Abstract
The changes in transmembrane potential during a stimulation pulse in the heart are not known. We have used transmembrane potential sensitive dye fluorescence to measure changes in transmembrane potential along fibers in an anisotropic arterially perfused rabbit epicardial layer. Cathodal or anodal extracellular point stimulation produced changes in transmembrane potential within 60 microns of the electrode that were positive or negative, respectively. The changes in transmembrane potential did not simply decrease to zero with increasing distance, as would occur with a theoretical fiber space constant, but instead became reversed beyond approximately 1 mm from the electrode consistent with a virtual electrode effect. Even stimulation from a line of terminals perpendicular to the fibers produced negative changes in transmembrane potential for cathodal stimulation with the largest negative changes during a 50-ms pulse at 3-4 mm from the electrode terminals. Negative changes as large as the amplitude of the action potential rising phase occurred during a 50-ms pulse for 20-volt cathodal stimulation. Switching to anodal stimulation reversed the directions of changes in transmembrane potential at most recording spots, however for stimulation during the refractory period negative changes in transmembrane potential were significantly larger than positive changes in transmembrane potential. Anodal stimulation during diastole with 3-ms pulses produced excitation in the region of depolarization that accelerated when the stimulation strength was increased to > 3 times the anodal threshold strength. Thus, virtual electrode effects of unipolar stimulation occur in myocardial fibers, and for sufficiently strong stimuli the virtual electrode effects may influence electrical behavior of the myocardium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allessie M. A., Schalij M. J., Kirchhof C. J., Boersma L., Huybers M., Hollen J. Experimental electrophysiology and arrhythmogenicity. Anisotropy and ventricular tachycardia. Eur Heart J. 1989 Sep;10 (Suppl E):2–8. doi: 10.1093/eurheartj/10.suppl_e.2. [DOI] [PubMed] [Google Scholar]
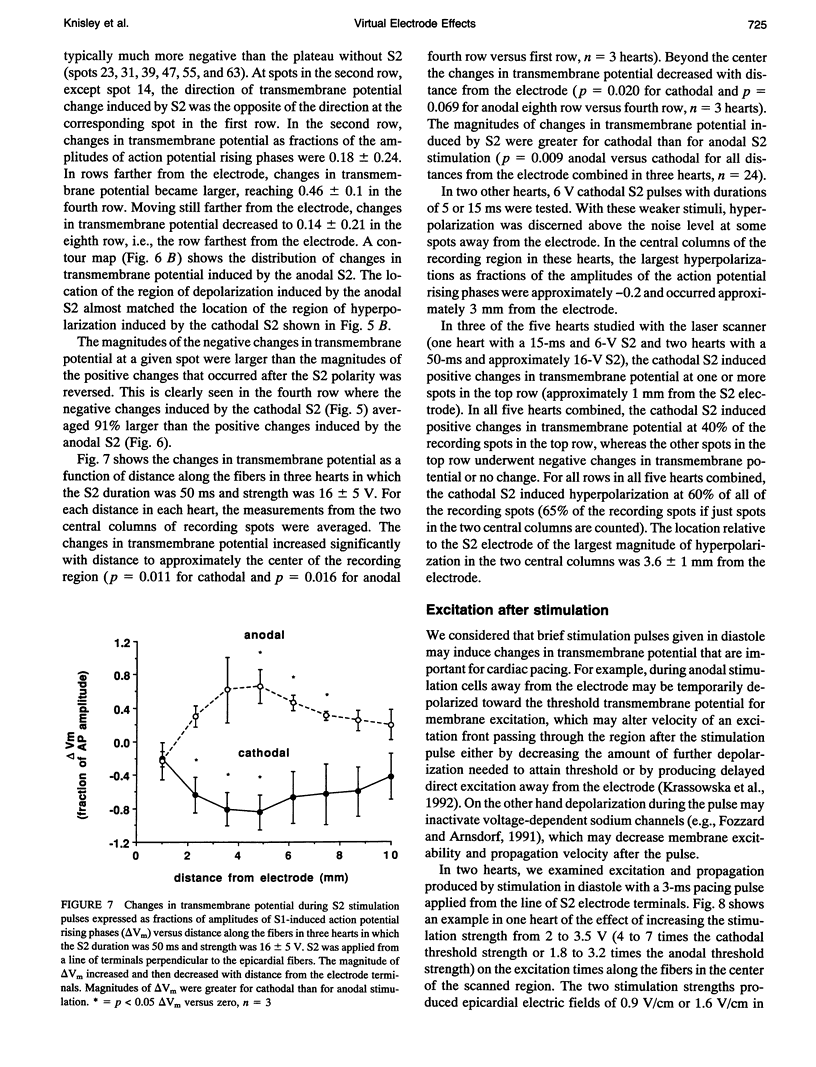
- CRANEFIELD P. F., HOFFMAN B. F. Propagated repolarization in heart muscle. J Gen Physiol. 1958 Mar 20;41(4):633–649. doi: 10.1085/jgp.41.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
