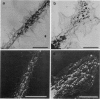
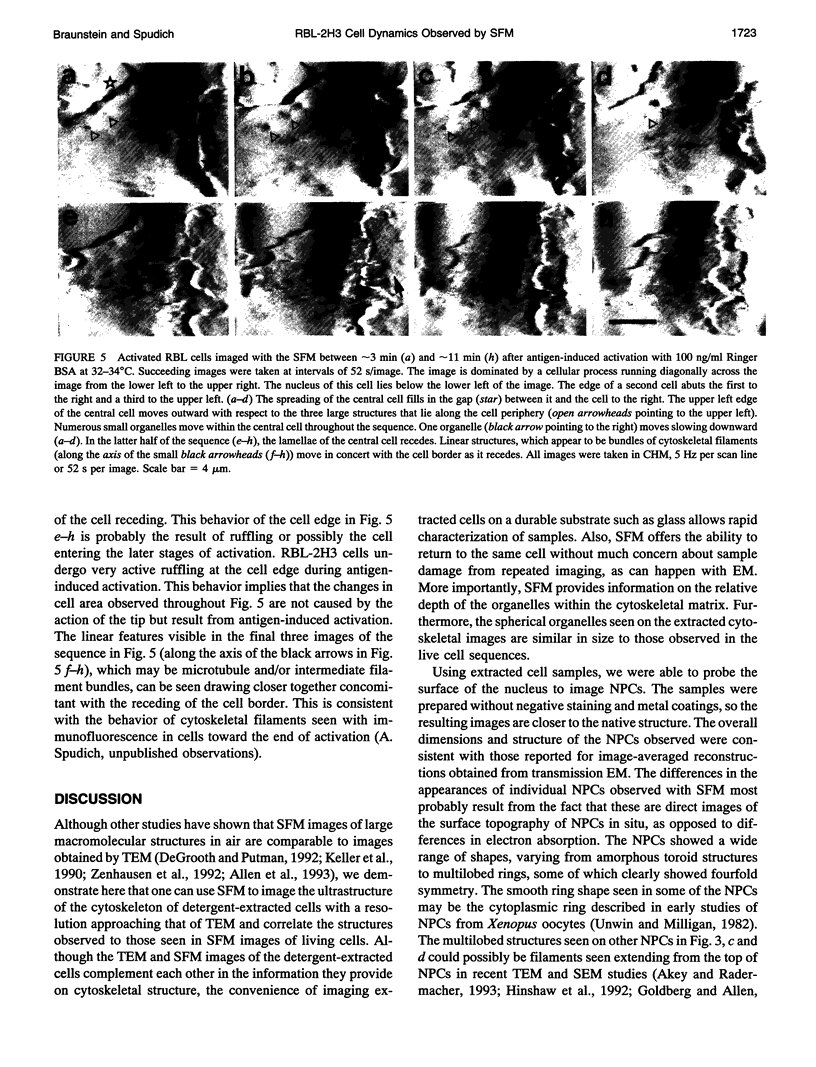
Abstract
Surface and subsurface dynamics of Rat Basophilic Leukemia cells, a model system of stimulated secretion, were imaged using Scanning Force Microscopy (SFM) at a rate of 50-60 s/image. Cytoskeletal elements and organelles were tracked within quiescent cells and those activated after IgE receptor crosslinking. In addition, surface waves were observed moving within the plasma membrane. The structures seen in quiescent and activated cells can be correlated with those seen in electron micrographs and topographic SFM images of fixed detergent-extracted cells. Furthermore, images of the detergent-extracted nuclei reveal the presence of numerous nuclear pore complexes. High-magnification images of the nuclear pore complexes show evidence of subunit structure and exhibit dimensions consistent with those reported previously using electron microscopy. The behavior and overall change in morphology of cells observed during activation was consistent with that observed under similar conditions with Differential Interference Contrast microscopy. This study demonstrates that SFM, unlike other techniques, can be used to provide high-resolution information in both fixed and living cells.
