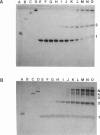
Abstract
The trp repressor of Escherichia coli (TR), although generally considered to be dimeric, has been shown by fluorescence anisotropy of extrinsically labeled protein to undergo oligomerization in solution at protein concentrations in the micromolar range (Fernando, T., and C. A. Royer 1992. Biochemistry. 31:3429-3441). Providing evidence that oligomerization is an intrinsic property of TR, the present studies using chemical cross-linking, analytical ultracentrifugation, and molecular sieve chromatography demonstrate that unmodified TR dimers form higher order aggregates. Tetramers and higher order species were observed in chemical cross-linking experiments at concentrations between 1 and 40 microM. Results from analytical ultracentrifugation and gel filtration chromatography were consistent with average molecular weight values between tetramer and dimer, although no plateaus in the association were evident over the concentration ranges studied, indicating that higher order species are populated. Analytical ultracentrifugation data in presence of corepressor imply that corepressor binding destabilizes the higher order aggregates, an observation that is consistent with the earlier fluorescence work. Through the investigation of the salt and pH dependence of oligomerization, the present studies have revealed an electrostatic component to the interactions between TR dimers.
Full text
PDF

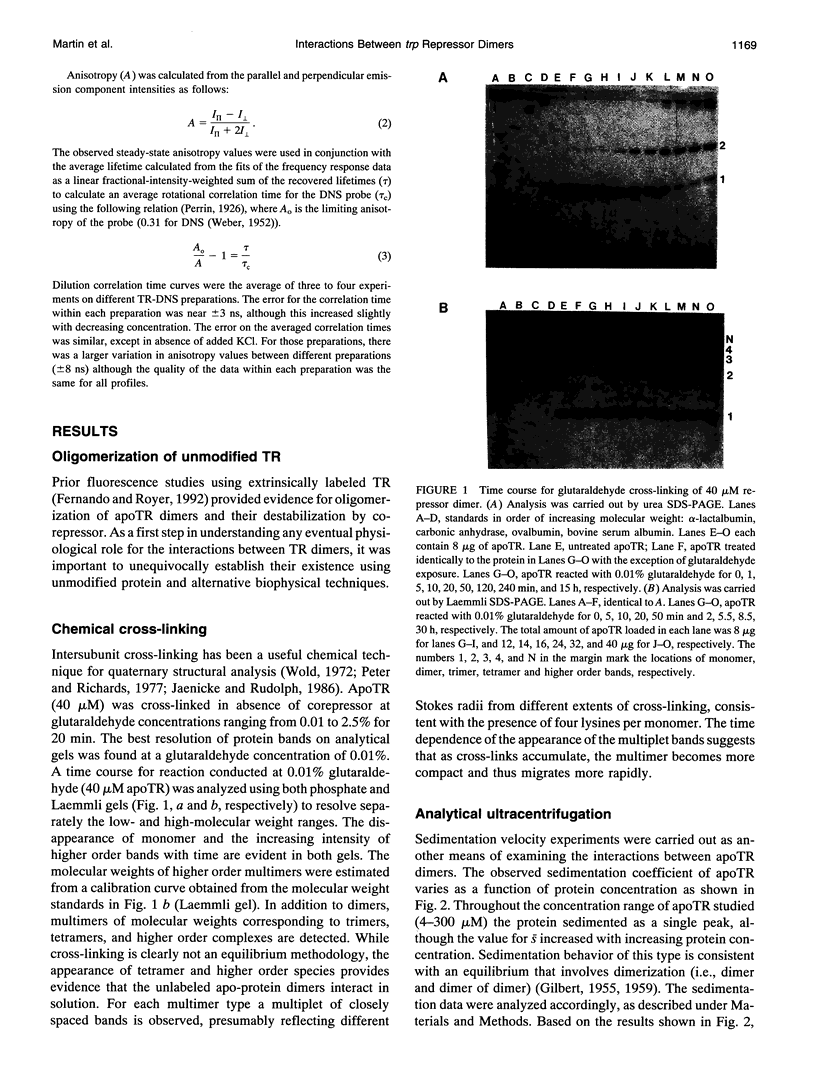
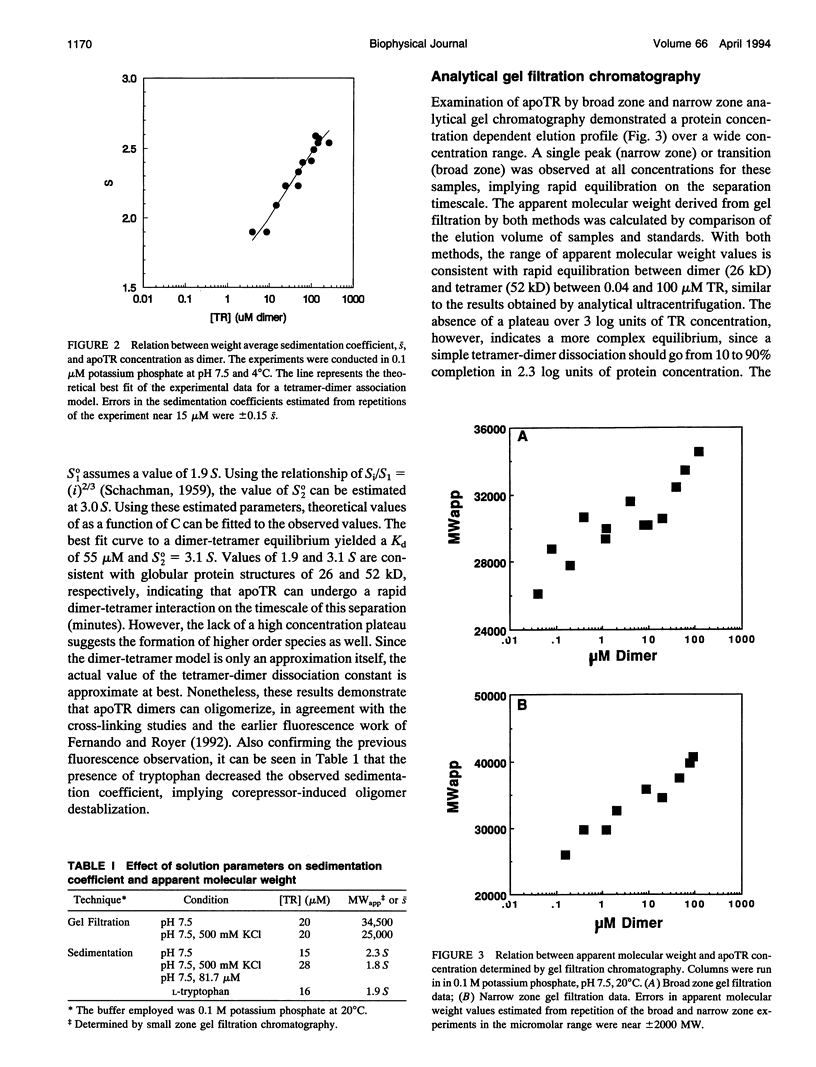
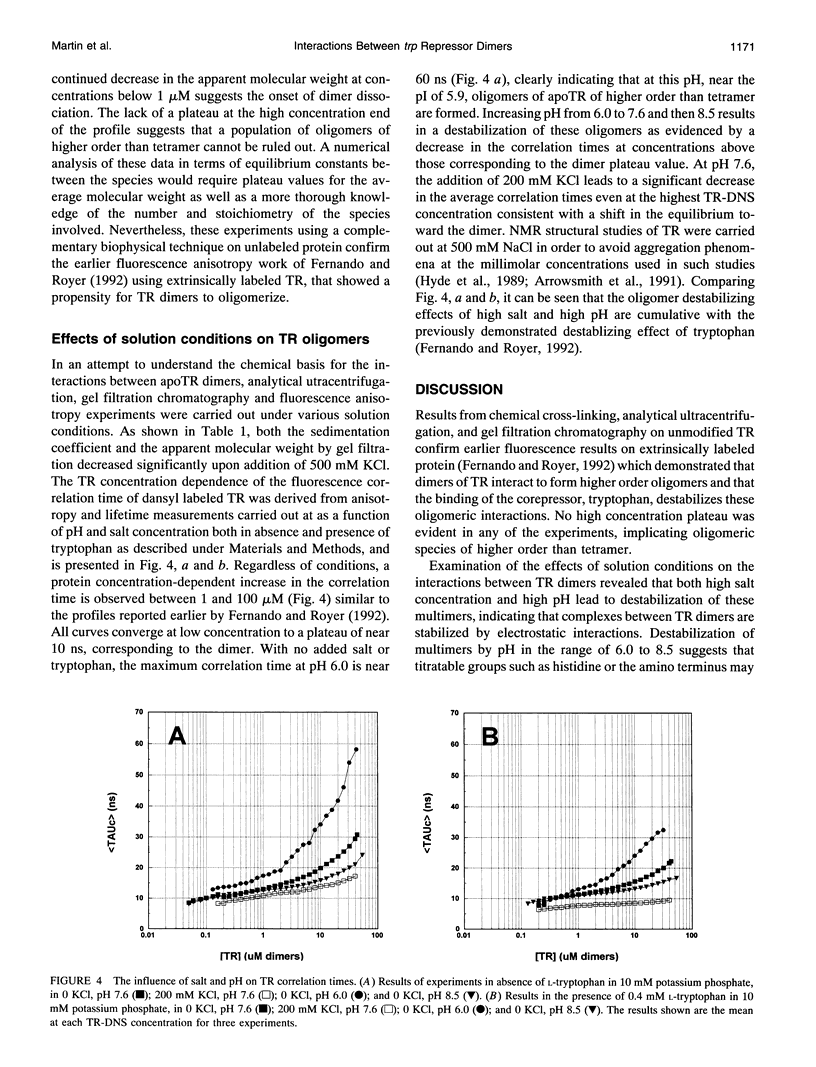


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckett D., Koblan K. S., Ackers G. K. Quantitative study of protein association at picomolar concentrations: the lambda phage cl repressor. Anal Biochem. 1991 Jul;196(1):69–75. doi: 10.1016/0003-2697(91)90118-d. [DOI] [PubMed] [Google Scholar]
- Carey J., Lewis D. E., Lavoie T. A., Yang J. How does trp repressor bind to its operator? J Biol Chem. 1991 Dec 25;266(36):24509–24513. [PubMed] [Google Scholar]
