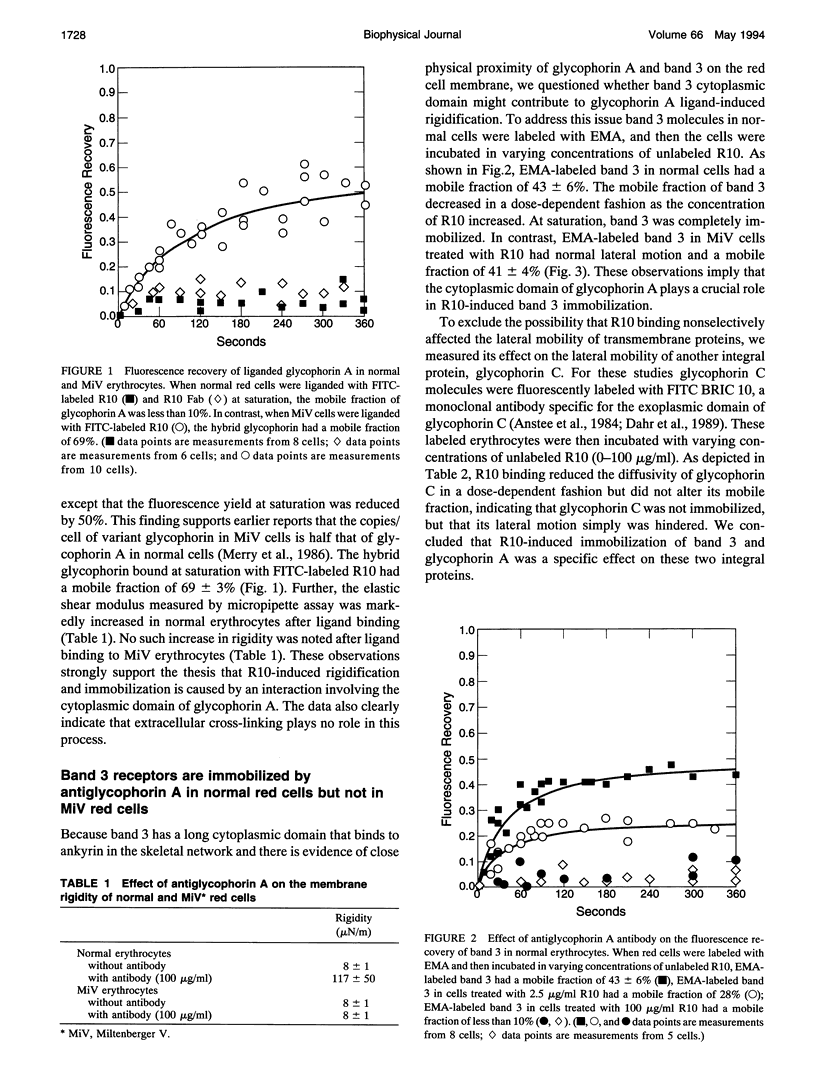
Abstract
The ability of transmembrane receptor proteins to change their association with the cytoskeleton in response to ligand binding seems to be a key mechanism of signal transduction across membranes. To investigate the molecular features of this mechanism we have used the red cell membrane as a model system to study signal transduction through the integral protein, glycophorin A. In these studies the lateral mobility of integral proteins was measured in situ by fluorescence recovery after photobleaching, and membrane rigidity was characterized by micropipette aspiration technique. We found that binding either a monoclonal antibody or its monovalent Fab to the exoplasmic domain of glycophorin A in normal red cells immobilized the receptor and rigidified the membrane. Further, immobilization and rigidification did not occur when antibodies were bound to Miltenberger V cells containing a mutant form of glycophorin A lacking the cytoplasmic domain. These results imply that the site of the immobilization/rigidification lies within the membrane skeletal structure, not in exofacial receptor crosslinking, and requires the extended cytoplasmic domain of normal glycophorin A. In addition, we found that glycophorin A immobilization and membrane skeletal rigidification were accompanied by immobilization of band 3 receptors. This unexpected result indicates a cooperative coupling between liganded glycophorin A, band 3, and the membrane skeleton. We speculate that cooperation of this type may represent a general mechanism for cytoskeletal linkage and transformation initiated by receptors with short cytoplasmic sequences, such as integrins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstee D. J., Edwards P. A. Monoclonal antibodies to human erythrocytes. Eur J Immunol. 1982 Mar;12(3):228–232. doi: 10.1002/eji.1830120311. [DOI] [PubMed] [Google Scholar]
- Anstee D. J., Parsons S. F., Ridgwell K., Tanner M. J., Merry A. H., Thomson E. E., Judson P. A., Johnson P., Bates S., Fraser I. D. Two individuals with elliptocytic red cells apparently lack three minor erythrocyte membrane sialoglycoproteins. Biochem J. 1984 Mar 1;218(2):615–619. doi: 10.1042/bj2180615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Bigbee W. L., Vanderlaan M., Fong S. S., Jensen R. H. Monoclonal antibodies specific for the M- and N-forms of human glycophorin A. Mol Immunol. 1983 Dec;20(12):1353–1362. doi: 10.1016/0161-5890(83)90166-9. [DOI] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N. Red blood cell glycophorins. Blood. 1992 Oct 15;80(8):1869–1879. [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N., Shohet S. B. Erythrocyte membrane rigidity induced by glycophorin A-ligand interaction. Evidence for a ligand-induced association between glycophorin A and skeletal proteins. J Clin Invest. 1985 Jun;75(6):1919–1926. doi: 10.1172/JCI111907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Reid M. E., Jensen R. H., Mohandas N. Signal transduction by glycophorin A: role of extracellular and cytoplasmic domains in a modulatable process. J Cell Biol. 1988 Oct;107(4):1351–1357. doi: 10.1083/jcb.107.