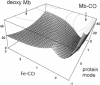
Abstract
Heme proteins react inhomogeneously with ligands at cryogenic temperatures and homogeneously at room temperature. We have identified and characterized a transition from inhomogeneous to homogeneous behavior at intermediate temperatures in the time dependence of CO binding to horse myoglobin. The turnover is attributed to a functionally important tertiary protein relaxation process during which the barrier increases dynamically. This is verified by a combination of theory and multipulse measurements. A likely biological significance of this effect is in the autocatalysis of the ligand release process.
Full text
PDF
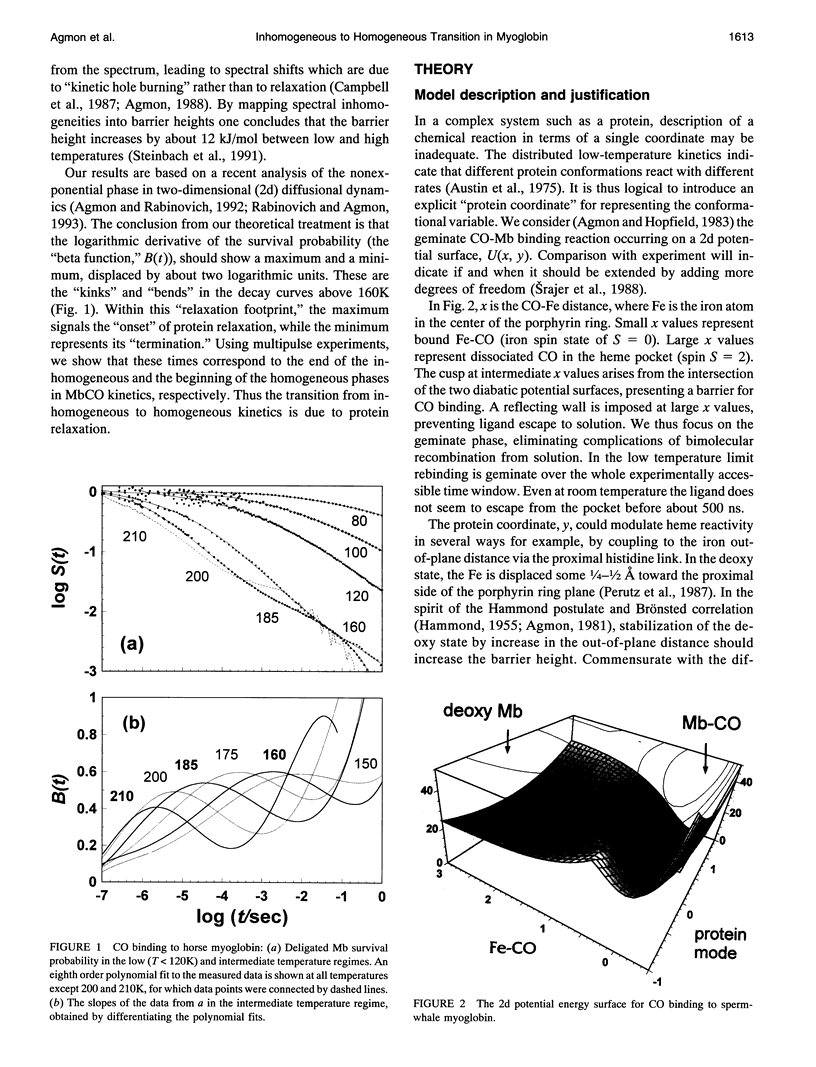


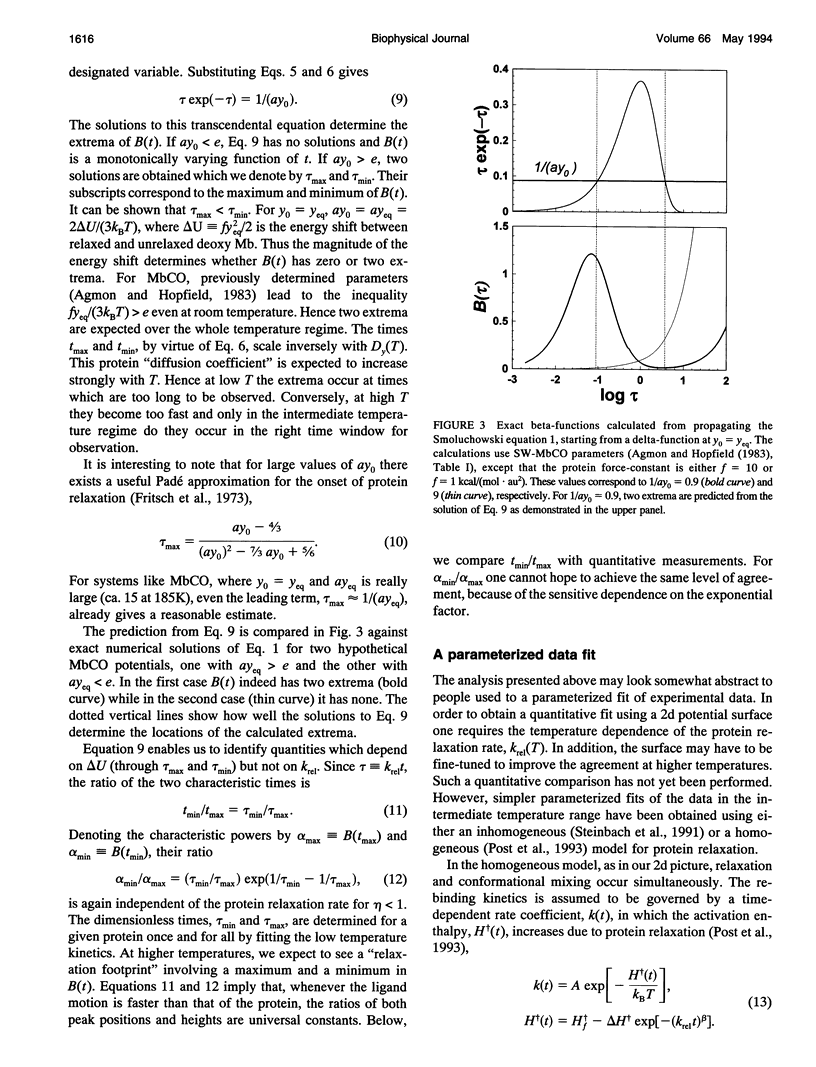



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agmon N. Reactive line-shape narrowing in low-temperature inhomogeneous geminate recombination of CO to myoglobin. Biochemistry. 1988 May 3;27(9):3507–3511. doi: 10.1021/bi00409a057. [DOI] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Ansari A., Jones C. M., Henry E. R., Hofrichter J., Eaton W. A. The role of solvent viscosity in the dynamics of protein conformational changes. Science. 1992 Jun 26;256(5065):1796–1798. doi: 10.1126/science.1615323. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Campbell B. F., Chance M. R., Friedman J. M. Linkage of functional and structural heterogeneity in proteins: dynamic hole burning in carboxymyoglobin. Science. 1987 Oct 16;238(4825):373–376. doi: 10.1126/science.3659921. [DOI] [PubMed] [Google Scholar]