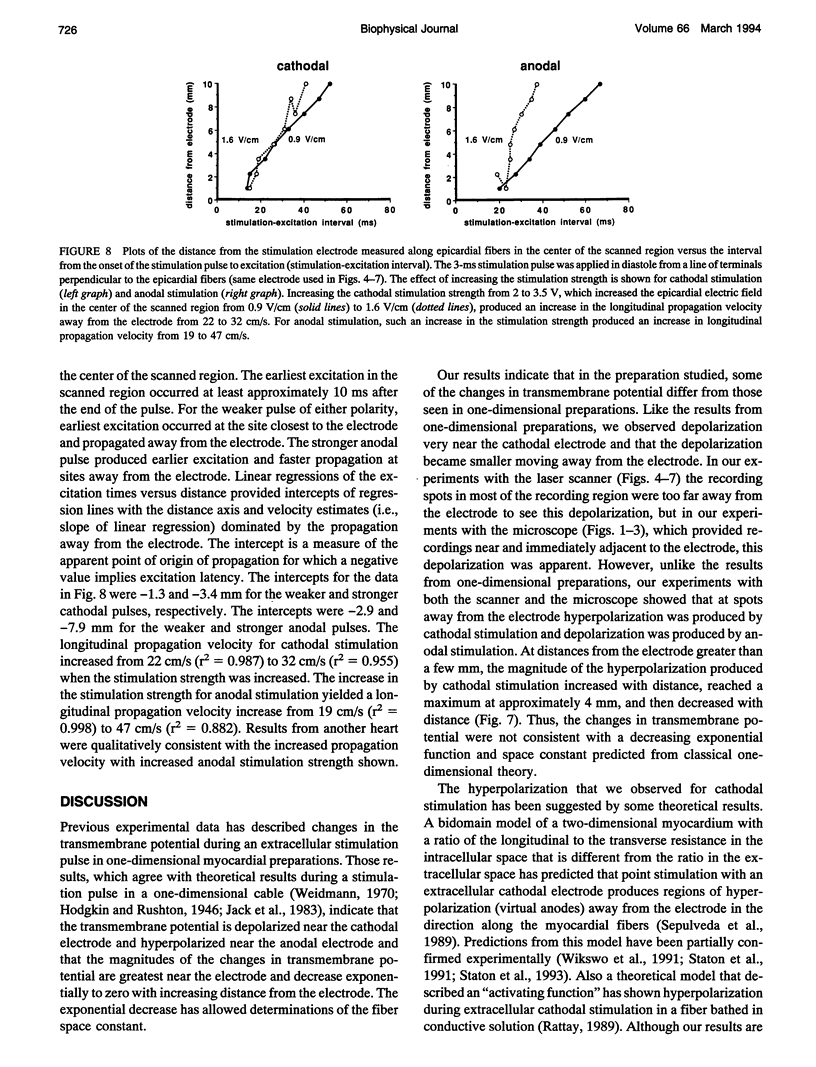
- Ehrenberg B., Farkas D. L., Fluhler E. N., Lojewska Z., Loew L. M. Membrane potential induced by external electric field pulses can be followed with a potentiometric dye. Biophys J. 1987 May;51(5):833–837. doi: 10.1016/S0006-3495(87)83410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhler E., Burnham V. G., Loew L. M. Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry. 1985 Oct 8;24(21):5749–5755. doi: 10.1021/bi00342a010. [DOI] [PubMed] [Google Scholar]
- Frazier D. W., Krassowska W., Chen P. S., Wolf P. D., Dixon E. G., Smith W. M., Ideker R. E. Extracellular field required for excitation in three-dimensional anisotropic canine myocardium. Circ Res. 1988 Jul;63(1):147–164. doi: 10.1161/01.res.63.1.147. [DOI] [PubMed] [Google Scholar]
- Hill B. C., Courtney K. R. Design of a multi-point laser scanned optical monitor of cardiac action potential propagation: application to microreentry in guinea pig atrium. Ann Biomed Eng. 1987;15(6):567–577. doi: 10.1007/BF02364249. [DOI] [PubMed] [Google Scholar]
- Hill B. C., Hunt A. J., Courtney K. R. Reentrant tachycardia in a thin layer of ventricular subepicardium: effects of d-sotalol and lidocaine. J Cardiovasc Pharmacol. 1990 Dec;16(6):871–880. doi: 10.1097/00005344-199012000-00003. [DOI] [PubMed] [Google Scholar]
- Hiramatsu Y., Buchanan J. W., Jr, Knisley S. B., Koch G. G., Kropp S., Gettes L. S. Influence of rate-dependent cellular uncoupling on conduction change during simulated ischemia in guinea pig papillary muscles: effect of verapamil. Circ Res. 1989 Jul;65(1):95–102. doi: 10.1161/01.res.65.1.95. [DOI] [PubMed] [Google Scholar]
- Kishida H., Surawicz B., Fu L. T. Effects of K+ and K+-induced polarization on (dV/dt)max, threshold potential, and membrane input resistance in guinea pig and cat ventricular myocardium. Circ Res. 1979 Jun;44(6):800–814. doi: 10.1161/01.res.44.6.800. [DOI] [PubMed] [Google Scholar]
- Knisley S. B., Blitchington T. F., Hill B. C., Grant A. O., Smith W. M., Pilkington T. C., Ideker R. E. Optical measurements of transmembrane potential changes during electric field stimulation of ventricular cells. Circ Res. 1993 Feb;72(2):255–270. doi: 10.1161/01.res.72.2.255. [DOI] [PubMed] [Google Scholar]
