Abstract
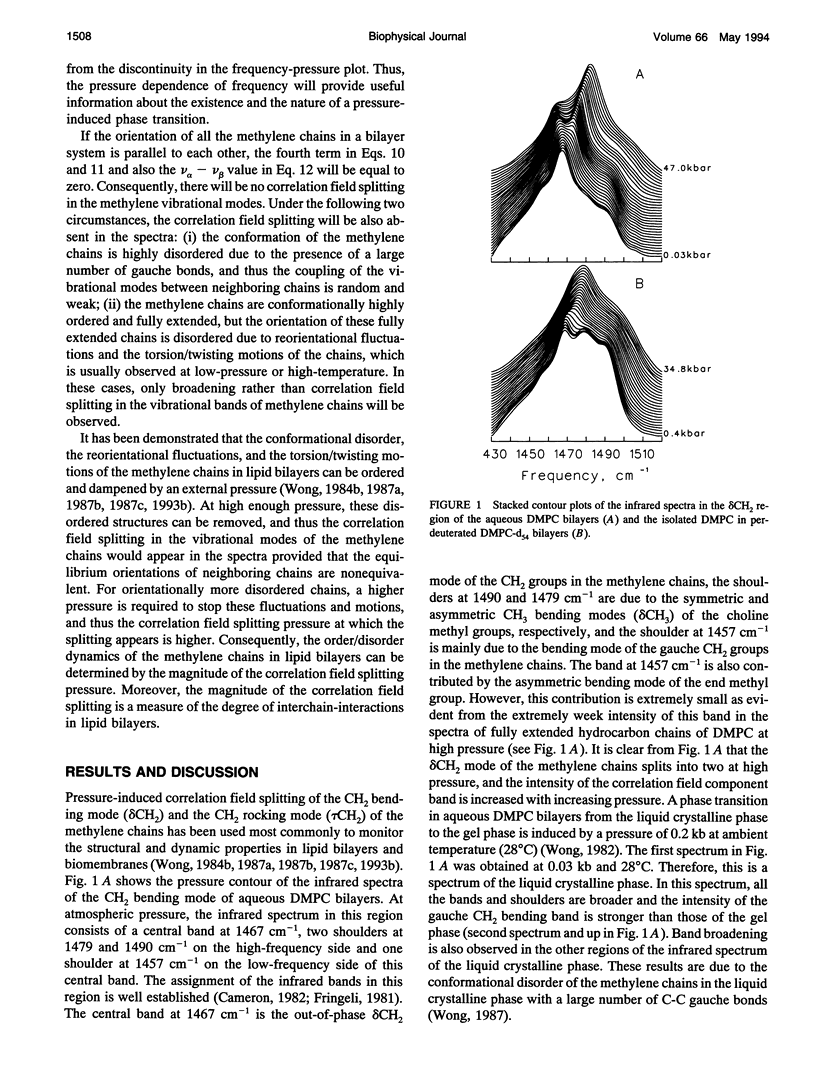
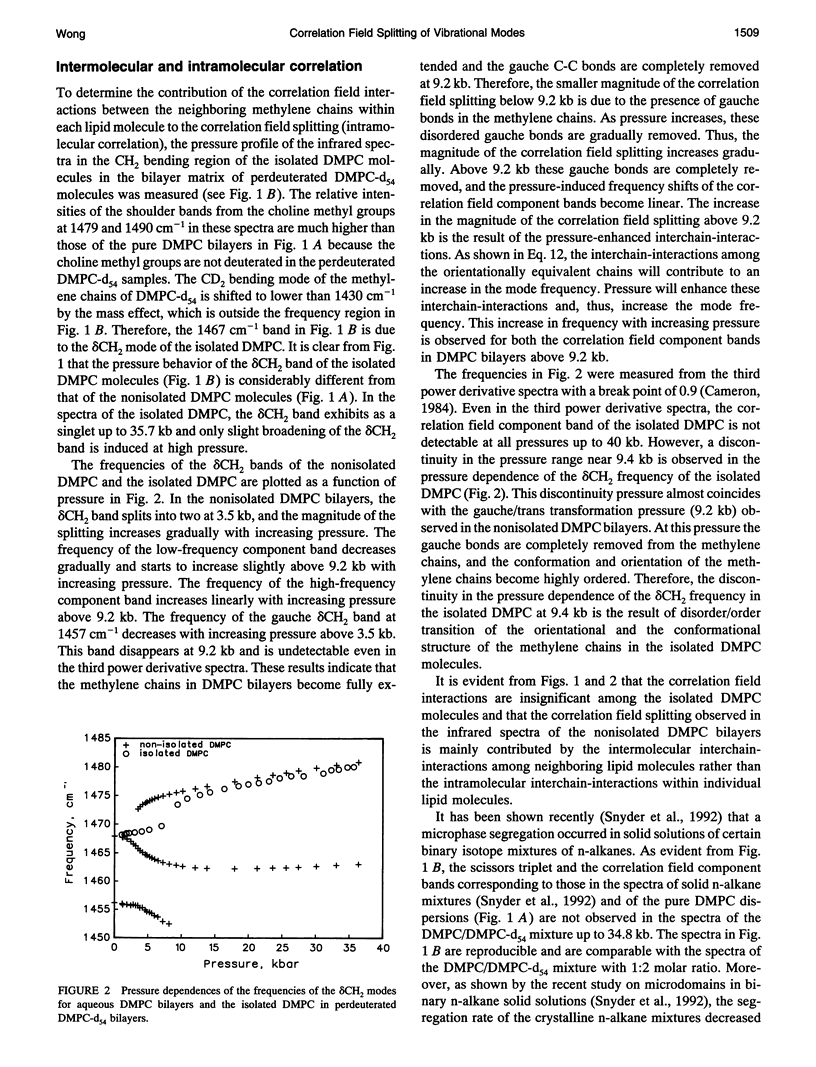
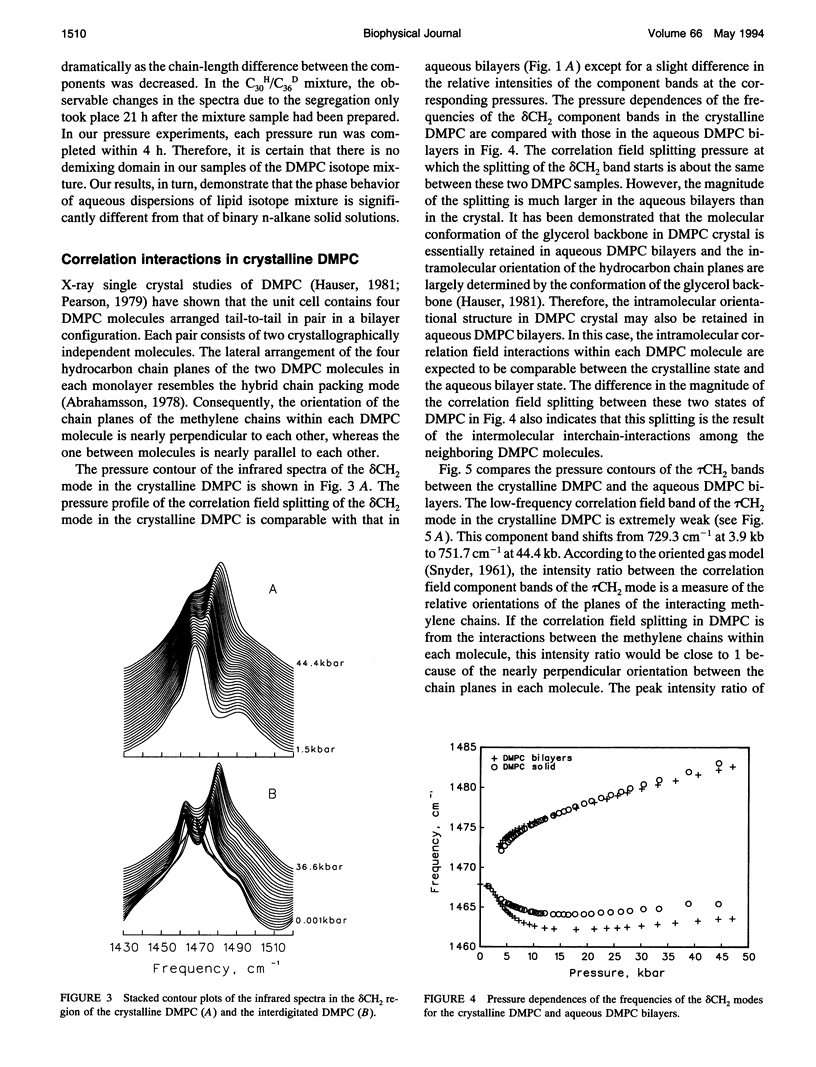
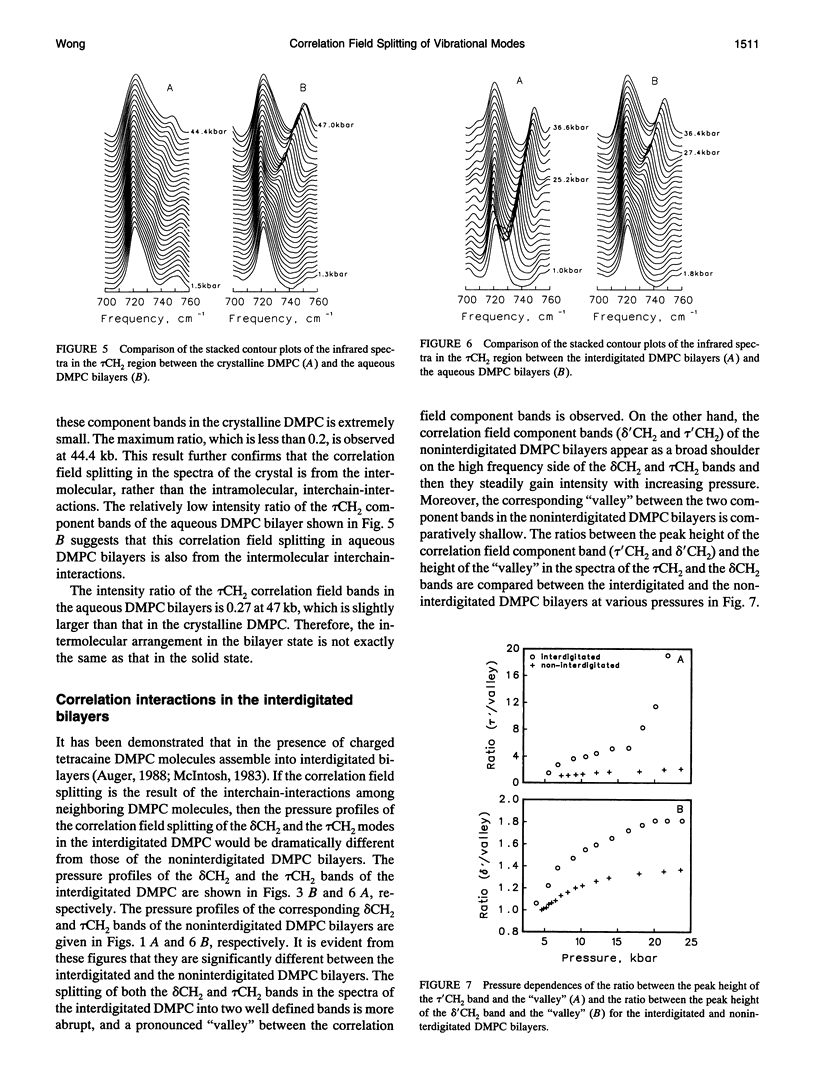
Correlation field splittings of the vibrational modes of methylene chains in lipid bilayers, isolated lipid molecules in perdeuterated lipid bilayers, crystalline lipid, and interdigitated lipid bilayers have been investigated by pressure-tuning Fourier-transform infrared spectroscopy. The correlation field splittings of these modes are originating from the vibrational coupling interactions between the fully extended methylene chains with different site symmetry along each bilayer leaflet. The interchain-interactions of the methylene chains with the same site symmetry only contribute to frequency shift of the vibrational modes. The magnitude of the correlation field splitting is a measure of the strength of the interchain-interactions, and the relative intensities of the correlation field component bands provide information concerning the relative orientation of the zig-zag planes of the interacting methylene chains. It has been demonstrated in the present work that the correlation field splitting of the CH2 bending and rocking modes commonly observed in the vibrational spectra of lipid bilayers is the result of the intermolecular interchain-interactions among the methylene chains of the neighboring molecules. The intramolecular interchain-interactions between the sn-1 and sn-2 methylene chains within each molecule are weak. The correlation field splitting resulting from the intramolecular interchain-interactions exhibits a much smaller magnitude than that from the intermolecular interchain-interactions and is observed only at very high pressure. Interdigitation of the opposing bilayer leaflets disturbs