Abstract
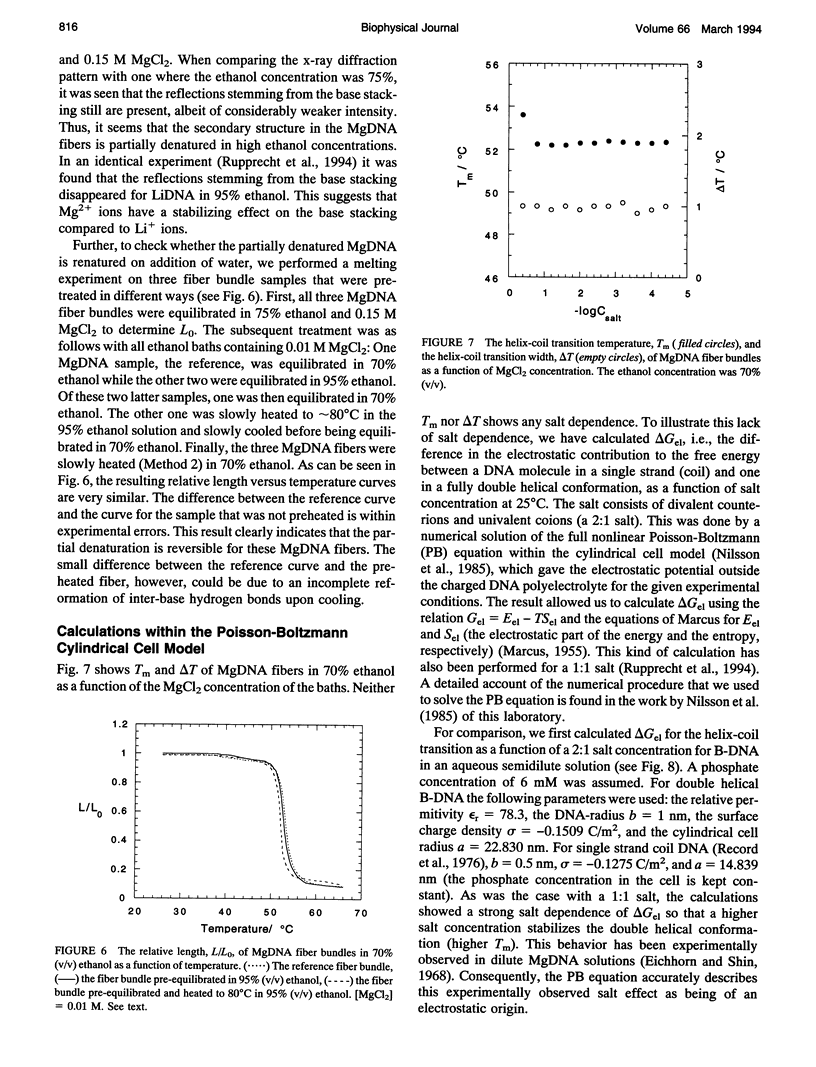
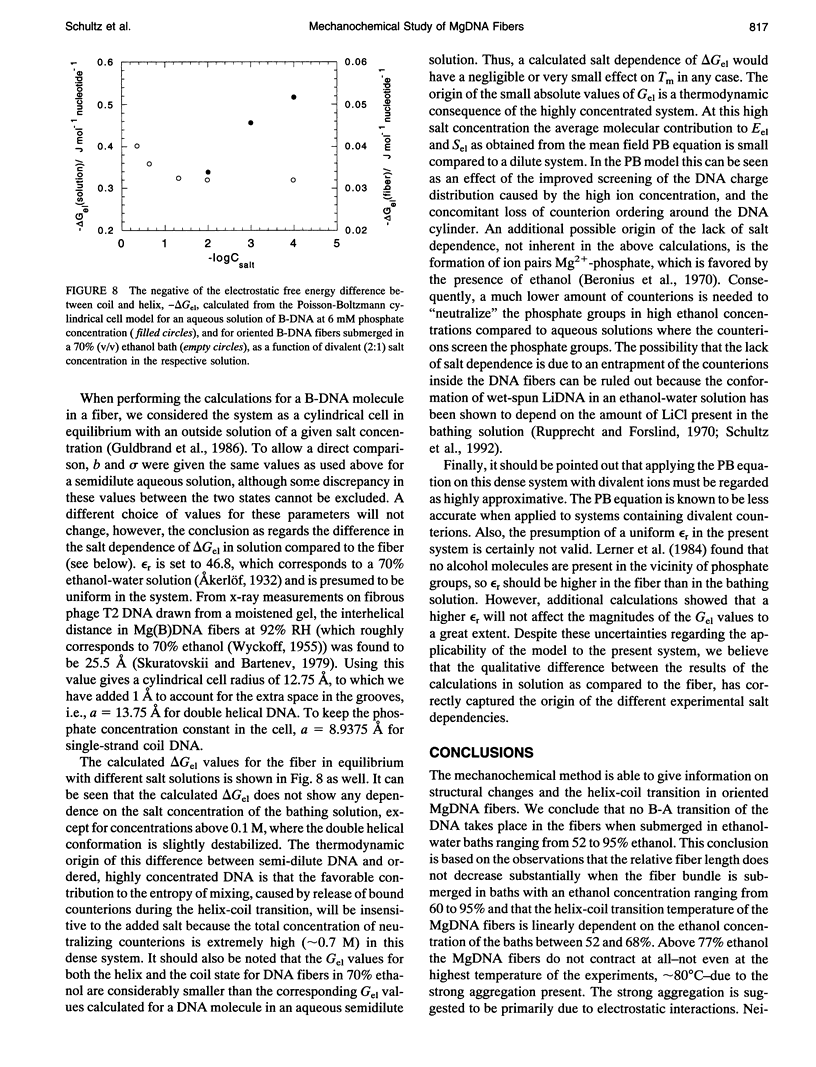
Highly oriented calf-thymus MgDNA fibers, prepared by a wet spinning method, were studied with a simple mechanochemical set-up. The relative fiber length, L/Lo, was measured with the fibers submerged in ethanol-water solutions. In one type of experiment L/Lo was measured as a function of ethanol concentration at room temperature. No substantial decrease in L/Lo with increasing ethanol concentration was observed, indicating that MgDNA fibers stay in the B form even when the water activity is very low. For low ethanol concentrations the fiber structure is stable and does not dissolve even at very high water activities. In a second type of experiment, the heat-induced helix-coil transition was manifested by a marked contraction of the fibers. The transition temperature decreases linearly with increasing ethanol concentration between 52 and 68% ethanol. At higher ethanol concentrations the helix-coil transition temperature increases due to strong aggregation within the DNA fibers, and above 77% ethanol the fibers do not contract at all, not even at the upper temperature limit of the experiments, approximately 80 degrees C. This behavior is discussed with reference to dried DNA and the P form of DNA. The helix-coil transition temperature of the MgDNA fibers in 70% ethanol does not show any dependence on the MgCl2 concentration. It is shown that the Poisson-Boltzmann cylindrical cell model can account qualitatively for this lack of salt dependence.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba Y., Kagemoto A. Influence of magnesium ions on helix-coil transition of DNA determined by modified differential scanning calorimeter. Biopolymers. 1974;13(2):339–344. doi: 10.1002/bip.1974.360130209. [DOI] [PubMed] [Google Scholar]
- Bartenev V. N., Golovamov EuI, Kapitonova K. A., Mokulskii M. A., Volkova L. I., Skuratovskii IYa Structure of the B DNA cationic shell as revealed by an X-ray diffraction study of CsDNA. Sequence-specific cationic stabilization of B form DNA. J Mol Biol. 1983 Sep 5;169(1):217–234. doi: 10.1016/s0022-2836(83)80181-8. [DOI] [PubMed] [Google Scholar]
- Bloomfield V. A. Condensation of DNA by multivalent cations: considerations on mechanism. Biopolymers. 1991 Nov;31(13):1471–1481. doi: 10.1002/bip.360311305. [DOI] [PubMed] [Google Scholar]
- Brandes R., Vold R. R., Vold R. L., Kearns D. R. Effects of hydration on purine motion in solid DNA. Biochemistry. 1986 Nov 18;25(23):7744–7751. doi: 10.1021/bi00371a069. [DOI] [PubMed] [Google Scholar]
