Abstract
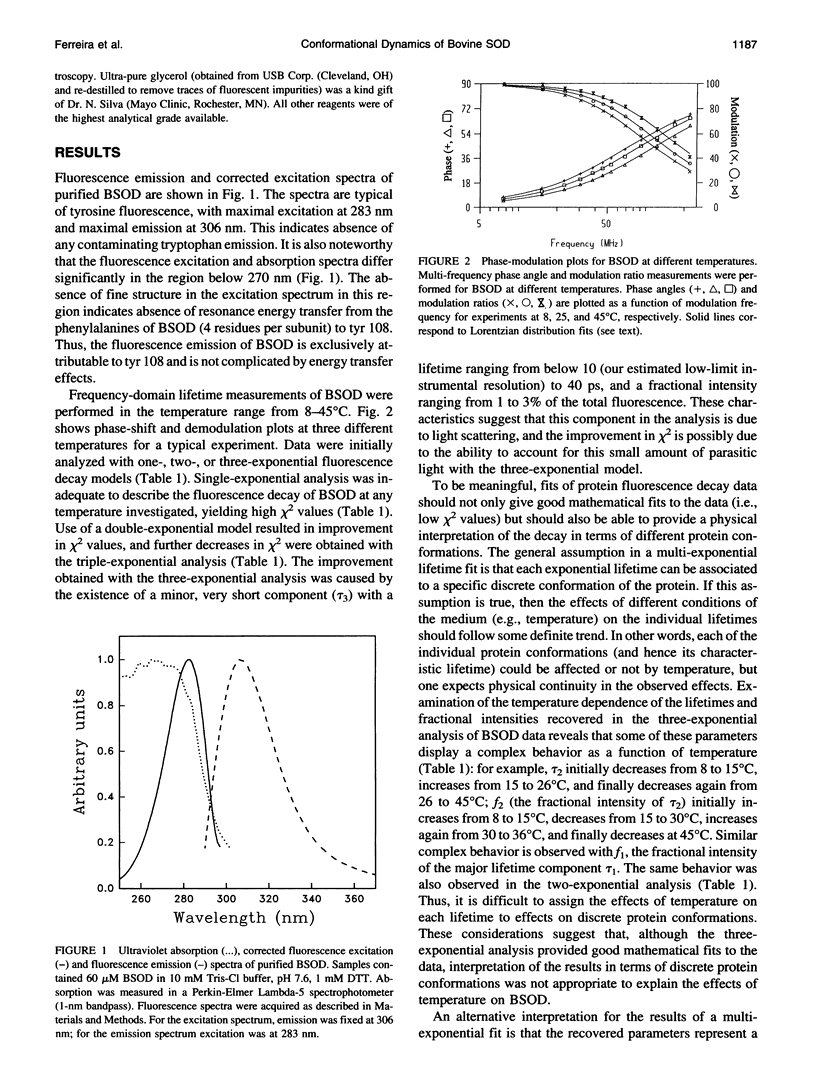
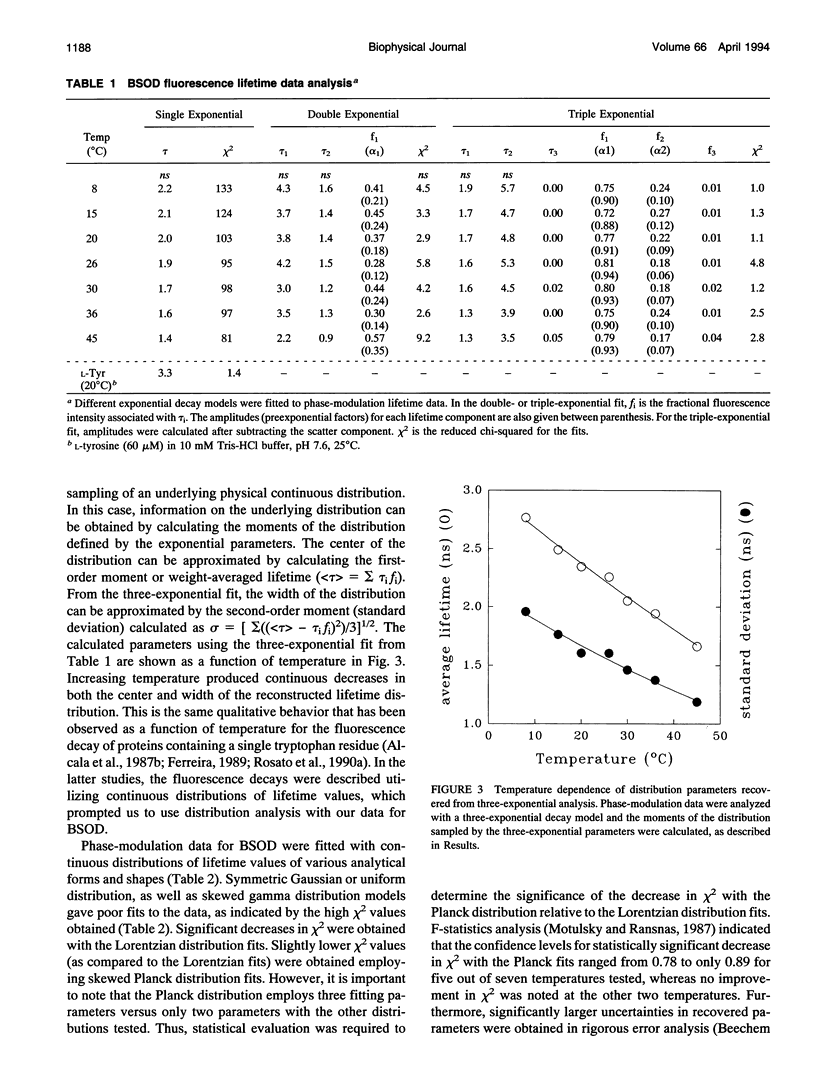
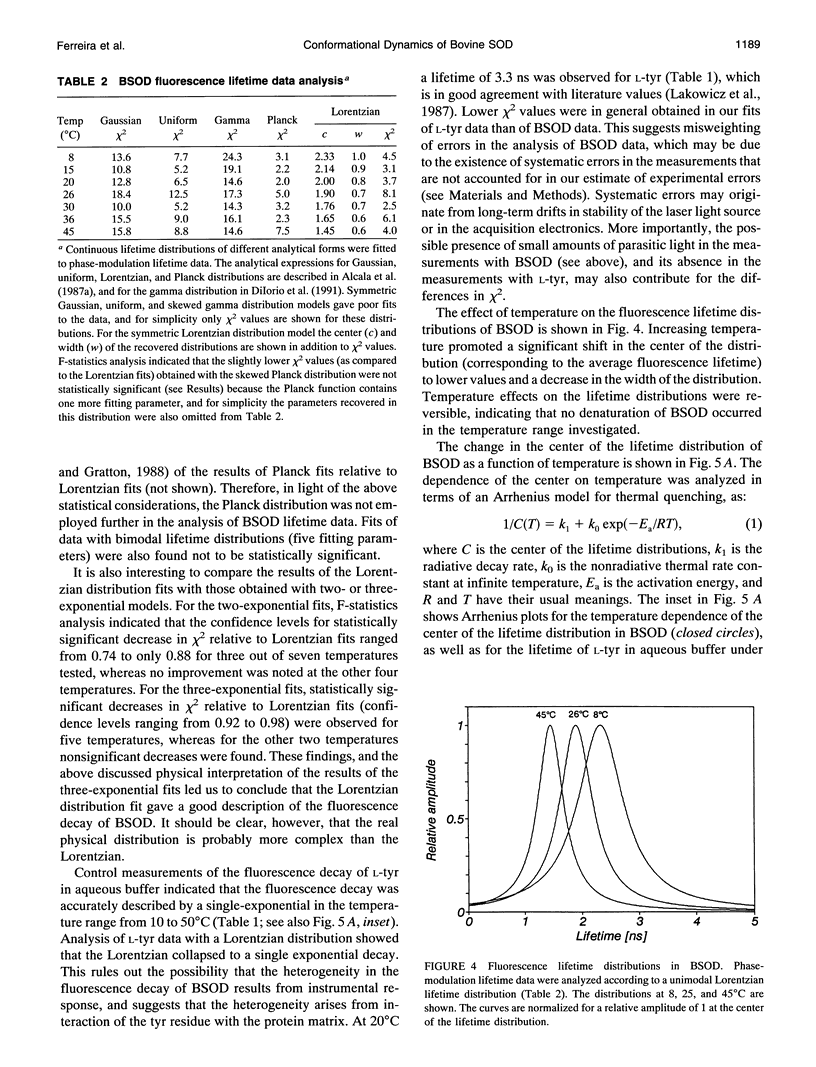
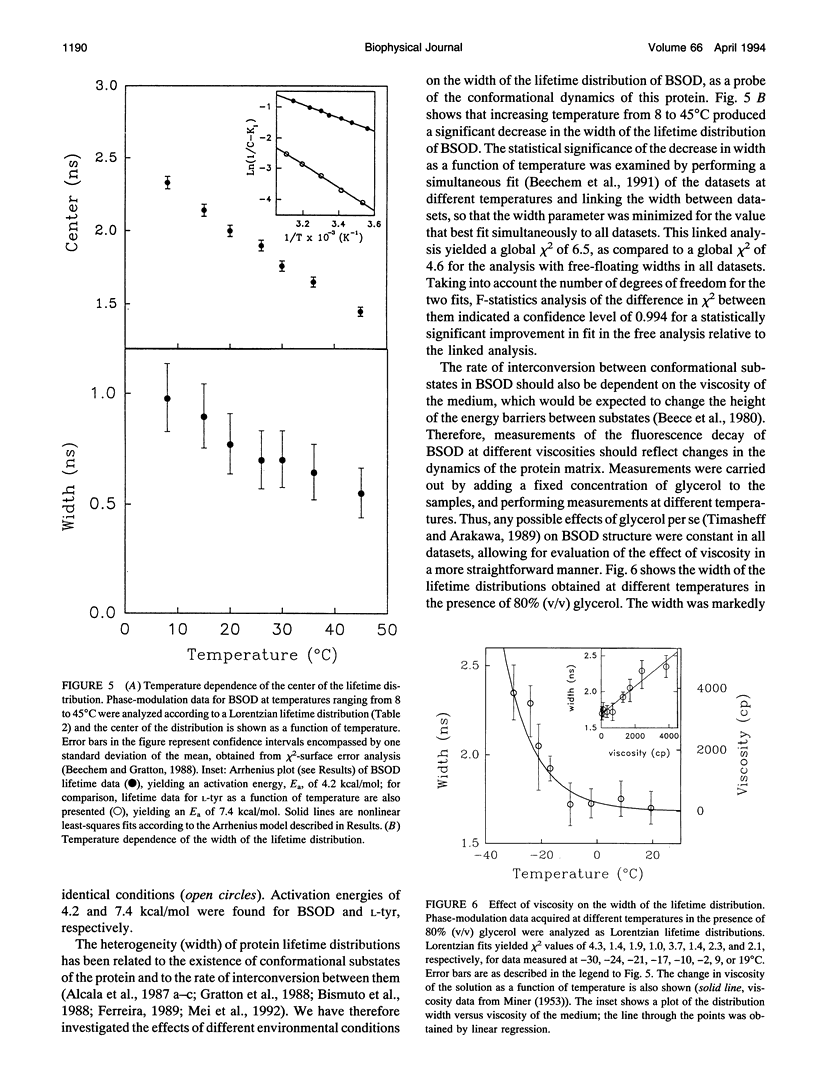
The structural dynamics of bovine erythrocyte Cu, Zn superoxide dismutase (BSOD) was studied by time-resolved fluorescence spectroscopy. BSOD is a homodimer containing a single tyrosine residue (and no tryptophan) per subunit. Frequency-domain fluorometry revealed a heterogeneous fluorescence decay that could be described with a Lorentzian distribution of lifetimes. The lifetime distribution parameters (center and width) were markedly dependent on temperature. The distribution center (average lifetime) displayed Arrhenius behavior with an Ea of 4.2 kcal/mol, in contrast with an Ea of 7.4 kcal/mol for the single-exponential decay of L-tyrosine. This indicated that thermal quenching of tyrosine emission was not solely responsible for the effect of temperature on the lifetimes of BSOD. The distribution width was broad (1 ns at 8 degrees C) and decreased significantly at higher temperatures. Furthermore, the width of the lifetime distribution increased in parallel to increasing viscosity of the medium. The combined effects of temperature and viscosity on the fluorescence decay suggest the existence of multiple conformational substrates in BSOD that interconvert during the excited-state lifetime. Denaturation of BSOD by guanidine hydrochloride produced an increase in the lifetime distribution width, indicating a larger number of conformations probed by the tyrosine residue in the denatured state. The rotational mobility of the tyrosine in BSOD was also investigated. Analysis of fluorescence anisotropy decay data enabled resolution of two rotational correlation times. One correlation time corresponded to a fast (picosecond) rotation that contributed 62% of the anisotropy decay and likely reported local mobility of the tyrosine ring. The longer correlation time was 50% of the expected value for rotation of the whole (dimeric) BSOD molecule and appeared to reflect segmental motions in the protein in addition to overall tumbling. Comparison between rotational correlation times and fluorescence lifetimes of BSOD indicates that the heterogeneity in lifetimes does not arise from mobility of the tyrosine per se, but rather from dynamics of the protein matrix surrounding this residue which affect its fluorescence decay.