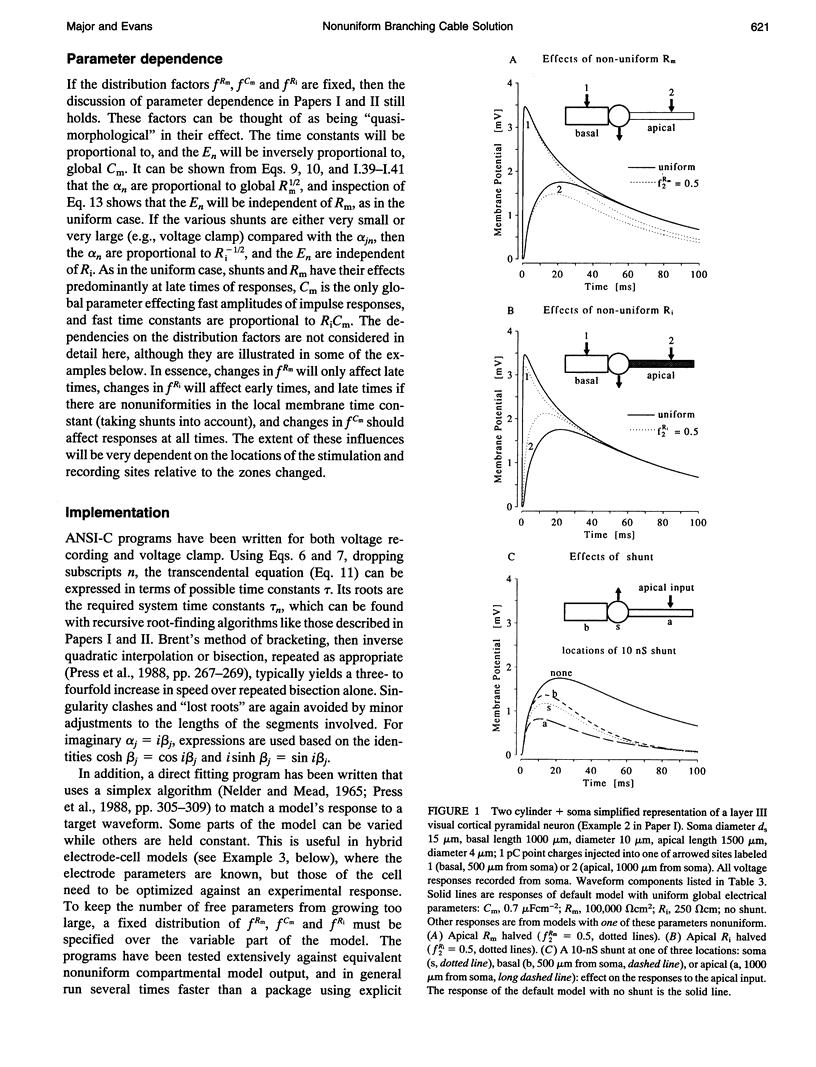
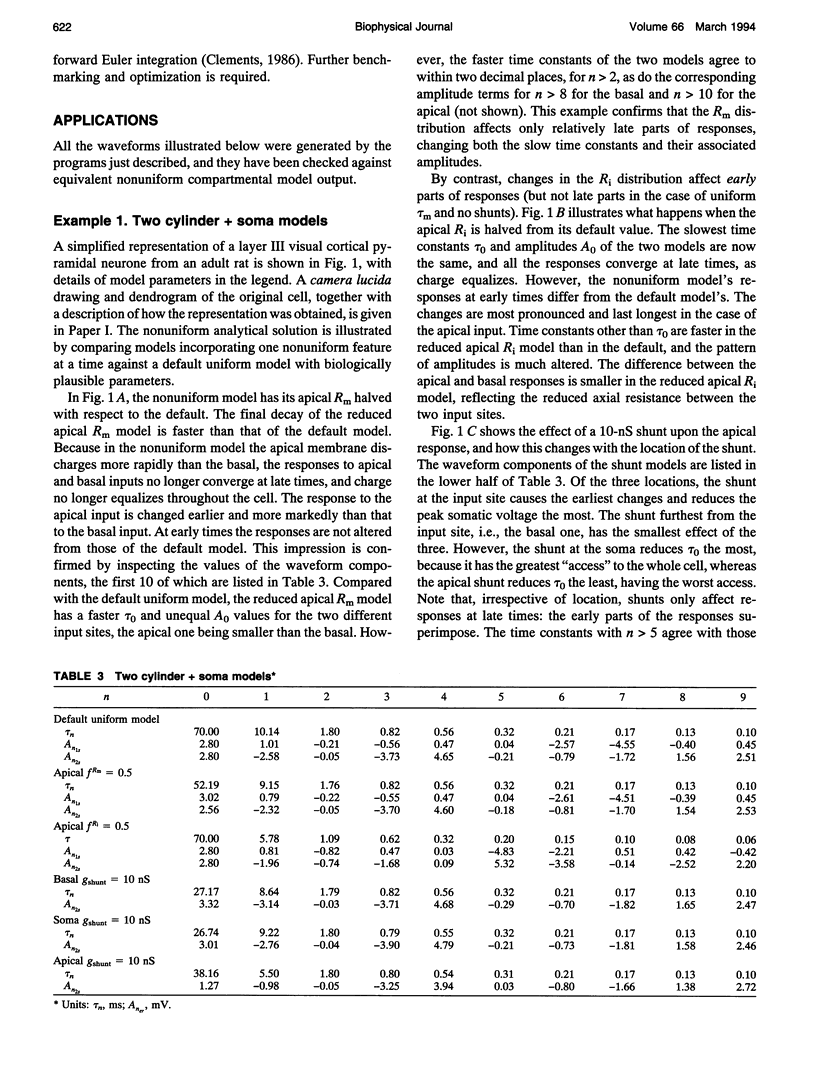
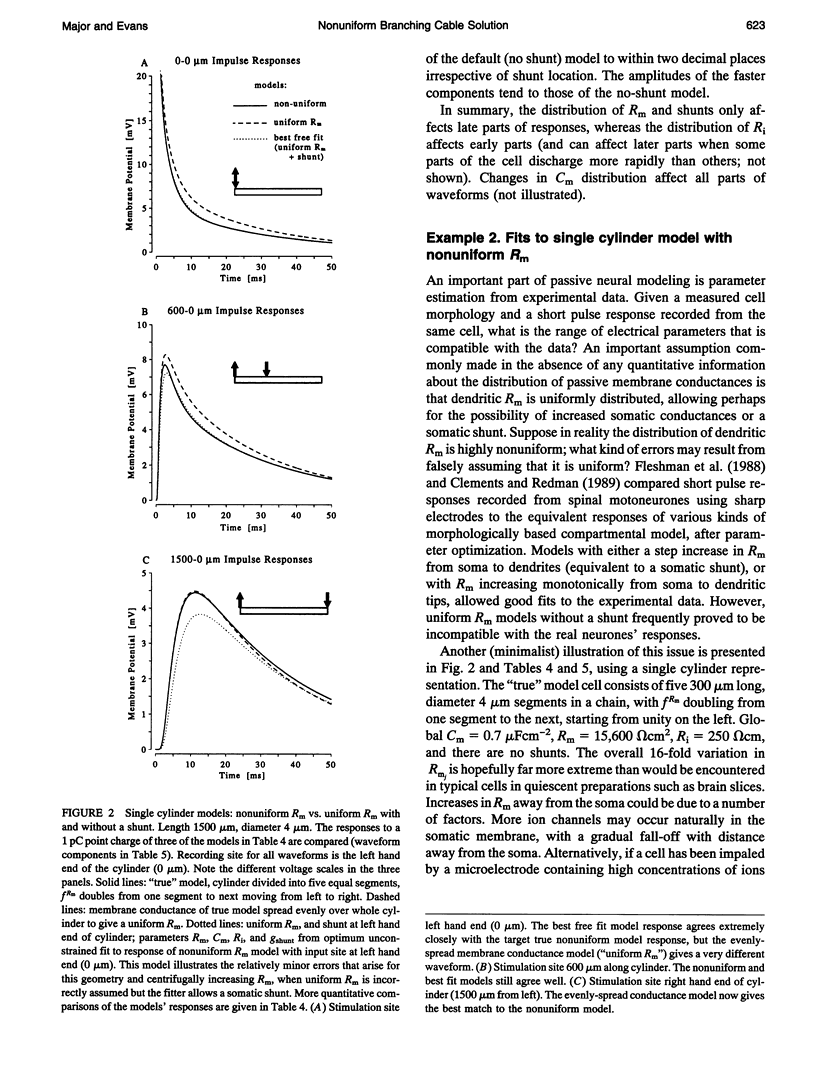
Abstract
Solutions for transients in arbitrarily branching passive cable neurone models with a soma are extended to models with nonuniform electrical parameters and multiple dendritic shunts. The response to an injected current can again be represented as an infinite series of exponentially decaying components with system time constants obtained from the roots of a recursive transcendental equation. The reciprocity relations and global parameter dependencies are the same as for uniform models. Infinitely many "raw" electro-morphological models map onto a given "core" electrotonic model; local as well as global raw parameter trade-offs are now possible. The solutions are illustrated by means of biologically relevant examples: (i) the effects of nonuniform electrical parameters in a two-cylinder + soma cortical pyramidal cell model, (ii) the errors that can occur when uniformity is incorrectly assumed in a single cylinder model, (iii) nonsumming interactions between cells and electrodes that can dramatically increase the duration of the effective capacitative electrode artefact, and (iv) shunting inhibition and double impalements in a hippocampal CA1 pyramidal cell "cartoon" representation. These solutions should complement compartmental modelling techniques.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. N., Crill W. E. Specific membrane properties of cat motoneurones. J Physiol. 1974 Jun;239(2):301–324. doi: 10.1113/jphysiol.1974.sp010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluman G. W., Tuckwell H. C. Techniques for obtaining analytical solutions for Rall's model neuron. J Neurosci Methods. 1987 Jun;20(2):151–166. doi: 10.1016/0165-0270(87)90047-1. [DOI] [PubMed] [Google Scholar]
- Clegg J. S. Intracellular water and the cytomatrix: some methods of study and current views. J Cell Biol. 1984 Jul;99(1 Pt 2):167s–171s. doi: 10.1083/jcb.99.1.167s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. S. Properties and metabolism of the aqueous cytoplasm and its boundaries. Am J Physiol. 1984 Feb;246(2 Pt 2):R133–R151. doi: 10.1152/ajpregu.1984.246.2.R133. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Redman S. J. Cable properties of cat spinal motoneurones measured by combining voltage clamp, current clamp and intracellular staining. J Physiol. 