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahr W., Blanchard D., Kiedrowski S., Poschmann A., Cartron J. P., Moulds J. J. High-frequency antigens of human erythrocyte membrane sialoglycoproteins. VI. Monoclonal antibodies reacting with the N-terminal domain of glycophorin C. Biol Chem Hoppe Seyler. 1989 Aug;370(8):849–854. doi: 10.1515/bchm3.1989.370.2.849. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. A solid-liquid composite model of the red cell membrane. J Membr Biol. 1977 Jan 28;30(4):351–362. doi: 10.1007/BF01869676. [DOI] [PubMed] [Google Scholar]
- Evans E., Mohandas N., Leung A. Static and dynamic rigidities of normal and sickle erythrocytes. Major influence of cell hemoglobin concentration. J Clin Invest. 1984 Feb;73(2):477–488. doi: 10.1172/JCI111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T. M., Haest C. W., Stöhr M., Kamp D., Deuticke B. Selective alteration of erythrocyte deformabiliby by SH-reagents: evidence for an involvement of spectrin in membrane shear elasticity. Biochim Biophys Acta. 1978 Jul 4;510(2):270–282. doi: 10.1016/0005-2736(78)90027-5. [DOI] [PubMed] [Google Scholar]
- Gilligan D. M., Bennett V. The junctional complex of the membrane skeleton. Semin Hematol. 1993 Jan;30(1):74–83. [PubMed] [Google Scholar]
- Golan D. E., Brown C. S., Cianci C. M., Furlong S. T., Caulfield J. P. Schistosomula of Schistosoma mansoni use lysophosphatidylcholine to lyse adherent human red blood cells and immobilize red cell membrane components. J Cell Biol. 1986 Sep;103(3):819–828. doi: 10.1083/jcb.103.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. H., Blumenfeld O. O. Identification of recombination events resulting in three hybrid genes encoding human MiV, MiV(J.L.), and Sta glycophorins. Blood. 1991 Apr 15;77(8):1813–1820. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Khodadad J. K., Weinstein R. S. Band 3 protein of the red cell membrane of the llama: crosslinking and cleavage of the cytoplasmic domain. Biochem Biophys Res Commun. 1985 Jul 16;130(1):493–499. doi: 10.1016/0006-291x(85)90444-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Derick L. H. Molecular anatomy of the red blood cell membrane skeleton: structure-function relationships. Semin Hematol. 1992 Oct;29(4):231–243. [PubMed] [Google Scholar]
- Liu S. C., Palek J. Spectrin tetramer-dimer equilibrium and the stability of erythrocyte membrane skeletons. Nature. 1980 Jun 19;285(5766):586–588. doi: 10.1038/285586a0. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Zhai S., Palek J., Golan D. E., Amato D., Hassan K., Nurse G. T., Babona D., Coetzer T., Jarolim P. Molecular defect of the band 3 protein in southeast Asian ovalocytosis. N Engl J Med. 1990 Nov 29;323(22):1530–1538. doi: 10.1056/NEJM199011293232205. [DOI] [PubMed] [Google Scholar]
- Merry A. H., Hodson C., Thomson E., Mallinson G., Anstee D. J. The use of monoclonal antibodies to quantify the levels of sialoglycoproteins alpha and delta and variant sialoglycoproteins in human erythrocyte membranes. Biochem J. 1986 Jan 1;233(1):93–98. doi: 10.1042/bj2330093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Chasis J. A. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol. 1993 Jul;30(3):171–192. [PubMed] [Google Scholar]
