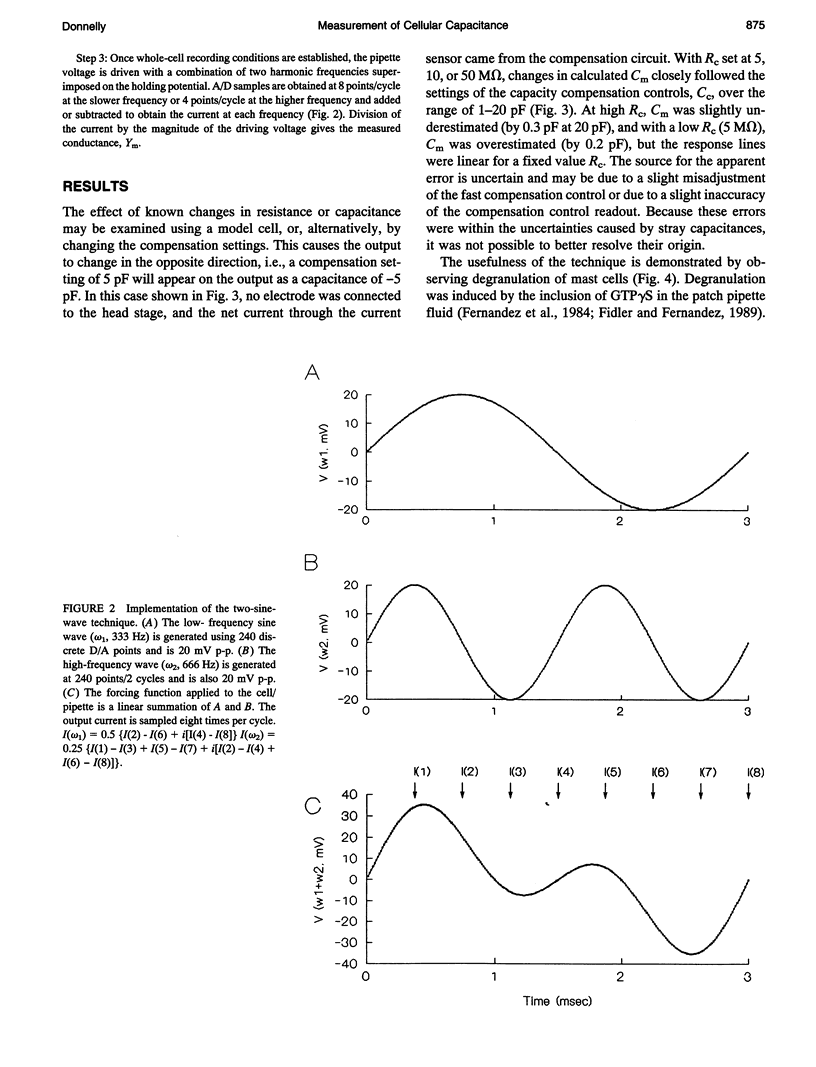
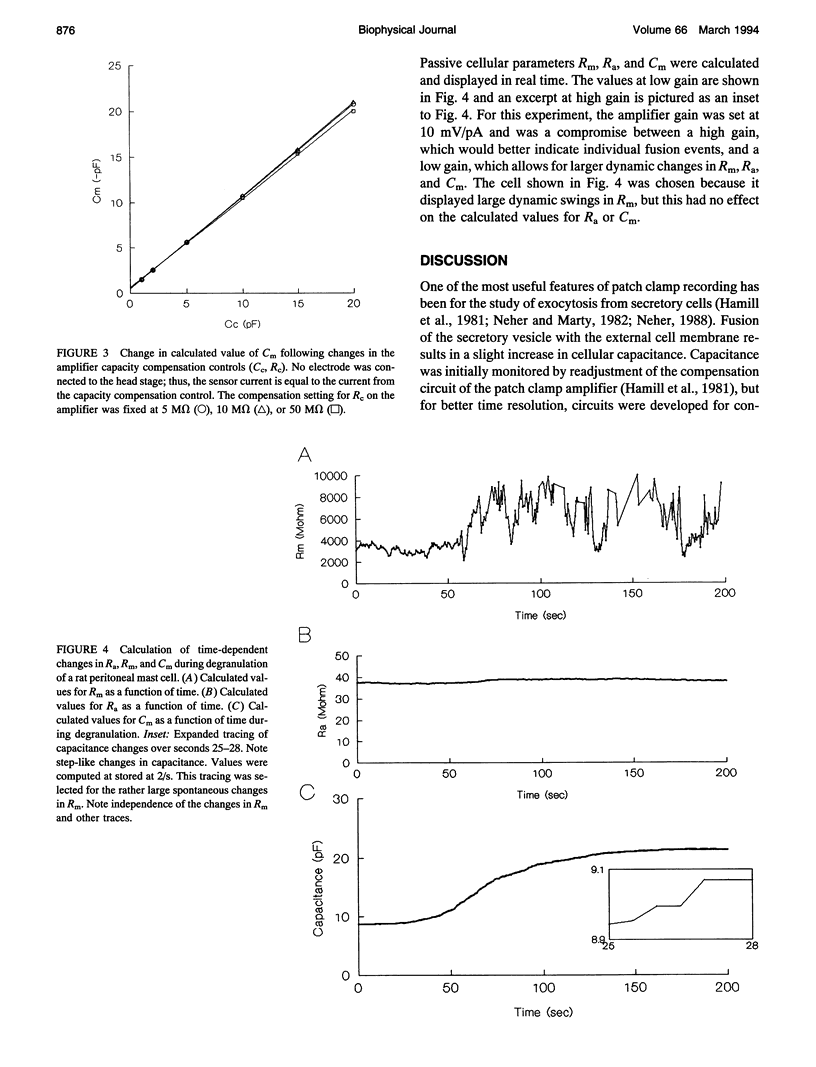
Abstract
During patch clamp recordings, measurement of passive parameters such as access resistance (Ra), membrane resistance (Rm), and membrane capacitance (Cm) often provides useful information regarding physiological changes in the cell. In particular, an increase in capacitance may indicate vesicle fusion events as occurs during exocytosis. Rapid capacitance changes are usually measured with a phase-sensitive detector set to a phase angle that is independent of resistance changes. However, this angle changes over time and may be difficult to determine in cells with a low membrane resistance. The present paper describes a technique for rapidly measuring Ra, Rm, and Cm by simultaneously applying two harmonic frequencies and calculating the passive parameters from the resultant electrode current. Calibration and operation are independent of the compensation circuit settings that may be set to null most of the electrode current. The technique may be implemented on a standard patch clamp setup without other specialized equipment and should be particularly useful for the study of cells that have low Rm or undergo rapid changes in Ra or Rm.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookman R. J., Lim N. F., Schweizer F. E., Nowycky M. Single cell assays of excitation-secretion coupling. Ann N Y Acad Sci. 1991;635:352–364. doi: 10.1111/j.1749-6632.1991.tb36504.x. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Final steps in exocytosis observed in a cell with giant secretory granules. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1945–1949. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly D. F. Response to cyanide of two types of glomoid cells in mature rat carotid body. Brain Res. 1993 Dec 10;630(1-2):157–168. doi: 10.1016/0006-8993(93)90653-5. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Joshi C., Fernandez J. M. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988 Jun;53(6):885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Neher E. The patch-clamp technique in the study of secretion. Trends Neurosci. 1989 Apr;12(4):159–163. doi: 10.1016/0166-2236(89)90059-3. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Curran M., Cohen F. S., Brodwick M. Simultaneous electrical and optical measurements show that membrane fusion precedes secretory granule swelling during exocytosis of beige mouse mast cells. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1585–1589. doi: 10.1073/pnas.84.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]