1989 Feb;409:63–87. doi: 10.1113/jphysiol.1989.sp017485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M. H., Levinson S. R. Immunocytochemical localization of sodium channel distributions in the excitable membranes of Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6707–6711. doi: 10.1073/pnas.79.21.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. D., Kember G. C., Major G. Techniques for obtaining analytical solutions to the multicylinder somatic shunt cable model for passive neurones. Biophys J. 1992 Aug;63(2):350–365. doi: 10.1016/S0006-3495(92)81631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman J. W., Segev I., Burke R. B. Electrotonic architecture of type-identified alpha-motoneurons in the cat spinal cord. J Neurophysiol. 1988 Jul;60(1):60–85. doi: 10.1152/jn.1988.60.1.60. [DOI] [PubMed] [Google Scholar]
- Fromherz P., Vetter T. Cable properties of arborized Retzius cells of the leech in culture as probed by a voltage-sensitive dye. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2041–2045. doi: 10.1073/pnas.89.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman D., Chen S., Aung T. T., Cherksey B., Sugimori M., Llinás R. R. Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7076–7080. doi: 10.1073/pnas.88.16.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. A program for simulation of nerve equations with branching geometries. Int J Biomed Comput. 1989 Mar;24(1):55–68. doi: 10.1016/0020-7101(89)90007-x. [DOI] [PubMed] [Google Scholar]
- Hines M. Efficient computation of branched nerve equations. Int J Biomed Comput. 1984 Jan-Feb;15(1):69–76. doi: 10.1016/0020-7101(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Holmes W. R. A continuous cable method for determining the transient potential in passive dendritic trees of known geometry. Biol Cybern. 1986;55(2-3):115–124. doi: 10.1007/BF00341927. [DOI] [PubMed] [Google Scholar]
- Holmes W. R., Segev I., Rall W. Interpretation of time constant and electrotonic length estimates in multicylinder or branched neuronal structures. J Neurophysiol. 1992 Oct;68(4):1401–1420. doi: 10.1152/jn.1992.68.4.1401. [DOI] [PubMed] [Google Scholar]