- Doster W., Beece D., Bowne S. F., DiIorio E. E., Eisenstein L., Frauenfelder H., Reinisch L., Shyamsunder E., Winterhalter K. H., Yue K. T. Control and pH dependence of ligand binding to heme proteins. Biochemistry. 1982 Sep 28;21(20):4831–4839. doi: 10.1021/bi00263a001. [DOI] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 1981;76:175–261. doi: 10.1016/0076-6879(81)76126-3. [DOI] [PubMed] [Google Scholar]
- Friedman J. M. Structure, dynamics, and reactivity in hemoglobin. Science. 1985 Jun 14;228(4705):1273–1280. doi: 10.1126/science.4001941. [DOI] [PubMed] [Google Scholar]
- Gibson Q. H., Regan R., Elber R., Olson J. S., Carver T. E. Distal pocket residues affect picosecond ligand recombination in myoglobin. An experimental and molecular dynamics study of position 29 mutants. J Biol Chem. 1992 Nov 5;267(31):22022–22034. [PubMed] [Google Scholar]
- Hong MK, Shyamsunder E, Austin RH, Gerstman BS, Chan SS. Time-resolved infrared studies of molecular diffusion in myoglobin. Phys Rev Lett. 1991 May 20;66(20):2673–2676. doi: 10.1103/PhysRevLett.66.2673. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Neet K. E. The catalytic and regulatory properties of enzymes. Annu Rev Biochem. 1968;37:359–410. doi: 10.1146/annurev.bi.37.070168.002043. [DOI] [PubMed] [Google Scholar]
- Ormos P., Ansari A., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Sauke T. B., Steinbach P. J., Young R. D. Inhomogeneous broadening in spectral bands of carbonmonoxymyoglobin. The connection between spectral and functional heterogeneity. Biophys J. 1990 Feb;57(2):191–199. doi: 10.1016/S0006-3495(90)82522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich J. W., Lambry J. C., Kuczera K., Karplus M., Poyart C., Martin J. L. Ligand binding and protein relaxation in heme proteins: a room temperature analysis of NO geminate recombination. Biochemistry. 1991 Apr 23;30(16):3975–3987. doi: 10.1021/bi00230a025. [DOI] [PubMed] [Google Scholar]