significantly the intermolecular interchain-interactions and results in dramatic changes in the pressure profiles of the correlation field component bands of both the CH2 bending and rocking modes. The relative intensities of the correlation field component bands of these modes and the magnitude of the splitting are also altered significantly. These results provide further evidence that the correlation field splitting of the CH2 bending and rocking modes in the vibrational spectra of lipid bilayers is due to the intermolecular interchain-interactions. The present work has also demonstrated that the correlation field splitting of the vibrational modes in lipid bilayers is mainly contributed by the intermolecular interchain-interactions among the nearest neighboring molecules and that the long-range correlation interactions beyond the second neighboring molecules are insignificant.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson S., Dahlén B., Löfgren H., Pascher I. Lateral packing of hydrocarbon chains. Prog Chem Fats Other Lipids. 1978;16:125–143. doi: 10.1016/0079-6832(78)90039-3. [DOI] [PubMed] [Google Scholar]
- Ahmed M. K., Choma C. T., Wong P. T. High pressure FTIR study of interaction of melittin with dimyristoylphosphatidyl glycerol bilayers. Chem Phys Lipids. 1992 Nov;63(1-2):139–148. doi: 10.1016/0009-3084(92)90030-s. [DOI] [PubMed] [Google Scholar]
- Auger M., Jarrell H. C., Smith I. C., Siminovitch D. J., Mantsch H. H., Wong P. T. Effects of the local anesthetic tetracaine on the structural and dynamic properties of lipids in model membranes: a high-pressure Fourier transform infrared study. Biochemistry. 1988 Aug 9;27(16):6086–6093. doi: 10.1021/bi00416a038. [DOI] [PubMed] [Google Scholar]