- Holmes W. R., Woody C. D. Effects of uniform and non-uniform synaptic 'activation-distributions' on the cable properties of modeled cortical pyramidal neurons. Brain Res. 1989 Dec 25;505(1):12–22. doi: 10.1016/0006-8993(89)90110-8. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Midtgaard J. Dendrite processing in more ways than one . Trends Neurosci. 1989 Sep;12(9):313–315. doi: 10.1016/0166-2236(89)90036-2. [DOI] [PubMed] [Google Scholar]
- Huguenard J. R., Hamill O. P., Prince D. A. Sodium channels in dendrites of rat cortical pyramidal neurons. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2473–2477. doi: 10.1073/pnas.86.7.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe D. B., Johnston D., Lasser-Ross N., Lisman J. E., Miyakawa H., Ross W. N. The spread of Na+ spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature. 1992 May 21;357(6375):244–246. doi: 10.1038/357244a0. [DOI] [PubMed] [Google Scholar]
- Jonas P., Major G., Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993 Dec;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C., Douglas R., Wehmeier U. Visibility of synaptically induced conductance changes: theory and simulations of anatomically characterized cortical pyramidal cells. J Neurosci. 1990 Jun;10(6):1728–1744. doi: 10.1523/JNEUROSCI.10-06-01728.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman A. U. Dendritic morphology of pyramidal neurones of the visual cortex of the rat: III. Spine distributions. J Comp Neurol. 1991 Apr 8;306(2):332–343. doi: 10.1002/cne.903060209. [DOI] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Major G., Evans J. D., Jack J. J. Solutions for transients in arbitrarily branching cables: I. Voltage recording with a somatic shunt. Biophys J. 1993 Jul;65(1):423–449. doi: 10.1016/S0006-3495(93)81037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major G., Evans J. D., Jack J. J. Solutions for transients in arbitrarily branching cables: II. Voltage clamp theory. Biophys J. 1993 Jul;65(1):450–468. doi: 10.1016/S0006-3495(93)81038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major G. Solutions for transients in arbitrarily branching cables: III. Voltage clamp problems. Biophys J. 1993 Jul;65(1):469–491. doi: 10.1016/S0006-3495(93)81039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll A., Larkman A., Blakemore C. Modulation of EPSP shape and efficacy by intrinsic membrane conductances in rat neocortical pyramidal neurons in vitro. J Physiol. 1993 Aug;468:693–710. doi: 10.1113/jphysiol.1993.sp019795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. H., Mulloney B., Budelli R. W. Quantitative methods for predicting neuronal behavior. Neuroscience. 1981;6(5):823–837. doi: 10.1016/0306-4522(81)90165-2. [DOI] [PubMed] [Google Scholar]