- Braunlin W. H., Drakenberg T., Nordenskiöld L. Ca2+ binding environments on natural and synthetic polymeric DNA's. J Biomol Struct Dyn. 1992 Oct;10(2):333–343. doi: 10.1080/07391102.1992.10508651. [DOI] [PubMed] [Google Scholar]
- Braunlin W. H., Nordenskiöld L., Drakenberg T. A reexamination of 25Mg2+ NMR in DNA solution: site heterogeneity and cation competition effects. Biopolymers. 1991 Oct;31(11):1343–1346. doi: 10.1002/bip.360311111. [DOI] [PubMed] [Google Scholar]
- Demarco C., Lindsay S. M., Pokorny M., Powell J., Rupprecht A. Interhelical effects on the low-frequency modes and phase transitions of Li- and Na-DNA. Biopolymers. 1985 Nov;24(11):2035–2040. doi: 10.1002/bip.360241103. [DOI] [PubMed] [Google Scholar]
- Dix D. E., Straus D. B. DNA helix stability. I. Differential stabilization by counter cations. Arch Biochem Biophys. 1972 Sep;152(1):299–310. doi: 10.1016/0003-9861(72)90219-6. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Eichhorn G. L., Shin Y. A. Interaction of metal ions with polynucleotides and related compounds. XII. The relative effect of various metal ions on DNA helicity. J Am Chem Soc. 1968 Dec 18;90(26):7323–7328. doi: 10.1021/ja01028a024. [DOI] [PubMed] [Google Scholar]
- Harmouchi M., Albiser G., Premilat S. Effect of a mechanical tension on the hydration of DNA in fibres. Biochem Biophys Res Commun. 1992 Oct 15;188(1):78–85. doi: 10.1016/0006-291x(92)92352-x. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Krylov DYu, Minyat E. E., Minchenkova L. E. B-A transition in DNA. J Biomol Struct Dyn. 1983 Oct;1(2):453–460. doi: 10.1080/07391102.1983.10507454. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Minyat E. E., Frank-Kamenetskii M. D., Schyolkina A. K. The B to A transition of DNA in solution. J Mol Biol. 1974 Aug 25;87(4):817–833. doi: 10.1016/0022-2836(74)90086-2. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Lavalle N., Lee S. A., Rupprecht A. Counterion effects on the physical properties and the A to B transition of calf-thymus DNA films. Biopolymers. 1990;30(9-10):877–887. doi: 10.1002/bip.360300903. [DOI] [PubMed] [Google Scholar]
- Lavalle N, Lee SA, Flox LS. Lattice-dynamical model of crystalline DNA: Intermolecular bonds and the A to B transition. Phys Rev A. 1991 Mar 15;43(6):3126–3130. doi: 10.1103/physreva.43.3126. [DOI] [PubMed] [Google Scholar]