- Auger M., Smith I. C., Mantsch H. H., Wong P. T. High-pressure infrared study of phosphatidylserine bilayers and their interactions with the local anesthetic tetracaine. Biochemistry. 1990 Feb 27;29(8):2008–2015. doi: 10.1021/bi00460a008. [DOI] [PubMed] [Google Scholar]
- Carrier D., Mantsch H. H., Wong P. T. Protective effect of lipidic surfaces against pressure-induced conformational changes of poly(L-lysine). Biochemistry. 1990 Jan 9;29(1):254–258. doi: 10.1021/bi00453a034. [DOI] [PubMed] [Google Scholar]
- Choma C. T., Wong P. T. The structure of anhydrous and hydrated dimyristoylphosphatidyl glycerol: a pressure tuning infrared spectroscopic study. Chem Phys Lipids. 1992 Apr;61(2):131–137. doi: 10.1016/0009-3084(92)90005-a. [DOI] [PubMed] [Google Scholar]
- Chong P. L., Capes S., Wong P. T. Effects of hydrostatic pressure on the location of PRODAN in lipid bilayers: a FT-IR study. Biochemistry. 1989 Oct 17;28(21):8358–8363. doi: 10.1021/bi00447a014. [DOI] [PubMed] [Google Scholar]
- Chong P. L., Wong P. T. Interactions of Laurdan with phosphatidylcholine liposomes: a high pressure FTIR study. Biochim Biophys Acta. 1993 Jul 4;1149(2):260–266. doi: 10.1016/0005-2736(93)90209-i. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hübner W., Wong P. T., Mantsch H. H. The effect of hydrostatic pressure on the bilayer structure of phosphatidylcholines containing omega-cyclohexyl fatty acyl chains. Biochim Biophys Acta. 1990 Sep 7;1027(3):229–237. doi: 10.1016/0005-2736(90)90312-c. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Rigas B., Morgello S., Goldman I. S., Wong P. T. Human colorectal cancers display abnormal Fourier-transform infrared spectra. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8140–8144. doi: 10.1073/pnas.87.20.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch D. J., Wong P. T., Mantsch H. H. Effects of cis and trans unsaturation on the structure of phospholipid bilayers: a high-pressure infrared spectroscopic study. Biochemistry. 1987 Jun 16;26(12):3277–3287. doi: 10.1021/bi00386a006. [DOI] [PubMed] [Google Scholar]