Full text
PDF

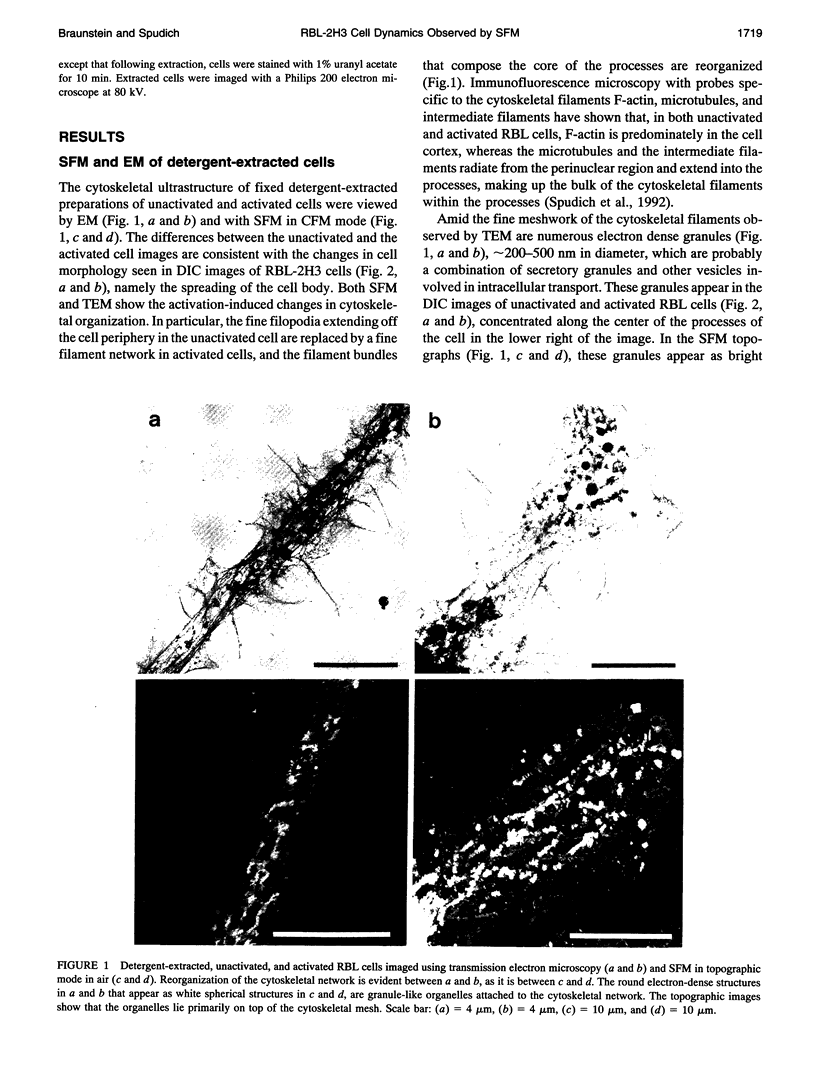
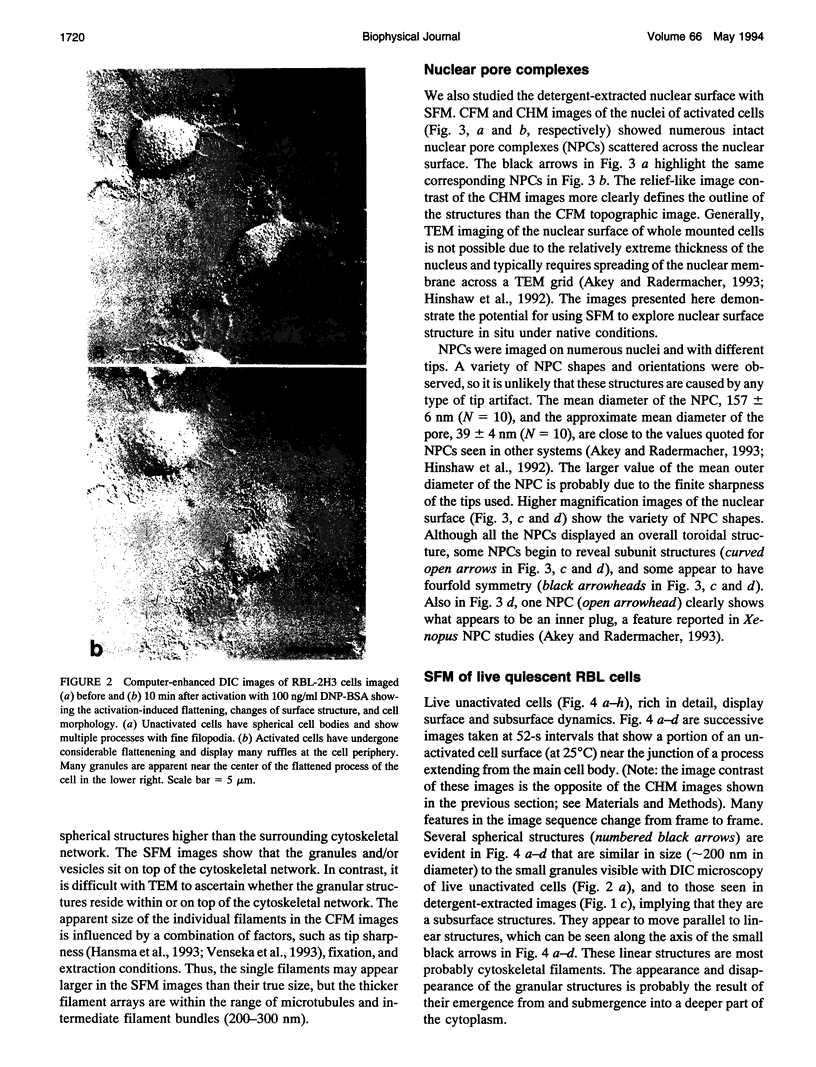
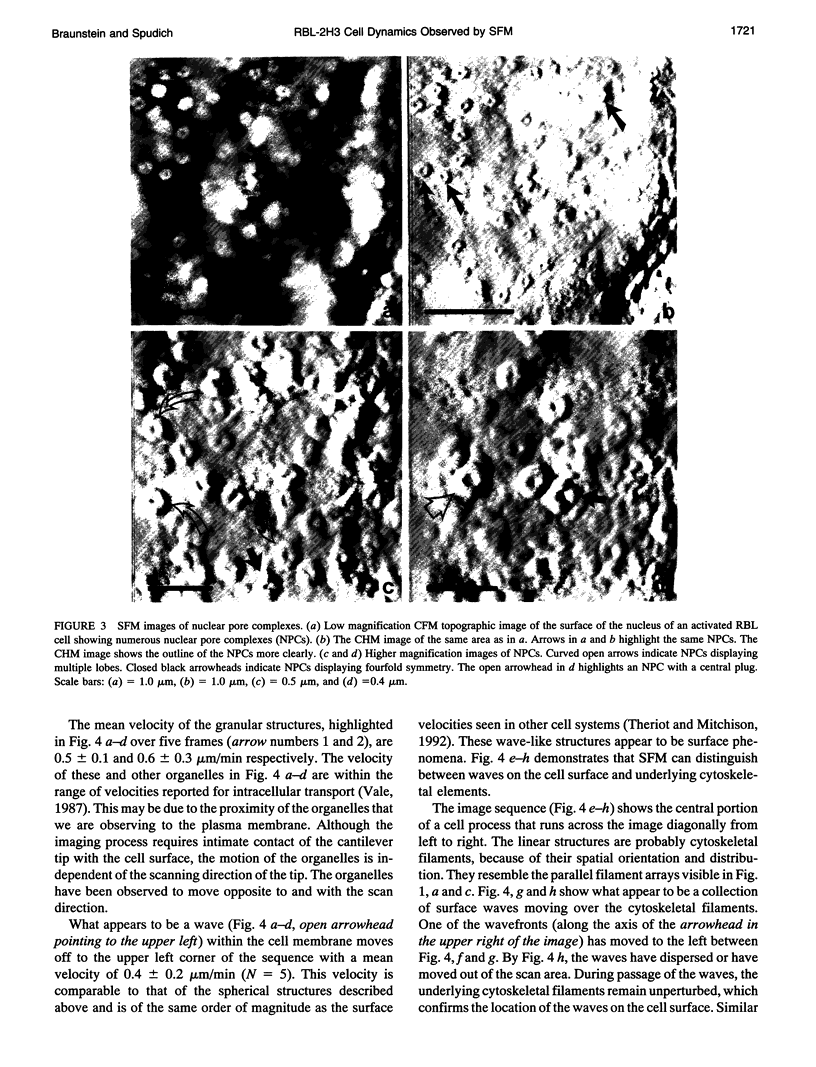



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akey C. W., Radermacher M. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J Cell Biol. 1993 Jul;122(1):1–19. doi: 10.1083/jcb.122.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey C. W. Visualization of transport-related configurations of the nuclear pore transporter. Biophys J. 1990 Aug;58(2):341–355. doi: 10.1016/S0006-3495(90)82381-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. J., Dong X. F., O'Neill T. E., Yau P., Kowalczykowski S. C., Gatewood J., Balhorn R., Bradbury E. M. Atomic force microscope measurements of nucleosome cores assembled along defined DNA sequences. Biochemistry. 1993 Aug 24;32(33):8390–8396. doi: 10.1021/bi00084a002. [DOI] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Chang L., Kious T., Yorgancioglu M., Keller D., Pfeiffer J. Cytoskeleton of living, unstained cells imaged by scanning force microscopy. Biophys J. 1993 Apr;64(4):1282–1286. doi: 10.1016/S0006-3495(93)81493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman J. R., Lenn M., Rees D. A. Coupling of cytoskeleton functions for fibroblast locomotion. Eur J Cell Biol. 1985 Mar;36(2):182–194. [PubMed] [Google Scholar]
- Dabora S. L., Sheetz M. P. Cultured cell extracts support organelle movement on microtubules in vitro. Cell Motil Cytoskeleton. 1988;10(4):482–495. doi: 10.1002/cm.970100405. [DOI] [PubMed] [Google Scholar]
- De Grooth B. G., Putman C. A. High-resolution imaging of chromosome-related structures by atomic force microscopy. J Microsc. 1992 Dec;168(Pt 3):239–247. doi: 10.1111/j.1365-2818.1992.tb03266.x. [DOI] [PubMed] [Google Scholar]