- Mohandas N., Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Lie-Injo L. E., Friedman M., Mak J. W. Rigid membranes of Malayan ovalocytes: a likely genetic barrier against malaria. Blood. 1984 Jun;63(6):1385–1392. [PubMed] [Google Scholar]
- Mohandas N., Winardi R., Knowles D., Leung A., Parra M., George E., Conboy J., Chasis J. Molecular basis for membrane rigidity of hereditary ovalocytosis. A novel mechanism involving the cytoplasmic domain of band 3. J Clin Invest. 1992 Feb;89(2):686–692. doi: 10.1172/JCI115636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Influence of temperature and cholesterol on the rotational diffusion of band 3 in the human erythrocyte membrane. Biochemistry. 1979 Aug 7;18(16):3457–3465. doi: 10.1021/bi00583a004. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Saul A., Lamont G., Sawyer W. H., Kidson C. Decreased membrane deformability in Melanesian ovalocytes from Papua New Guinea. J Cell Biol. 1984 Apr;98(4):1348–1354. doi: 10.1083/jcb.98.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield A. E., Tanner M. J., Pinder J. C., Clough B., Bayley P. M., Nash G. B., Dluzewski A. R., Reardon D. M., Cox T. M., Wilson R. J. Basis of unique red cell membrane properties in hereditary ovalocytosis. J Mol Biol. 1992 Feb 20;223(4):949–958. doi: 10.1016/0022-2836(92)90254-h. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Smith D. K., Palek J. Sulfhydryl reagents induce altered spectrin self-association, skeletal instability, and increased thermal sensitivity of red cells. Blood. 1983 Dec;62(6):1190–1196. [PubMed] [Google Scholar]
- Smith J. E., Mohandas N., Clark M. R., Greenquist A. C., Shohet S. B. Deformability and spectrin properties in three types of elongated red cells. Am J Hematol. 1980;8(1):1–13. doi: 10.1002/ajh.2830080102. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Bruce L., Martin P. G., Rearden D. M., Jones G. L. Melanesian hereditary ovalocytes have a deletion in red cell band 3. Blood. 1991 Nov 15;78(10):2785–2786. [PubMed] [Google Scholar]
- Tanner M. J. Molecular and cellular biology of the erythrocyte anion exchanger (AE1). Semin Hematol. 1993 Jan;30(1):34–57. [PubMed] [Google Scholar]
- Telen M. J., Chasis J. A. Relationship of the human erythrocyte Wrb antigen to an interaction between glycophorin A and band 3. Blood. 1990 Aug 15;76(4):842–848. [PubMed] [Google Scholar]
- Vignal A., Rahuel C., London J., Cherif Zahar B., Schaff S., Hattab C., Okubo Y., Cartron J. P. A novel gene member of the human glycophorin A and B gene family. Molecular cloning and expression. Eur J Biochem. 1990 Aug 17;191(3):619–625. doi: 10.1111/j.1432-1033.1990.tb19166.x. [DOI] [PubMed] [Google Scholar]
- Wolf D. E. Designing, building, and using a fluorescence recovery after photobleaching instrument. Methods Cell Biol. 1989;30:271–306. doi: 10.1016/s0091-679x(08)60983-8. [DOI] [PubMed] [Google Scholar]
- Yguerabide J., Schmidt J. A., Yguerabide E. E. Lateral mobility in membranes as detected by fluorescence recovery after photobleaching. Biophys J. 1982 Oct;40(1):69–75. doi: 10.1016/S0006-3495(82)84459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]