- RALL W. Theory of physiological properties of dendrites. Ann N Y Acad Sci. 1962 Mar 2;96:1071–1092. doi: 10.1111/j.1749-6632.1962.tb54120.x. [DOI] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Holmes W. R., Jack J. J., Redman S. J., Segev I. Matching dendritic neuron models to experimental data. Physiol Rev. 1992 Oct;72(4 Suppl):S159–S186. doi: 10.1152/physrev.1992.72.suppl_4.S159. [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969 Dec;9(12):1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. P. Membrane resistivity estimated for the Purkinje neuron by means of a passive computer model. Neuroscience. 1985 Jan;14(1):111–131. doi: 10.1016/0306-4522(85)90168-x. [DOI] [PubMed] [Google Scholar]
- Stuart G. J., Dodt H. U., Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993 Jun;423(5-6):511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Takashima S. Membrane capacity of squid giant axon during hyper- and depolarizations. J Membr Biol. 1976 Jun 9;27(1-2):21–39. doi: 10.1007/BF01869127. [DOI] [PubMed] [Google Scholar]
- Trimmer J. S. Immunological identification and characterization of a delayed rectifier K+ channel polypeptide in rat brain. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10764–10768. doi: 10.1073/pnas.88.23.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek R. E., Ahlijanian M. K., Catterall W. A. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990 Sep 20;347(6290):281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- Westenbroek R. E., Hell J. W., Warner C., Dubel S. J., Snutch T. P., Catterall W. A. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron. 1992 Dec;9(6):1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- Wilson C. J. Passive cable properties of dendritic spines and spiny neurons. J Neurosci. 1984 Jan;4(1):281–297. doi: 10.1523/JNEUROSCI.04-01-00281.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. K., Stewart M. Different firing patterns generated in dendrites and somata of CA1 pyramidal neurones in guinea-pig hippocampus. J Physiol. 1992 Nov;457:675–687. doi: 10.1113/jphysiol.1992.sp019401. [DOI] [PMC free article] [PubMed] [Google Scholar]