- Lerner D. B., Becktel W. J., Everett R., Goodman M., Kearns D. R. Solvation effects on the 31P-NMR chemical shifts and infrared spectra of phosphate diesters. Biopolymers. 1984 Nov;23(11 Pt 1):2157–2172. doi: 10.1002/bip.360231105. [DOI] [PubMed] [Google Scholar]
- Lindsay S. M., Lee S. A., Powell J. W., Weidlich T., DeMarco C., Lewen G. D., Tao N. J., Rupprecht A. The origin of the A to B transition in DNA fibers and films. Biopolymers. 1988 Jun;27(6):1015–1043. doi: 10.1002/bip.360270610. [DOI] [PubMed] [Google Scholar]
- MARVIN D. A., SPENCER M., WILKINS M. H., HAMILTON L. D. The molecular configuration of deoxyribonucleic acid. III. X-ray diffraction study of the C form of the lithium salt. J Mol Biol. 1961 Oct;3:547–565. doi: 10.1016/s0022-2836(61)80021-1. [DOI] [PubMed] [Google Scholar]
- Malenkov G., Minchenkova L., Minyat E., Schyolkina A., Ivanov V. The nature of the B-A transition of DNA in solution. FEBS Lett. 1975 Mar 1;51(1):38–42. doi: 10.1016/0014-5793(75)80850-7. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Ott G. S., Ziegler R., Bauer W. R. The DNA melting transition in aqueous magnesium salt solutions. Biochemistry. 1975 Jul 29;14(15):3431–3438. doi: 10.1021/bi00686a022. [DOI] [PubMed] [Google Scholar]
- Rau D. C., Parsegian V. A. Direct measurement of the intermolecular forces between counterion-condensed DNA double helices. Evidence for long range attractive hydration forces. Biophys J. 1992 Jan;61(1):246–259. doi: 10.1016/S0006-3495(92)81831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M. T., Jr, Mazur S. J., Melançon P., Roe J. H., Shaner S. L., Unger L. Double helical DNA: conformations, physical properties, and interactions with ligands. Annu Rev Biochem. 1981;50:997–1024. doi: 10.1146/annurev.bi.50.070181.005025. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Woodbury C. P., Lohman T. M. Na+ effects on transition of DNA and polynucleotides of variable linear charge density. Biopolymers. 1976 May;15(5):893–915. doi: 10.1002/bip.1976.360150507. [DOI] [PubMed] [Google Scholar]
- Rose D. M., Bleam M. L., Record M. T., Bryant R. G. Mg NMR in DNA solutions: Dominance of site binding effects. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6289–6292. doi: 10.1073/pnas.77.11.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht A. A wet spinning apparatus and auxiliary equipment suitable for preparing samples of oriented DNA. Biotechnol Bioeng. 1970 Jan;12(1):93–121. doi: 10.1002/bit.260120109. [DOI] [PubMed] [Google Scholar]