- Forscher P., Smith S. J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J Cell Biol. 1988 Oct;107(4):1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. L., Ryan T. A., Jones B. D., Smith S. J., Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993 Aug 12;364(6438):639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- Goldberg M. W., Allen T. D. High resolution scanning electron microscopy of the nuclear envelope: demonstration of a new, regular, fibrous lattice attached to the baskets of the nucleoplasmic face of the nuclear pores. J Cell Biol. 1992 Dec;119(6):1429–1440. doi: 10.1083/jcb.119.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma H. G., Bezanilla M., Zenhausern F., Adrian M., Sinsheimer R. L. Atomic force microscopy of DNA in aqueous solutions. Nucleic Acids Res. 1993 Feb 11;21(3):505–512. doi: 10.1093/nar/21.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E., Haydon P. G., Sakaguchi D. S. Actin filament dynamics in living glial cells imaged by atomic force microscopy. Science. 1992 Sep 25;257(5078):1944–1946. doi: 10.1126/science.1411511. [DOI] [PubMed] [Google Scholar]
- Hinshaw J. E., Carragher B. O., Milligan R. A. Architecture and design of the nuclear pore complex. Cell. 1992 Jun 26;69(7):1133–1141. doi: 10.1016/0092-8674(92)90635-p. [DOI] [PubMed] [Google Scholar]
- Hoh J. H., Sosinsky G. E., Revel J. P., Hansma P. K. Structure of the extracellular surface of the gap junction by atomic force microscopy. Biophys J. 1993 Jul;65(1):149–163. doi: 10.1016/S0006-3495(93)81074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörber J. K., Häberle W., Ohnesorge F., Binnig G., Liebich H. G., Czerny C. P., Mahnel H., Mayr A. Investigation of living cells in the nanometer regime with the scanning force microscope. Scanning Microsc. 1992 Dec;6(4):919–930. [PubMed] [Google Scholar]