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcala J. R., Gratton E., Prendergast F. G. Fluorescence lifetime distributions in proteins. Biophys J. 1987 Apr;51(4):597–604. doi: 10.1016/S0006-3495(87)83384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala J. R., Gratton E., Prendergast F. G. Interpretation of fluorescence decays in proteins using continuous lifetime distributions. Biophys J. 1987 Jun;51(6):925–936. doi: 10.1016/S0006-3495(87)83420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala J. R., Gratton E., Prendergast F. G. Resolvability of fluorescence lifetime distributions using phase fluorometry. Biophys J. 1987 Apr;51(4):587–596. doi: 10.1016/S0006-3495(87)83383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund B. M., Gräslund A. Structure and dynamics of motilin. Time-resolved fluorescence of peptide hormone with single tyrosine residue. Biophys Chem. 1992 Nov;45(1):17–25. doi: 10.1016/0301-4622(92)87019-f. [DOI] [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H. Isolation and characterization of superoxide dismutase. Methods Enzymol. 1984;105:88–93. doi: 10.1016/s0076-6879(84)05012-6. [DOI] [PubMed] [Google Scholar]
- Bannister J., Bannister W., Wood E. Bovine erythrocyte cupro-zinc protein. 1. Isolation and general characterization. Eur J Biochem. 1971 Jan;18(2):178–186. doi: 10.1111/j.1432-1033.1971.tb01228.x. [DOI] [PubMed] [Google Scholar]
- Beece D., Eisenstein L., Frauenfelder H., Good D., Marden M. C., Reinisch L., Reynolds A. H., Sorensen L. B., Yue K. T. Solvent viscosity and protein dynamics. Biochemistry. 1980 Nov 11;19(23):5147–5157. doi: 10.1021/bi00564a001. [DOI] [PubMed] [Google Scholar]
- Beechem J. M., Brand L. Time-resolved fluorescence of proteins. Annu Rev Biochem. 1985;54:43–71. doi: 10.1146/annurev.bi.54.070185.000355. [DOI] [PubMed] [Google Scholar]