- Rupprecht A., Forslind B. Variation of electrolyte content in wet-spun lithium- and sodium-DNA. Biochim Biophys Acta. 1970 Apr 15;204(2):304–316. doi: 10.1016/0005-2787(70)90148-6. [DOI] [PubMed] [Google Scholar]
- Rupprecht A. Mechanochemical study of wet-spun lithium-DNA fibers. Biopolymers. 1970;9(7):825–842. doi: 10.1002/bip.1970.360090708. [DOI] [PubMed] [Google Scholar]
- Rupprecht A., Piskur J. A simple mechanochemical method for studying structure and dynamics of biopolymer fibers in various media. Acta Chem Scand B. 1983;37(9):863–864. doi: 10.3891/acta.chem.scand.37b-0863. [DOI] [PubMed] [Google Scholar]
- Rupprecht A. Preparation of oriented DNA by wet spinning. Acta Chem Scand. 1966;20(2):494–504. doi: 10.3891/acta.chem.scand.20-0494. [DOI] [PubMed] [Google Scholar]
- Schultz J., Nordenskiöld L., Rupprecht A. A study of the quadrupolar NMR splittings of 7Li+, 23Na+, and 133Cs+ counterions in macroscopically oriented DNA fibers. Biopolymers. 1992 Dec;32(12):1631–1642. doi: 10.1002/bip.360321206. [DOI] [PubMed] [Google Scholar]
- Smith S. B., Finzi L., Bustamante C. Direct mechanical measurements of the elasticity of single DNA molecules by using magnetic beads. Science. 1992 Nov 13;258(5085):1122–1126. doi: 10.1126/science.1439819. [DOI] [PubMed] [Google Scholar]
- Srivastava V. K., Srivastava G. P., Nandi U. S. Studies of DNA in alcohol/water mixtures. Indian J Biochem Biophys. 1979 Dec;16(6):427–431. [PubMed] [Google Scholar]
- Trohalaki S., Frisch H. L., Lerman L. S. The effects of lithium, rubidium, cesium and magnesium ions on the close packing of persistence-length DNA fragments. Biophys Chem. 1991 May;40(2):197–205. doi: 10.1016/0301-4622(91)87009-t. [DOI] [PubMed] [Google Scholar]
- Wada A., Yabuki S., Husimi Y. Fine structure in the thermal denaturation of DNA: high temperature-resolution spectrophotometric studies. CRC Crit Rev Biochem. 1980;9(2):87–144. doi: 10.3109/10409238009105432. [DOI] [PubMed] [Google Scholar]
- Weidlich T, Lindsay SM, Rupprecht A. Counterion effects on the structure and dynamics of solid DNA. Phys Rev Lett. 1988 Oct 3;61(14):1674–1677. doi: 10.1103/PhysRevLett.61.1674. [DOI] [PubMed] [Google Scholar]
- Zehfus M. H., Johnson W. C., Jr Conformation of P-form DNA. Biopolymers. 1984 Jul;23(7):1269–1281. doi: 10.1002/bip.360230711. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. A direct demonstration that the ethanol-induced transition of DNA is between the A and B forms: an X-ray diffraction study. J Mol Biol. 1979 Dec 25;135(4):1023–1027. doi: 10.1016/0022-2836(79)90526-6. [DOI] [PubMed] [Google Scholar]