- Knisley S. B., Smith W. M., Ideker R. E. Effect of field stimulation on cellular repolarization in rabbit myocardium. Implications for reentry induction. Circ Res. 1992 Apr;70(4):707–715. doi: 10.1161/01.res.70.4.707. [DOI] [PubMed] [Google Scholar]
- Krassowska W., Cabo C., Knisley S. B., Ideker R. E. Propagation versus delayed activation during field stimulation of cardiac muscle. Pacing Clin Electrophysiol. 1992 Feb;15(2):197–210. doi: 10.1111/j.1540-8159.1992.tb03064.x. [DOI] [PubMed] [Google Scholar]
- Krassowska W., Pilkington T. C., Ideker R. E. Periodic conductivity as a mechanism for cardiac stimulation and defibrillation. IEEE Trans Biomed Eng. 1987 Jul;34(7):555–560. doi: 10.1109/tbme.1987.325986. [DOI] [PubMed] [Google Scholar]
- Li T., Sperelakis N., Teneick R. E., Solaro R. J. Effects of diacetyl monoxime on cardiac excitation-contraction coupling. J Pharmacol Exp Ther. 1985 Mar;232(3):688–695. [PubMed] [Google Scholar]
- Plonsey R., Barr R. C. Current flow patterns in two-dimensional anisotropic bisyncytia with normal and extreme conductivities. Biophys J. 1984 Mar;45(3):557–571. doi: 10.1016/S0006-3495(84)84193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plonsey R., Barr R. C. Effect of microscopic and macroscopic discontinuities on the response of cardiac tissue to defibrillating (stimulating) currents. Med Biol Eng Comput. 1986 Mar;24(2):130–136. doi: 10.1007/BF02443925. [DOI] [PubMed] [Google Scholar]
- Rattay F. Analysis of models for extracellular fiber stimulation. IEEE Trans Biomed Eng. 1989 Jul;36(7):676–682. doi: 10.1109/10.32099. [DOI] [PubMed] [Google Scholar]
- Sepulveda N. G., Roth B. J., Wikswo J. P., Jr Current injection into a two-dimensional anisotropic bidomain. Biophys J. 1989 May;55(5):987–999. doi: 10.1016/S0006-3495(89)82897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach M. S., Kootsey J. M. Relating the sodium current and conductance to the shape of transmembrane and extracellular potentials by simulation: effects of propagation boundaries. IEEE Trans Biomed Eng. 1985 Oct;32(10):743–755. doi: 10.1109/TBME.1985.325489. [DOI] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikswo J. P., Jr, Wisialowski T. A., Altemeier W. A., Balser J. R., Kopelman H. A., Roden D. M. Virtual cathode effects during stimulation of cardiac muscle. Two-dimensional in vivo experiments. Circ Res. 1991 Feb;68(2):513–530. doi: 10.1161/01.res.68.2.513. [DOI] [PubMed] [Google Scholar]