- Siminovitch D. J., Wong P. T., Mantsch H. H. Effects of cis and trans unsaturation on the structure of phospholipid bilayers: a high-pressure infrared spectroscopic study. Biochemistry. 1987 Jun 16;26(12):3277–3287. doi: 10.1021/bi00386a006. [DOI] [PubMed] [Google Scholar]
- Siminovitch D. J., Wong P. T., Mantsch H. H. High pressure infrared spectroscopy of lipid bilayers: new tests for interdigitation. Biochim Biophys Acta. 1987 Jun 12;900(1):163–167. doi: 10.1016/0005-2736(87)90289-6. [DOI] [PubMed] [Google Scholar]
- Siminovitch D. J., Wong P. T., Mantsch H. H. High-pressure infrared spectroscopy of ether- and ester-linked phosphatidylcholine aqueous dispersions. Biophys J. 1987 Mar;51(3):465–473. doi: 10.1016/S0006-3495(87)83368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper S., Wong P. T., Tanphaichitr N. Binding of Ca2+ to sulfogalactosylceramide and the sequential effects on the lipid dynamics. Biochemistry. 1992 Dec 1;31(47):11902–11907. doi: 10.1021/bi00162a032. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Cadrin M., French S. W. Distinctive infrared spectral features in liver tumor tissues of mice: evidence of structural modifications at the molecular level. Exp Mol Pathol. 1991 Dec;55(3):269–284. doi: 10.1016/0014-4800(91)90007-k. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Capes S. E., Mantsch H. H. Hydrogen bonding between anhydrous cholesterol and phosphatidylcholines: an infrared spectroscopic study. Biochim Biophys Acta. 1989 Mar 27;980(1):37–41. doi: 10.1016/0005-2736(89)90197-1. