Abstract
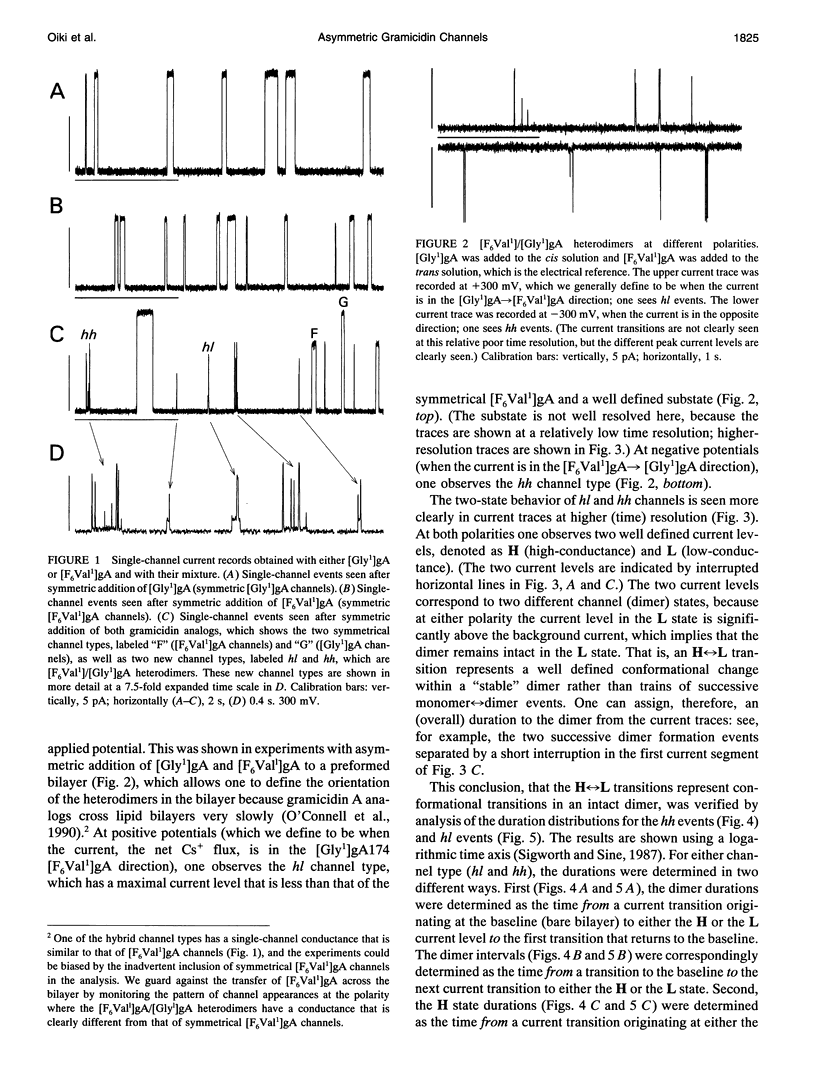