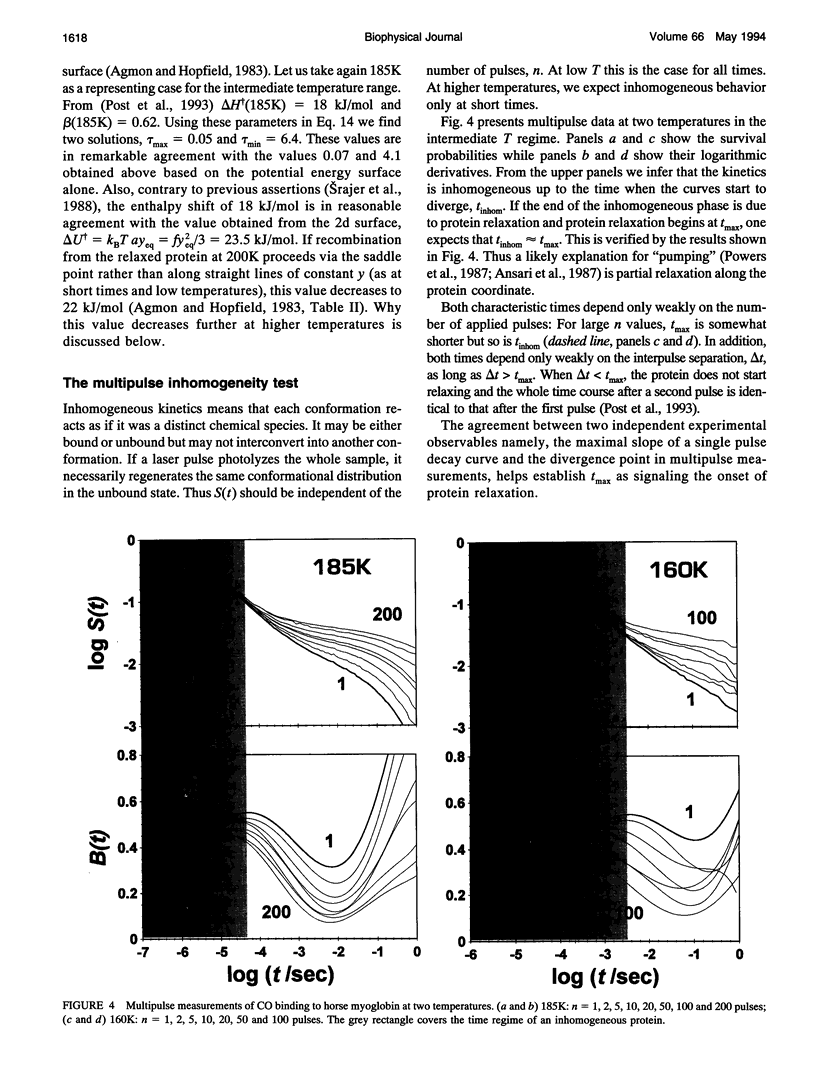
- Post F., Doster W., Karvounis G., Settles M. Structural relaxation and nonexponential kinetics of CO-binding to horse myoglobin. Multiple flash photolysis experiments. Biophys J. 1993 Jun;64(6):1833–1842. doi: 10.1016/S0006-3495(93)81554-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers L., Chance B., Chance M., Campbell B., Friedman J., Khalid S., Kumar C., Naqui A., Reddy K. S., Zhou Y. Kinetic, structural, and spectroscopic identification of geminate states of myoglobin: a ligand binding site on the reaction pathway. Biochemistry. 1987 Jul 28;26(15):4785–4796. doi: 10.1021/bi00389a028. [DOI] [PubMed] [Google Scholar]
- Rabinovich S, Agmon N. Scaling and critical-like behavior in multidimensional diffusive dynamics. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1993 May;47(5):3717–3720. doi: 10.1103/physreve.47.3717. [DOI] [PubMed] [Google Scholar]
- Srajer V., Reinisch L., Champion P. M. Investigation of laser-induced long-lived states of photolyzed MbCO. Biochemistry. 1991 May 21;30(20):4886–4895. doi: 10.1021/bi00234a008. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Ansari A., Berendzen J., Braunstein D., Chu K., Cowen B. R., Ehrenstein D., Frauenfelder H., Johnson J. B., Lamb D. C. Ligand binding to heme proteins: connection between dynamics and function. Biochemistry. 1991 Apr 23;30(16):3988–4001. doi: 10.1021/bi00230a026. [DOI] [PubMed] [Google Scholar]
- Tian WD, Sage JT, Srajer V, V, Champion PM. Relaxation dynamics of myoglobin in solution. Phys Rev Lett. 1992 Jan 20;68(3):408–411. doi: 10.1103/PhysRevLett.68.408. [DOI] [PubMed] [Google Scholar]