- Carey J. trp repressor arms contribute binding energy without occupying unique locations on DNA. J Biol Chem. 1989 Feb 5;264(4):1941–1945. [PubMed] [Google Scholar]
- Fernando T., Royer C. Role of protein--protein interactions in the regulation of transcription by trp repressor investigated by fluorescence spectroscopy. Biochemistry. 1992 Apr 7;31(13):3429–3441. doi: 10.1021/bi00128a018. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Miguel A. G., Gunsalus G. L. Intracellular Trp repressor levels in Escherichia coli. J Bacteriol. 1986 Jul;167(1):272–278. doi: 10.1128/jb.167.1.272-278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Yanofsky C. Nucleotide sequence and expression of Escherichia coli trpR, the structural gene for the trp aporepressor. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7117–7121. doi: 10.1073/pnas.77.12.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haran T. E., Joachimiak A., Sigler P. B. The DNA target of the trp repressor. EMBO J. 1992 Aug;11(8):3021–3030. doi: 10.1002/j.1460-2075.1992.tb05372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. J., Matthews K. S. Effect of amino acid alterations in the tryptophan-binding site of the trp repressor. J Biol Chem. 1990 Jan 15;265(2):731–737. [PubMed] [Google Scholar]
- Heatwole V. M., Somerville R. L. Synergism between the Trp repressor and Tyr repressor in repression of the aroL promoter of Escherichia coli K-12. J Bacteriol. 1992 Jan;174(1):331–335. doi: 10.1128/jb.174.1.331-335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatwole V. M., Somerville R. L. The tryptophan-specific permease gene, mtr, is differentially regulated by the tryptophan and tyrosine repressors in Escherichia coli K-12. J Bacteriol. 1991 Jun;173(11):3601–3604. doi: 10.1128/jb.173.11.3601-3604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk E., Heyduk T., Lee J. C. Global conformational changes in allosteric proteins. A study of Escherichia coli cAMP receptor protein and muscle pyruvate kinase. J Biol Chem. 1992 Feb 15;267(5):3200–3204. [PubMed] [Google Scholar]
