Abstract
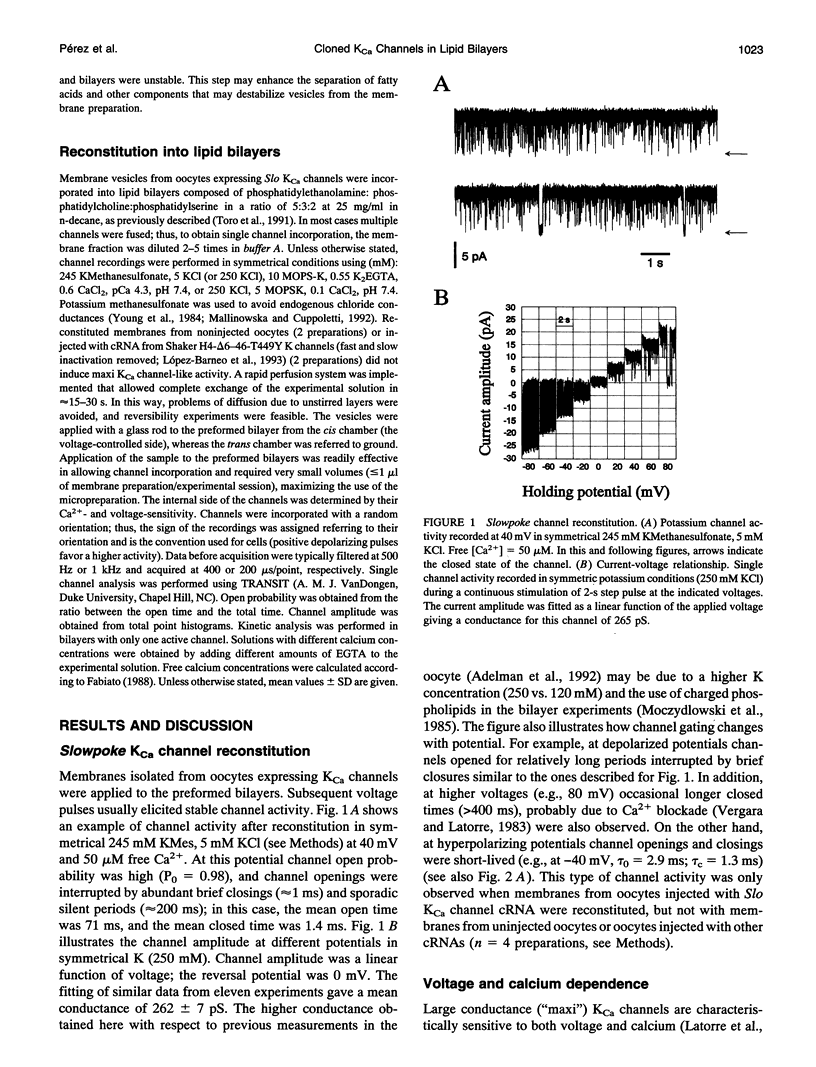
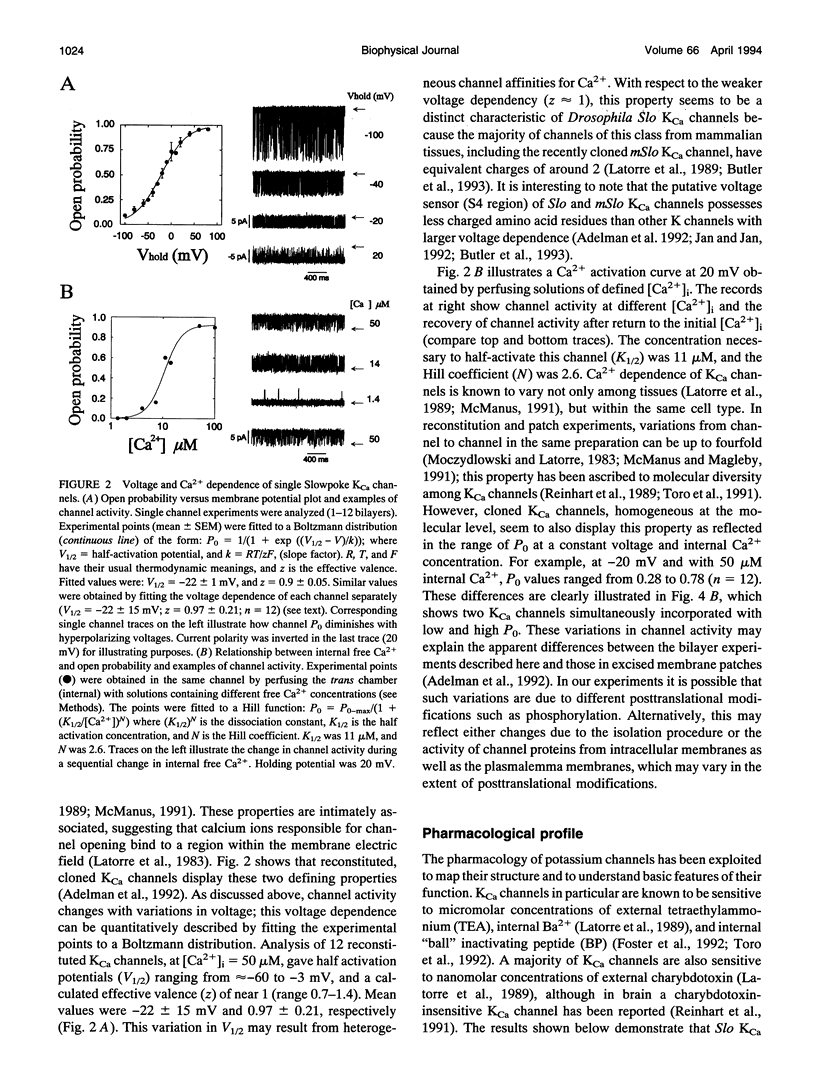
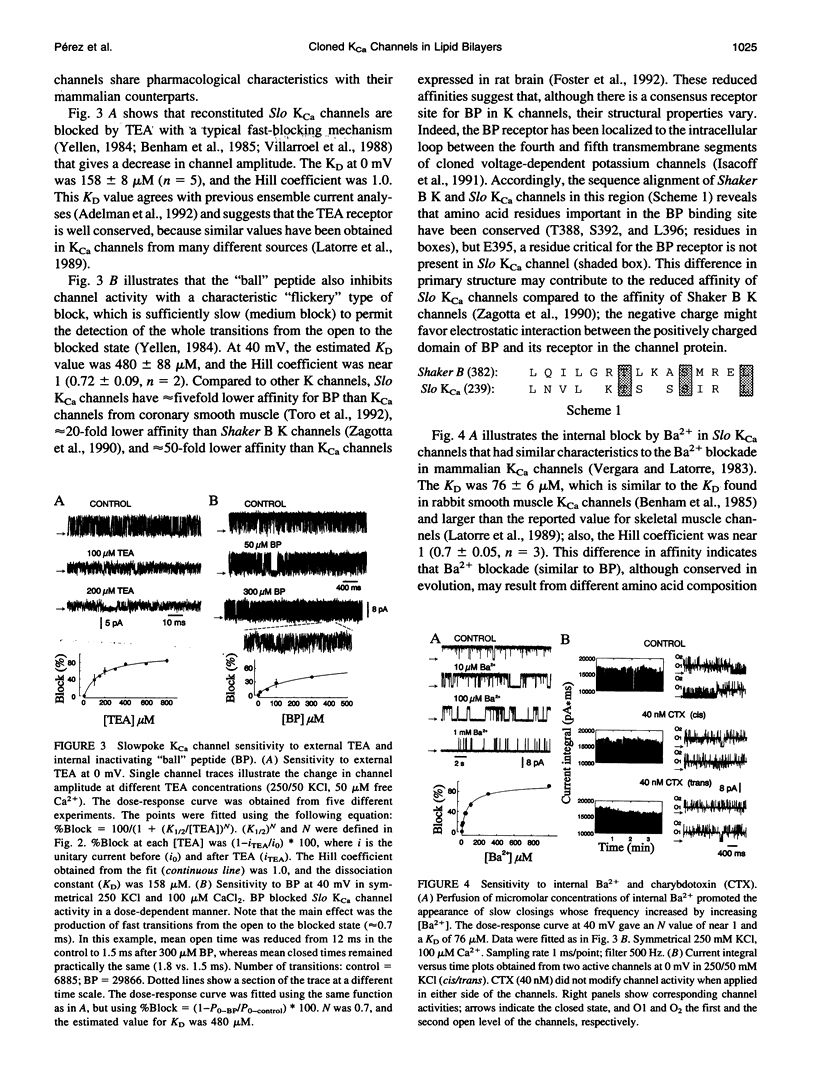
Reconstitution of large conductance calcium-activated potassium (KCa) channels from native cell membranes into planar lipid bilayers provides a powerful method to study single channel properties, including ion conduction, pharmacology, and gating. Recently, KCa channels derived from the Drosophila Slowpoke (Slo) gene have been cloned and heterologously expressed in Xenopus oocytes. In this report, we describe the reconstitution of cloned and expressed Slo KCa channels from Xenopus oocyte membranes into lipid bilayers. The reconstituted channels demonstrate functional properties characteristic of native KCa channels. They possess a mean unitary conductance of approximately 260 pS in symmetrical potassium (250 mM), and they are voltage- and calcium-sensitive. At 50 microM Ca2+, their half-activation potential was near -20 mV; and their affinity for calcium is in the micromolar range. Reconstituted Slo KCa channels were insensitive to external charybdotoxin (40-500 nM) and sensitive to micromolar concentrations of external tetraethylammonium (KD = 158 microM, at 0 mV) and internal Ba2+ (KD = 76 microM, at 40 mV). In addition, they were blocked by internally applied "ball" inactivating peptide (KD = 480 microM, at 40 mV). These results demonstrate that cloned KCa channels expressed in Xenopus oocytes can be readily incorporated into lipid bilayers where detailed mechanistic studies can be performed under controlled internal and external experimental conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman J. P., Shen K. Z., Kavanaugh M. P., Warren R. A., Wu Y. N., Lagrutta A., Bond C. T., North R. A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992 Aug;9(2):209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- Anwer K., Oberti C., Perez G. J., Perez-Reyes N., McDougall J. K., Monga M., Sanborn B. M., Stefani E., Toro L. Calcium-activated K+ channels as modulators of human myometrial contractile activity. Am J Physiol. 1993 Oct;265(4 Pt 1):C976–C985. doi: 10.1152/ajpcell.1993.265.4.C976. [DOI] [PubMed] [Google Scholar]
