Abstract
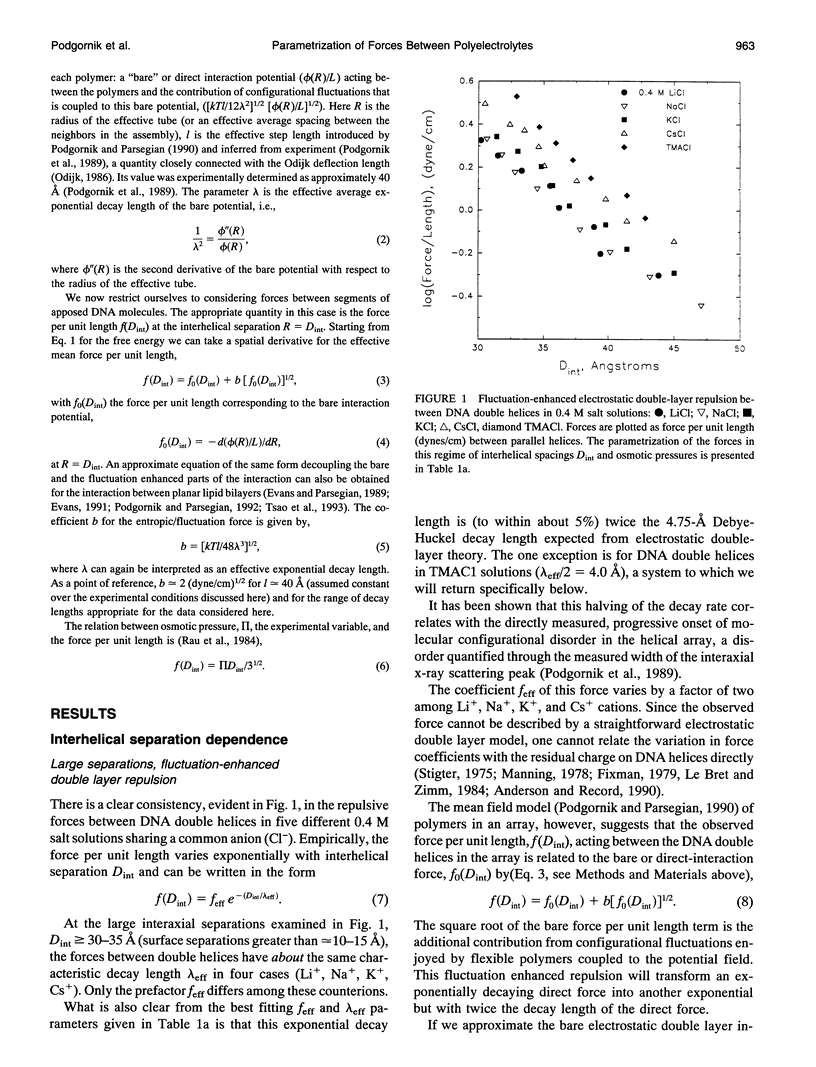
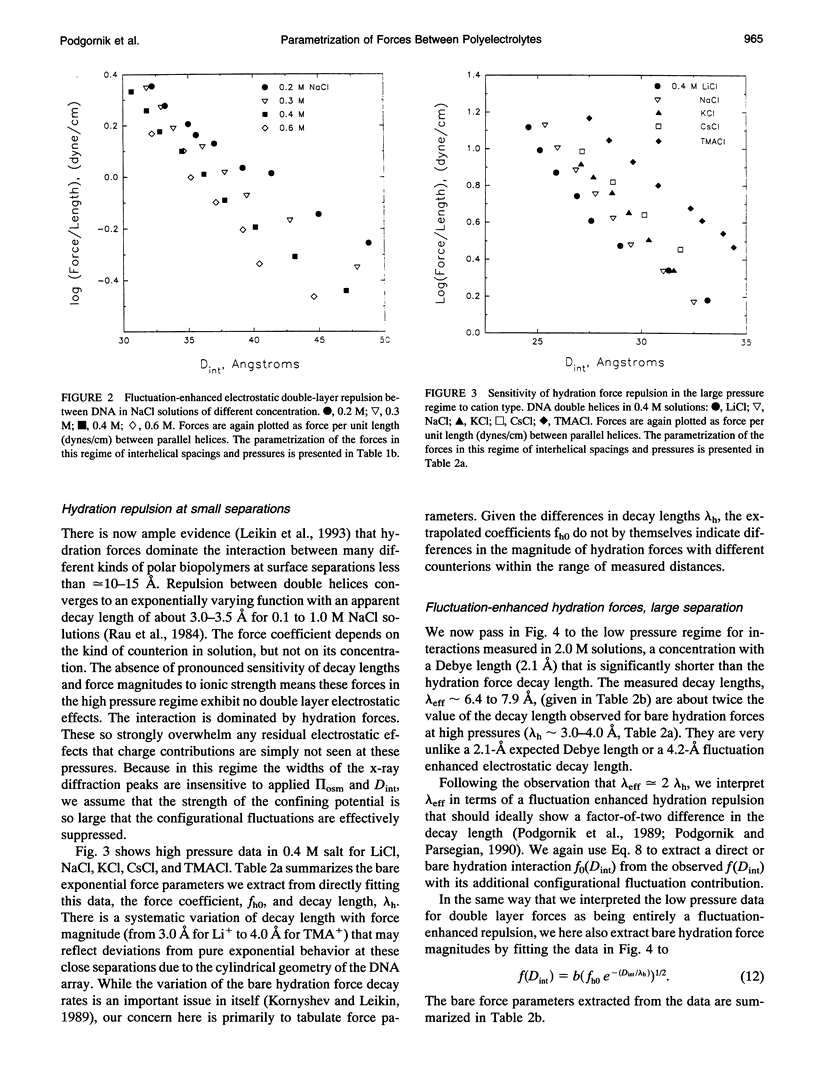
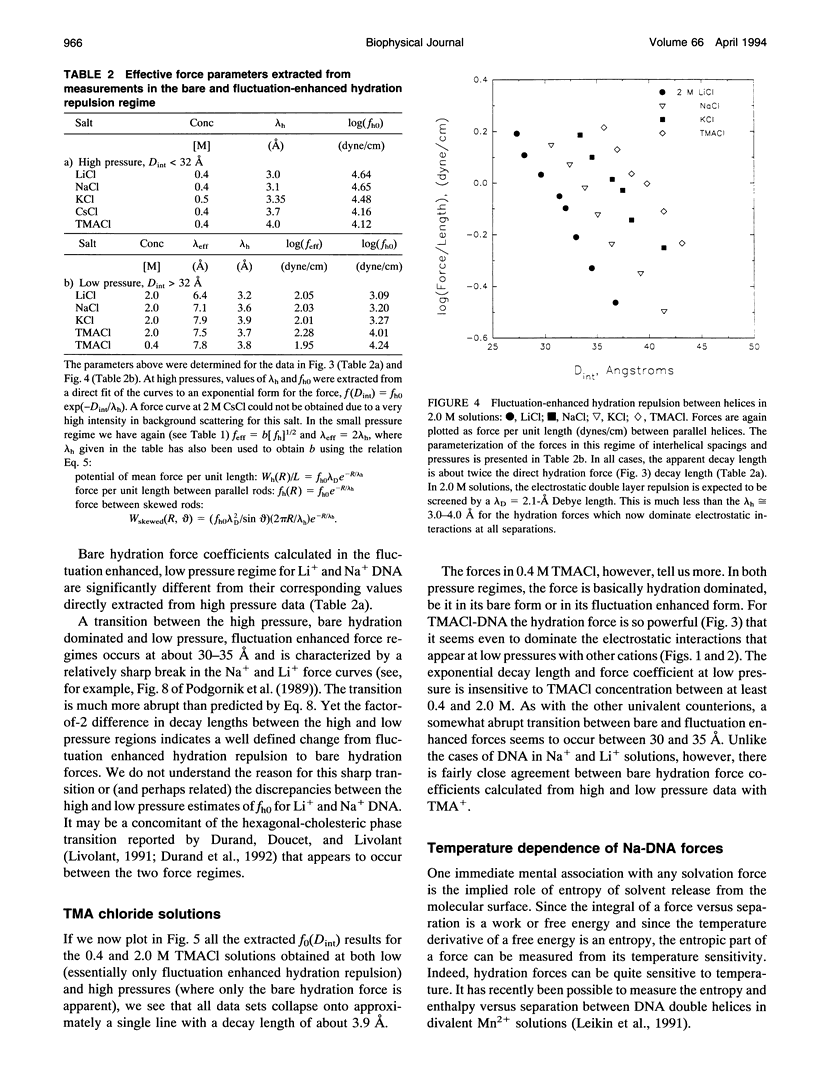
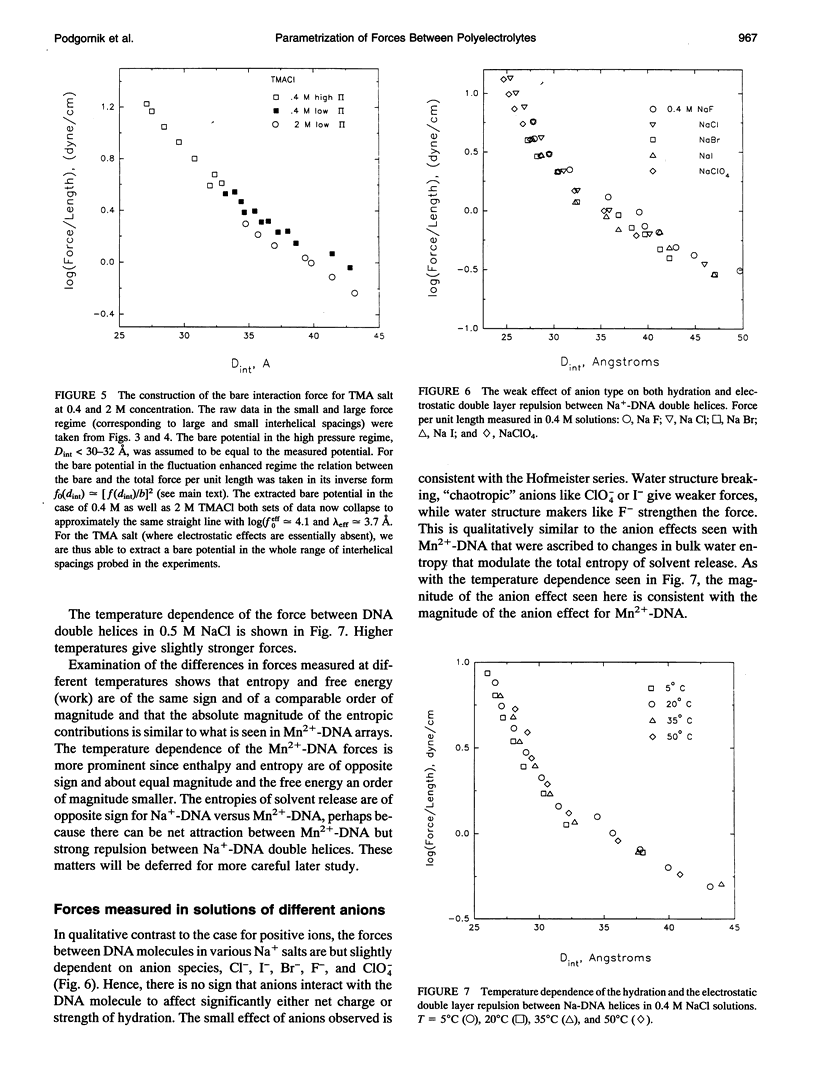
Directly measured forces between DNA helices in ordered arrays have been reduced to simple force coefficients and mathematical expressions for the interactions between pairs of molecules. The tabulated force parameters and mathematical expressions can be applied to parallel molecules or, by transformation, to skewed molecules of variable separation and mutual angle. This "toolbox" of intermolecular forces is intended for use in modelling molecular interactions, assembly, and conformation. The coefficients characterizing both the exponential hydration and the electrostatic interactions depend strongly on the univalent counterion species in solution, but are only weakly sensitive to anion type and temperature (from 5 to 50 degrees C). Interaction coefficients for the exponentially varying hydration force seen at spacings less than 10 to 15 A between surfaces are extracted directly from pressure versus interaxial distance curves. Electrostatic interactions are only observed at larger spacings and are always coupled with