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Chagwedera T. E., Mantsch H. H. Effect of cholesterol on structural and dynamic properties of tripalmitoyl glyceride. A high-pressure infrared spectroscopic study. Biophys J. 1989 Nov;56(5):845–850. doi: 10.1016/S0006-3495(89)82730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. T., Goldstein S. M., Grekin R. C., Godwin T. A., Pivik C., Rigas B. Distinct infrared spectroscopic patterns of human basal cell carcinoma of the skin. Cancer Res. 1993 Feb 15;53(4):762–765. [PubMed] [Google Scholar]
- Wong P. T., Huang C. H. Structural aspects of pressure effects on infrared spectra of mixed-chain phosphatidylcholine assemblies in D2O. Biochemistry. 1989 Feb 7;28(3):1259–1263. doi: 10.1021/bi00429a046. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Mantsch H. H. Reorientational and conformational ordering processes at elevated pressures in 1,2-dioleoyl phosphatidylcholine: a Raman and infrared spectroscopic study. Biophys J. 1988 Nov;54(5):781–790. doi: 10.1016/S0006-3495(88)83016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. T. Raman spectroscopy of thermotropic and high-pressure phases of aqueous phospholipid dispersions. Annu Rev Biophys Bioeng. 1984;13:1–24. doi: 10.1146/annurev.bb.13.060184.000245. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Wong R. K., Caputo T. A., Godwin T. A., Rigas B. Infrared spectroscopy of exfoliated human cervical cells: evidence of extensive structural changes during carcinogenesis. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):10988–10992. doi: 10.1073/pnas.88.24.10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakim D., Wong P. T. A high-pressure, infrared spectroscopic study of the solvation of bilirubin in lipid bilayers. Biochemistry. 1990 Feb 27;29(8):2003–2007. doi: 10.1021/bi00460a007. [DOI] [PubMed] [Google Scholar]