- Atkinson N. S., Robertson G. A., Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991 Aug 2;253(5019):551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch. 1985 Feb;403(2):120–127. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Butler A., Tsunoda S., McCobb D. P., Wei A., Salkoff L. mSlo, a complex mouse gene encoding "maxi" calcium-activated potassium channels. Science. 1993 Jul 9;261(5118):221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- Chung S. K., Reinhart P. H., Martin B. L., Brautigan D., Levitan I. B. Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science. 1991 Aug 2;253(5019):560–562. doi: 10.1126/science.1857986. [DOI] [PubMed] [Google Scholar]
- Ertel E. A. Excised patches of plasma membrane from vertebrate rod outer segments retain a functional phototransduction enzymatic cascade. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4226–4230. doi: 10.1073/pnas.87.11.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Foster C. D., Chung S., Zagotta W. N., Aldrich R. W., Levitan I. B. A peptide derived from the Shaker B K+ channel produces short and long blocks of reconstituted Ca(2+)-dependent K+ channels. Neuron. 1992 Aug;9(2):229–236. doi: 10.1016/0896-6273(92)90162-7. [DOI] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Putative receptor for the cytoplasmic inactivation gate in the Shaker K+ channel. Nature. 1991 Sep 5;353(6339):86–90. doi: 10.1038/353086a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Latorre R., Vergara C., Moczydlowski E. Properties of a Ca2+-activated K+ channel in a reconstituted system. Cell Calcium. 1983 Dec;4(5-6):343–357. doi: 10.1016/0143-4160(83)90013-1. [DOI] [PubMed] [Google Scholar]
- López-Barneo J., Hoshi T., Heinemann S. H., Aldrich R. W. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1(1):61–71. [PubMed] [Google Scholar]