- Bismuto E., Gratton E., Irace G. Effect of unfolding on the tryptophanyl fluorescence lifetime distribution in apomyoglobin. Biochemistry. 1988 Mar 22;27(6):2132–2136. doi: 10.1021/bi00406a047. [DOI] [PubMed] [Google Scholar]
- Careri G., Fasella P., Gratton E. Enzyme dynamics: the statistical physics approach. Annu Rev Biophys Bioeng. 1979;8:69–97. doi: 10.1146/annurev.bb.08.060179.000441. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Di Iorio E. E., Hiltpold U. R., Filipovic D., Winterhalter K. H., Gratton E., Vitrano E., Cupane A., Leone M., Cordone L. Protein dynamics. Comparative investigation on heme-proteins with different physiological roles. Biophys J. 1991 Mar;59(3):742–754. doi: 10.1016/S0006-3495(91)82287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. T. Fluorescence studies of the conformational dynamics of parvalbumin in solution: lifetime and rotational motions of the single tryptophan residue. Biochemistry. 1989 Dec 26;28(26):10066–10072. doi: 10.1021/bi00452a028. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Fridovich I. On the stability of bovine superoxide dismutase. The effects of metals. J Biol Chem. 1973 Apr 25;248(8):2645–2649. [PubMed] [Google Scholar]
- Frauenfelder H., Gratton E. Protein dynamics and hydration. Methods Enzymol. 1986;127:207–216. doi: 10.1016/0076-6879(86)27017-2. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Gauduchon P., Wahl P. Pulsefluorimetry of tyrosyl peptides. Biophys Chem. 1978 Mar;8(1):87–104. doi: 10.1016/0301-4622(78)85026-1. [DOI] [PubMed] [Google Scholar]
- Gryczynski I., Steiner R. F., Lakowicz J. R. Intensity and anisotropy decays of the tyrosine calmodulin proteolytic fragments, as studied by GHz frequency-domain fluorescence. Biophys Chem. 1991 Jan;39(1):69–78. doi: 10.1016/0301-4622(91)85007-d. [DOI] [PubMed] [Google Scholar]
- Gurd F. R., Rothgeb T. M. Motions in proteins. Adv Protein Chem. 1979;33:73–165. doi: 10.1016/s0065-3233(08)60459-3. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. The internal dynamics of globular proteins. CRC Crit Rev Biochem. 1981;9(4):293–349. doi: 10.3109/10409238109105437. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. A theory of fluorescence polarization decay in membranes. Biophys J. 1977 Dec;20(3):289–305. doi: 10.1016/S0006-3495(77)85550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kungl A. J. Rotamer interconversion and its influence on the fluorescence decay of tyrosine: a molecular dynamics study. Biophys Chem. 1992 Nov;45(1):41–50. doi: 10.1016/0301-4622(92)87022-b. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Laczko G., Gryczynski I. Picosecond resolution of oxytocin tyrosyl fluorescence by 2 GHz frequency-domain fluorometry. Biophys Chem. 1986 Jul;24(2):97–100. doi: 10.1016/0301-4622(86)80002-3. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Laczko G., Gryczynski I. Picosecond resolution of tyrosine fluorescence and anisotropy decays by 2-GHz frequency-domain fluorometry. Biochemistry. 1987 Jan 13;26(1):82–90. doi: 10.1021/bi00375a012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws W. R., Ross J. B., Wyssbrod H. R., Beechem J. M., Brand L., Sutherland J. C. Time-resolved fluorescence and 1H NMR studies of tyrosine and tyrosine analogues: correlation of NMR-determined rotamer populations and fluorescence kinetics. Biochemistry. 1986 Feb 11;25(3):599–607. doi: 10.1021/bi00351a013. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Arnold L. D., Torrie B. H., Andrews B., Kruuv J. Structural analyses of various Cu2+, Zn2+-superoxide dismutases by differential scanning calorimetry and Raman spectroscopy. Arch Biochem Biophys. 1985 Aug 15;241(1):243–251. doi: 10.1016/0003-9861(85)90380-7. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Frey H. E., Hallewell R. A. Contribution of conformational stability and reversibility of unfolding to the increased thermostability of human and bovine superoxide dismutase mutated at free cysteines. J Biol Chem. 1990 Dec 15;265(35):21612–21618. [PubMed] [Google Scholar]
- Libertini L. J., Small E. W. Application of method of moments analysis to fluorescence decay lifetime distributions. Biophys Chem. 1989 Nov;34(3):269–282. doi: 10.1016/0301-4622(89)80064-x. [DOI] [PubMed] [Google Scholar]