- Hurlburt B. K., Yanofsky C. Enhanced operator binding by trp superrepressors of Escherichia coli. J Biol Chem. 1990 May 15;265(14):7853–7858. [PubMed] [Google Scholar]
- Hyde E. I., Ramesh V., Roberts G. C., Arrowsmith C. H., Treat-Clemons L., Klaic B., Jardetzky O. NMR studies of the Escherichia coli trp aporepressor. Sequence-specific assignment of the aromatic proton resonances. Eur J Biochem. 1989 Aug 15;183(3):545–553. doi: 10.1111/j.1432-1033.1989.tb21083.x. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Rudolph R. Refolding and association of oligomeric proteins. Methods Enzymol. 1986;131:218–250. doi: 10.1016/0076-6879(86)31043-7. [DOI] [PubMed] [Google Scholar]
- Jarvis T. C., Ring D. M., Daube S. S., von Hippel P. H. "Macromolecular crowding": thermodynamic consequences for protein-protein interactions within the T4 DNA replication complex. J Biol Chem. 1990 Sep 5;265(25):15160–15167. [PubMed] [Google Scholar]
- Joachimiak A., Kelley R. L., Gunsalus R. P., Yanofsky C., Sigler P. B. Purification and characterization of trp aporepressor. Proc Natl Acad Sci U S A. 1983 Feb;80(3):668–672. doi: 10.1073/pnas.80.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachimiak A., Marmorstein R. Q., Schevitz R. W., Mandecki W., Fox J. L., Sigler P. B. Crystals of the trp repressor-operator complex suitable for X-ray diffraction analysis. J Biol Chem. 1987 Apr 5;262(10):4917–4921. [PubMed] [Google Scholar]
- Kumamoto A. A., Miller W. G., Gunsalus R. P. Escherichia coli tryptophan repressor binds multiple sites within the aroH and trp operators. Genes Dev. 1987 Aug;1(6):556–564. doi: 10.1101/gad.1.6.556. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb P., McKnight S. L. Diversity and specificity in transcriptional regulation: the benefits of heterotypic dimerization. Trends Biochem Sci. 1991 Nov;16(11):417–422. doi: 10.1016/0968-0004(91)90167-t. [DOI] [PubMed] [Google Scholar]