- MacKinnon R., Miller C. Mechanism of charybdotoxin block of the high-conductance, Ca2+-activated K+ channel. J Gen Physiol. 1988 Mar;91(3):335–349. doi: 10.1085/jgp.91.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B. Calcium-activated potassium channels: regulation by calcium. J Bioenerg Biomembr. 1991 Aug;23(4):537–560. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Accounting for the Ca(2+)-dependent kinetics of single large-conductance Ca(2+)-activated K+ channels in rat skeletal muscle. J Physiol. 1991 Nov;443:739–777. doi: 10.1113/jphysiol.1991.sp018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Competition for block of a Ca2(+)-activated K+ channel by charybdotoxin and tetraethylammonium. Neuron. 1988 Dec;1(10):1003–1006. doi: 10.1016/0896-6273(88)90157-2. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Alvarez O., Vergara C., Latorre R. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers. J Membr Biol. 1985;83(3):273–282. doi: 10.1007/BF01868701. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Latorre R. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. J Gen Physiol. 1983 Oct;82(4):511–542. doi: 10.1085/jgp.82.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Chung S., Levitan I. B. A family of calcium-dependent potassium channels from rat brain. Neuron. 1989 Jan;2(1):1031–1041. doi: 10.1016/0896-6273(89)90227-4. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Chung S., Martin B. L., Brautigan D. L., Levitan I. B. Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci. 1991 Jun;11(6):1627–1635. doi: 10.1523/JNEUROSCI.11-06-01627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornik F. S., Codina J., Birnbaumer L., Toro L. Modulation of coronary smooth muscle KCa channels by Gs alpha independent of phosphorylation by protein kinase A. Am J Physiol. 1993 Oct;265(4 Pt 2):H1460–H1465. doi: 10.1152/ajpheart.1993.265.4.H1460. [DOI] [PubMed] [Google Scholar]
- Suarez-Kurtz G., Garcia M. L., Kaczorowski G. J. Effects of charybdotoxin and iberiotoxin on the spontaneous motility and tonus of different guinea pig smooth muscle tissues. J Pharmacol Exp Ther. 1991 Oct;259(1):439–443. [PubMed] [Google Scholar]