- Libertini L. J., Small E. W. The intrinsic tyrosine fluorescence of histone H1. Steady state and fluorescence decay studies reveal heterogeneous emission. Biophys J. 1985 Jun;47(6):765–772. doi: 10.1016/S0006-3495(85)83979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski D. P., Fridovich I. Subunit association and side-chain reactivities of bovine erythrocyte superoxide dismutase in denaturing solvents. Biochemistry. 1979 Nov 13;18(23):5055–5060. doi: 10.1021/bi00590a005. [DOI] [PubMed] [Google Scholar]
- McNamara J. O., Fridovich I. Human genetics. Did radicals strike Lou Gehrig? Nature. 1993 Mar 4;362(6415):20–21. doi: 10.1038/362020a0. [DOI] [PubMed] [Google Scholar]
- McRee D. E., Redford S. M., Getzoff E. D., Lepock J. R., Hallewell R. A., Tainer J. A. Changes in crystallographic structure and thermostability of a Cu,Zn superoxide dismutase mutant resulting from the removal of a buried cysteine. J Biol Chem. 1990 Aug 25;265(24):14234–14241. doi: 10.2210/pdb3sod/pdb. [DOI] [PubMed] [Google Scholar]
- Mei G., Rosato N., Silva N., Jr, Rusch R., Gratton E., Savini I., Finazzi-Agrò A. Denaturation of human Cu/Zn superoxide dismutase by guanidine hydrochloride: a dynamic fluorescence study. Biochemistry. 1992 Aug 18;31(32):7224–7230. doi: 10.1021/bi00147a003. [DOI] [PubMed] [Google Scholar]
- Motulsky H. J., Ransnas L. A. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J. 1987 Nov;1(5):365–374. [PubMed] [Google Scholar]
- Munro I., Pecht I., Stryer L. Subnanosecond motions of tryptophan residues in proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):56–60. doi: 10.1073/pnas.76.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund T. M., Liu X. Y., Sommer J. H. Fluorescence polarization decay of tyrosine in lima bean trypsin inhibitor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8977–8981. doi: 10.1073/pnas.83.23.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti F., Mocali A. Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oxidation. Methods Enzymol. 1990;186:209–220. doi: 10.1016/0076-6879(90)86110-h. [DOI] [PubMed] [Google Scholar]
- Parge H. E., Hallewell R. A., Tainer J. A. Atomic structures of wild-type and thermostable mutant recombinant human Cu,Zn superoxide dismutase. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6109–6113. doi: 10.1073/pnas.89.13.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permyakov E. A., Ostrovsky A. V., Burstein E. A., Pleshanov P. G., Gerday C. Parvalbumin conformers revealed by steady-state and time-resolved fluorescence spectroscopy. Arch Biochem Biophys. 1985 Aug 1;240(2):781–791. doi: 10.1016/0003-9861(85)90087-6. [DOI] [PubMed] [Google Scholar]