- Lawson C. L., Carey J. Tandem binding in crystals of a trp repressor/operator half-site complex. Nature. 1993 Nov 11;366(6451):178–182. doi: 10.1038/366178a0. [DOI] [PubMed] [Google Scholar]
- Lawson C. L., Sigler P. B. The structure of trp pseudorepressor at 1.65A shows why indole propionate acts as a trp 'inducer'. Nature. 1988 Jun 30;333(6176):869–871. doi: 10.1038/333869a0. [DOI] [PubMed] [Google Scholar]
- LeTilly V., Royer C. A. Fluorescence anisotropy assays implicate protein-protein interactions in regulating trp repressor DNA binding. Biochemistry. 1993 Aug 3;32(30):7753–7758. doi: 10.1021/bi00081a021. [DOI] [PubMed] [Google Scholar]
- Liu Y. C., Matthews K. S. Dependence of trp repressor-operator affinity, stoichiometry, and apparent cooperativity on DNA sequence and size. J Biol Chem. 1993 Nov 5;268(31):23239–23249. [PubMed] [Google Scholar]
- Liu Y. C., Matthews K. S. Trp repressor interaction with bromodeoxyuridine-substituted operators alters UV-induced perturbation pattern in a sequence-dependent manner. Biochemistry. 1993 Oct 12;32(40):10532–10542. doi: 10.1021/bi00091a002. [DOI] [PubMed] [Google Scholar]
- Miner J. N., Yamamoto K. R. Regulatory crosstalk at composite response elements. Trends Biochem Sci. 1991 Nov;16(11):423–426. doi: 10.1016/0968-0004(91)90168-u. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z., Schevitz R. W., Zhang R. G., Lawson C. L., Joachimiak A., Marmorstein R. Q., Luisi B. F., Sigler P. B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature. 1988 Sep 22;335(6188):321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]
- Paluh J. L., Yanofsky C. High level production and rapid purification of the E. coli trp repressor. Nucleic Acids Res. 1986 Oct 24;14(20):7851–7860. doi: 10.1093/nar/14.20.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Roeder R. G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci. 1991 Nov;16(11):402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Squires C. L., Yanofsky C., Yang H. L., Zubay G. Regulation of in vitro transcription of the tryptophan operon by purified RNA polymerase in the presence of partially purified repressor and tryptophan. Nat New Biol. 1973 Oct 3;245(144):133–137. doi: 10.1038/newbio245133a0. [DOI] [PubMed] [Google Scholar]