configurational fluctuation forces that result in observed exponential decay lengths that are twice the expected Debye-Huckel length. The extraction of electrostatic force parameters relies on a theoretical expression describing steric forces of molecules "colliding" through soft exponentially varying direct interactions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. F., Record M. T., Jr Ion distributions around DNA and other cylindrical polyions: theoretical descriptions and physical implications. Annu Rev Biophys Biophys Chem. 1990;19:423–465. doi: 10.1146/annurev.bb.19.060190.002231. [DOI] [PubMed] [Google Scholar]
- Brenner S. L., Parsegian V. A. A physical method for deriving the electrostatic interaction between rod-like polyions at all mutual angles. Biophys J. 1974 Apr;14(4):327–334. doi: 10.1016/S0006-3495(74)85919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Parsegian V. A. Thermal-mechanical fluctuations enhance repulsion between bimolecular layers. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7132–7136. doi: 10.1073/pnas.83.19.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamien RD, Le Doussal P, Nelson DR. Theory of directed polymers. Phys Rev A. 1992 Jun 15;45(12):8727–8750. doi: 10.1103/physreva.45.8727. [DOI] [PubMed] [Google Scholar]
- Kornyshev AA, Leikin S. Fluctuation theory of hydration forces: The dramatic effects of inhomogeneous boundary conditions. Phys Rev A Gen Phys. 1989 Dec 1;40(11):6431–6437. doi: 10.1103/physreva.40.6431. [DOI] [PubMed] [Google Scholar]
- Le Bret M., Zimm B. H. Monte Carlo determination of the distribution of ions about a cylindrical polyelectrolyte. Biopolymers. 1984 Feb;23(2):271–285. doi: 10.1002/bip.360230208. [DOI] [PubMed] [Google Scholar]
- Leikin S., Parsegian V. A., Rau D. C., Rand R. P. Hydration forces. Annu Rev Phys Chem. 1993;44:369–395. doi: 10.1146/annurev.pc.44.100193.002101. [DOI] [PubMed] [Google Scholar]
- Leikin S, Rau DC, Parsegian VA. Measured entropy and enthalpy of hydration as a function of distance between DNA double helices. Phys Rev A. 1991 Oct 15;44(8):5272–5278. doi: 10.1103/physreva.44.5272. [DOI] [PubMed] [Google Scholar]
- Livolant F. Supramolecular organization of double-stranded DNA molecules in the columnar hexagonal liquid crystalline phase. An electron microscopic analysis using freeze-fracture methods. J Mol Biol. 1991 Mar 5;218(1):165–181. doi: 10.1016/0022-2836(91)90882-7. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Fuller N. L., Rau D. C. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 1986;127:400–416. doi: 10.1016/0076-6879(86)27032-9. [DOI] [PubMed] [Google Scholar]
- Rau D. C., Lee B., Parsegian V. A. Measurement of the repulsive force between polyelectrolyte molecules in ionic solution: hydration forces between parallel DNA double helices. Proc Natl Acad Sci U S A. 1984 May;81(9):2621–2625. doi: 10.1073/pnas.81.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Parsegian V. A. Direct measurement of temperature-dependent solvation forces between DNA double helices. Biophys J. 1992 Jan;61(1):260–271. doi: 10.1016/S0006-3495(92)81832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Parsegian V. A. Direct measurement of the intermolecular forces between counterion-condensed DNA double helices. Evidence for long range attractive hydration forces. Biophys J. 1992 Jan;61(1):246–259. doi: 10.1016/S0006-3495(92)81831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger JV, Bruinsma RF. Hexagonal and nematic phases of chains. II. Phase transitions. Phys Rev A. 1991 Mar 15;43(6):2922–2931. doi: 10.1103/physreva.43.2922. [DOI] [PubMed] [Google Scholar]