- Kasas S., Gotzos V., Celio M. R. Observation of living cells using the atomic force microscope. Biophys J. 1993 Feb;64(2):539–544. doi: 10.1016/S0006-3495(93)81396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. W., Keller D. J., Bear D., Vasenka J., Bustamante C. Electrodeposition procedure of E. coli RNA polymerase onto gold and deposition of E. coli RNA polymerase onto mica for observation with scanning force microscopy. Ultramicroscopy. 1992 Jul;42-44(Pt B):1173–1180. doi: 10.1016/0304-3991(92)90420-o. [DOI] [PubMed] [Google Scholar]
- Liu Z. Y., Young J. I., Elson E. L. Rat basophilic leukemia cells stiffen when they secrete. J Cell Biol. 1987 Dec;105(6 Pt 2):2933–2943. doi: 10.1083/jcb.105.6.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubchenko Y. L., Oden P. I., Lampner D., Lindsay S. M., Dunker K. A. Atomic force microscopy of DNA and bacteriophage in air, water and propanol: the role of adhesion forces. Nucleic Acids Res. 1993 Mar 11;21(5):1117–1123. doi: 10.1093/nar/21.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Murray M. N., Hansma H. G., Bezanilla M., Sano T., Ogletree D. F., Kolbe W., Smith C. L., Cantor C. R., Spengler S., Hansma P. K. Atomic force microscopy of biochemically tagged DNA. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3811–3814. doi: 10.1073/pnas.90.9.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Seagrave J., Stump R. F., Pfeiffer J. R., Deanin G. G. Signal transduction and cellular response in RBL-2H3 mast cells. Prog Allergy. 1988;42:185–245. [PubMed] [Google Scholar]
- Parpura V., Haydon P. G., Henderson E. Three-dimensional imaging of living neurons and glia with the atomic force microscope. J Cell Sci. 1993 Feb;104(Pt 2):427–432. doi: 10.1242/jcs.104.2.427. [DOI] [PubMed] [Google Scholar]
- Rees W. A., Keller R. W., Vesenka J. P., Yang G., Bustamante C. Evidence of DNA bending in transcription complexes imaged by scanning force microscopy. Science. 1993 Jun 11;260(5114):1646–1649. doi: 10.1126/science.8503010. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P., McGivney A., Barsumian E. L., Crews F. T., Hirata F., Axelrod J. Variants of the rat basophilic leukemia cell line for the study of histamine release. Fed Proc. 1982 Jan;41(1):30–34. [PubMed] [Google Scholar]