- Sarsero J. P., Wookey P. J., Pittard A. J. Regulation of expression of the Escherichia coli K-12 mtr gene by TyrR protein and Trp repressor. J Bacteriol. 1991 Jul;173(13):4133–4143. doi: 10.1128/jb.173.13.4133-4143.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevitz R. W., Otwinowski Z., Joachimiak A., Lawson C. L., Sigler P. B. The three-dimensional structure of trp repressor. 1985 Oct 31-Nov 6Nature. 317(6040):782–786. doi: 10.1038/317782a0. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Roeder W. D., Bogosian G., Somerville R. L., Weith H. L. DNA sequence of the E. coli trpR gene and prediction of the amino acid sequence of Trp repressor. Nucleic Acids Res. 1980 Apr 11;8(7):1551–1560. doi: 10.1093/nar/8.7.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. M., Daniel R., Illing N., Errington J. Characterization of a sporulation gene, spoIVA, involved in spore coat morphogenesis in Bacillus subtilis. J Bacteriol. 1992 Jan;174(2):586–594. doi: 10.1128/jb.174.2.586-594.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. II. Fluorescent conjugates of ovalbumin and bovine serum albumin. Biochem J. 1952 May;51(2):155–167. doi: 10.1042/bj0510155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. G., Joachimiak A., Lawson C. L., Schevitz R. W., Otwinowski Z., Sigler P. B. The crystal structure of trp aporepressor at 1.8 A shows how binding tryptophan enhances DNA affinity. Nature. 1987 Jun 18;327(6123):591–597. doi: 10.1038/327591a0. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Gunsalus R. P., Brown K. D., Yanofsky C. Structure and regulation of aroH, the structural gene for the tryptophan-repressible 3-deoxy-D-arabino-heptulosonic acid-7-phosphate synthetase of Escherichia coli. J Mol Biol. 1981 Jan 5;145(1):47–73. doi: 10.1016/0022-2836(81)90334-x. [DOI] [PubMed] [Google Scholar]