- Taglialatela M., Drewe J. A., Brown A. M. Barium blockade of a clonal potassium channel and its regulation by a critical pore residue. Mol Pharmacol. 1993 Jul;44(1):180–190. [PubMed] [Google Scholar]
- Toro L., Ramos-Franco J., Stefani E. GTP-dependent regulation of myometrial KCa channels incorporated into lipid bilayers. J Gen Physiol. 1990 Aug;96(2):373–394. doi: 10.1085/jgp.96.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro L., Stefani E., Latorre R. Internal blockade of a Ca(2+)-activated K+ channel by Shaker B inactivating "ball" peptide. Neuron. 1992 Aug;9(2):237–245. doi: 10.1016/0896-6273(92)90163-8. [DOI] [PubMed] [Google Scholar]
- Toro L., Vaca L., Stefani E. Calcium-activated potassium channels from coronary smooth muscle reconstituted in lipid bilayers. Am J Physiol. 1991 Jun;260(6 Pt 2):H1779–H1789. doi: 10.1152/ajpheart.1991.260.6.H1779. [DOI] [PubMed] [Google Scholar]
- Vergara C., Latorre R. Kinetics of Ca2+-activated K+ channels from rabbit muscle incorporated into planar bilayers. Evidence for a Ca2+ and Ba2+ blockade. J Gen Physiol. 1983 Oct;82(4):543–568. doi: 10.1085/jgp.82.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C., Moczydlowski E., Latorre R. Conduction, Blockade and Gating in a Ca -activated K Channel Incorporated into Planar Lipid Bilayers. Biophys J. 1984 Jan;45(1):73–76. doi: 10.1016/S0006-3495(84)84114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A., Alvarez O., Oberhauser A., Latorre R. Probing a Ca2+-activated K+ channel with quaternary ammonium ions. Pflugers Arch. 1988 Dec;413(2):118–126. doi: 10.1007/BF00582521. [DOI] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young G. P., Young J. D., Deshpande A. K., Goldstein M., Koide S. S., Cohn Z. A. A Ca2+-activated channel from Xenopus laevis oocyte membranes reconstituted into planar bilayers. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5155–5159. doi: 10.1073/pnas.81.16.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]