- Soranno T., Bell E. Cytostructural dynamics of spreading and translocating cells. J Cell Biol. 1982 Oct;95(1):127–136. doi: 10.1083/jcb.95.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich A., Meyer T., Stryer L. Association of the beta isoform of protein kinase C with vimentin filaments. Cell Motil Cytoskeleton. 1992;22(4):250–256. doi: 10.1002/cm.970220405. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J. Comparison of actin and cell surface dynamics in motile fibroblasts. J Cell Biol. 1992 Oct;119(2):367–377. doi: 10.1083/jcb.119.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Milligan R. A. A large particle associated with the perimeter of the nuclear pore complex. J Cell Biol. 1982 Apr;93(1):63–75. doi: 10.1083/jcb.93.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D. Intracellular transport using microtubule-based motors. Annu Rev Cell Biol. 1987;3:347–378. doi: 10.1146/annurev.cb.03.110187.002023. [DOI] [PubMed] [Google Scholar]
- Vesenka J., Manne S., Giberson R., Marsh T., Henderson E. Colloidal gold particles as an incompressible atomic force microscope imaging standard for assessing the compressibility of biomolecules. Biophys J. 1993 Sep;65(3):992–997. doi: 10.1016/S0006-3495(93)81171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenhorn A. L., Drake B., Prater C. B., Gould S. A., Hansma P. K., Ohnesorge F., Egger M., Heyn S. P., Gaub H. E. Immobilized proteins in buffer imaged at molecular resolution by atomic force microscopy. Biophys J. 1990 Nov;58(5):1251–1258. doi: 10.1016/S0006-3495(90)82465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenhausern F., Adrian M., ten Heggeler-Bordier B., Emch R., Jobin M., Taborelli M., Descouts P. Imaging of DNA by scanning force microscopy. J Struct Biol. 1992 Jan-Feb;108(1):69–73. doi: 10.1016/1047-8477(92)90008-x. [DOI] [PubMed] [Google Scholar]