- Rigler R., Roslund J., Forsen S. Side chain mobility in bovine calbindin D9k. Rotational motion of Tyr13. Eur J Biochem. 1990 Mar 30;188(3):541–545. doi: 10.1111/j.1432-1033.1990.tb15434.x. [DOI] [PubMed] [Google Scholar]
- Rigo A., Marmocchi F., Cocco D., Viglino P., Rotilio G. On the quaternary structure of copper-zinc superoxide dismutases. Reversible dissociation into protomers of the isozyme I from wheat germ. Biochemistry. 1978 Feb 7;17(3):534–537. doi: 10.1021/bi00596a025. [DOI] [PubMed] [Google Scholar]
- Roe J. A., Butler A., Scholler D. M., Valentine J. S., Marky L., Breslauer K. J. Differential scanning calorimetry of Cu,Zn-superoxide dismutase, the apoprotein, and its zinc-substituted derivatives. Biochemistry. 1988 Feb 9;27(3):950–958. doi: 10.1021/bi00403a017. [DOI] [PubMed] [Google Scholar]
- Rosato N., Gratton E., Mei G., Finazzi-Agrò A. Fluorescence lifetime distributions in human superoxide dismutase. Effect of temperature and denaturation. Biophys J. 1990 Oct;58(4):817–822. doi: 10.1016/S0006-3495(90)82427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato N., Mei G., Gratton E., Bannister J. V., Bannister W. H., Finazzi-Agrò A. A time-resolved fluorescence study of human copper-zinc superoxide dismutase. Biophys Chem. 1990 May;36(1):41–46. doi: 10.1016/0301-4622(90)85005-q. [DOI] [PubMed] [Google Scholar]
- Ross J. B., Laws W. R., Buku A., Sutherland J. C., Wyssbrod H. R. Time-resolved fluorescence and 1H NMR studies of tyrosyl residues in oxytocin and small peptides: correlation of NMR-determined conformations of tyrosyl residues and fluorescence decay kinetics. Biochemistry. 1986 Feb 11;25(3):607–612. doi: 10.1021/bi00351a014. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Beem K. M., Richardson J. S., Richardson D. C. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982 Sep 15;160(2):181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]