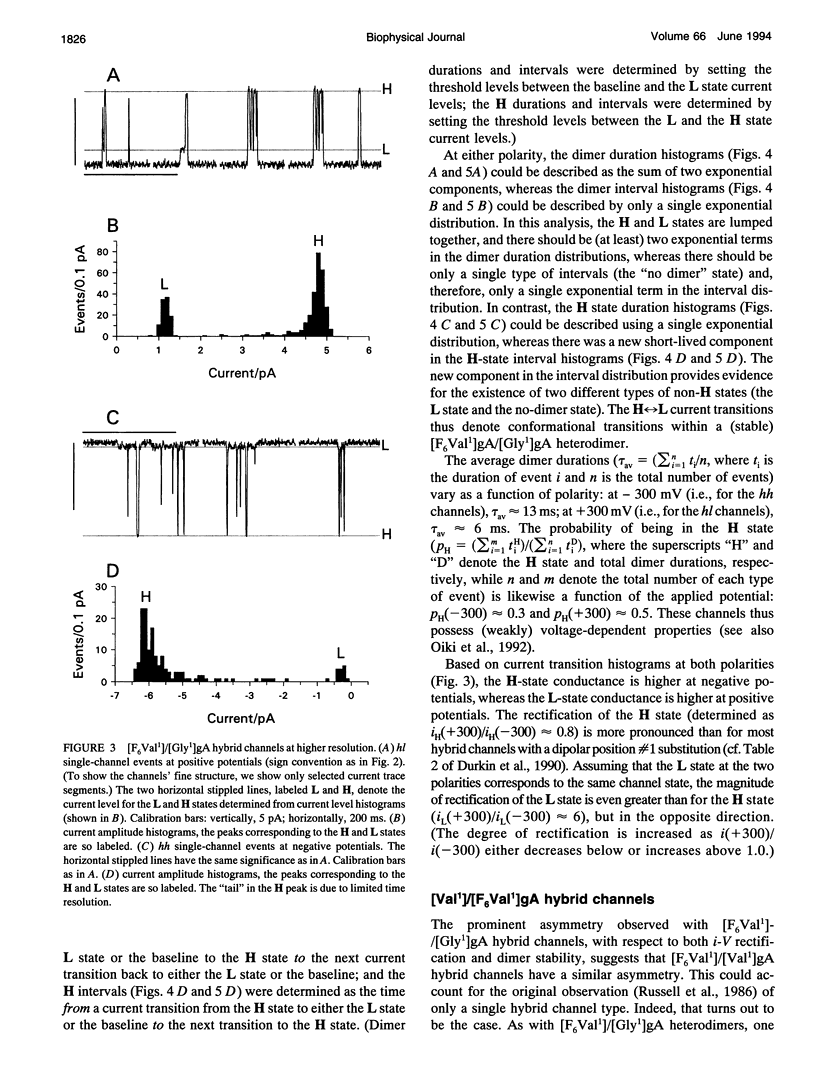
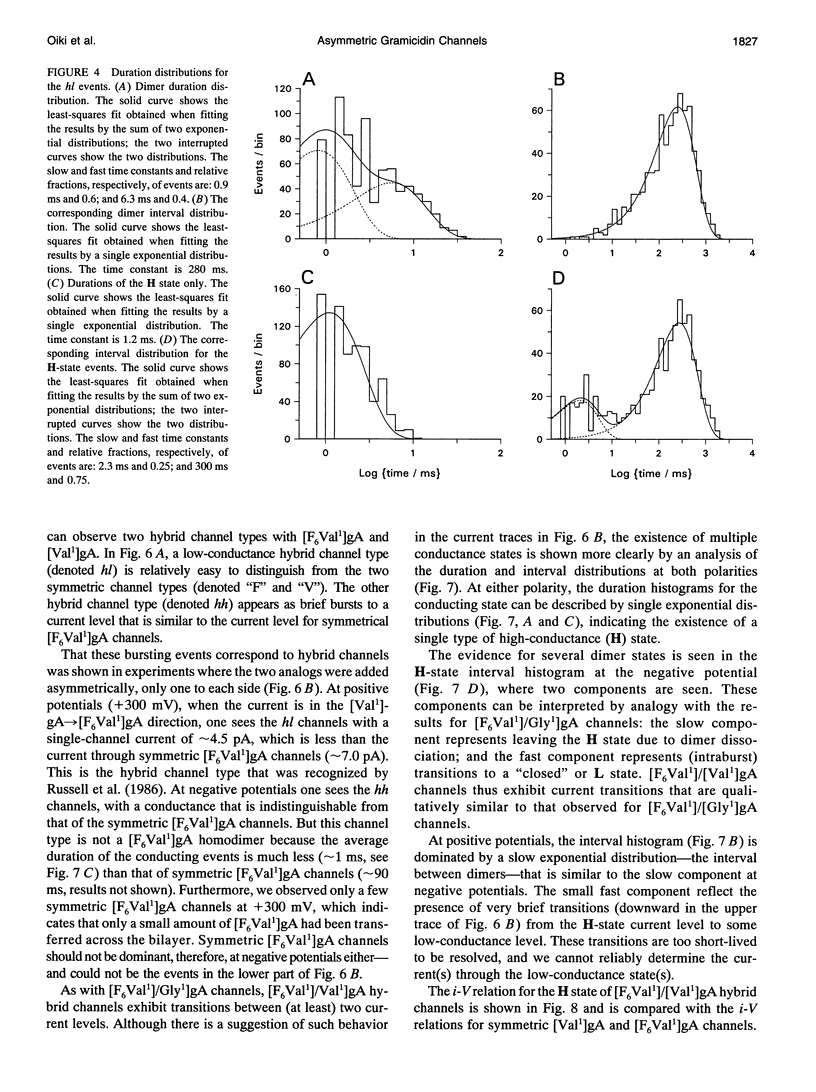
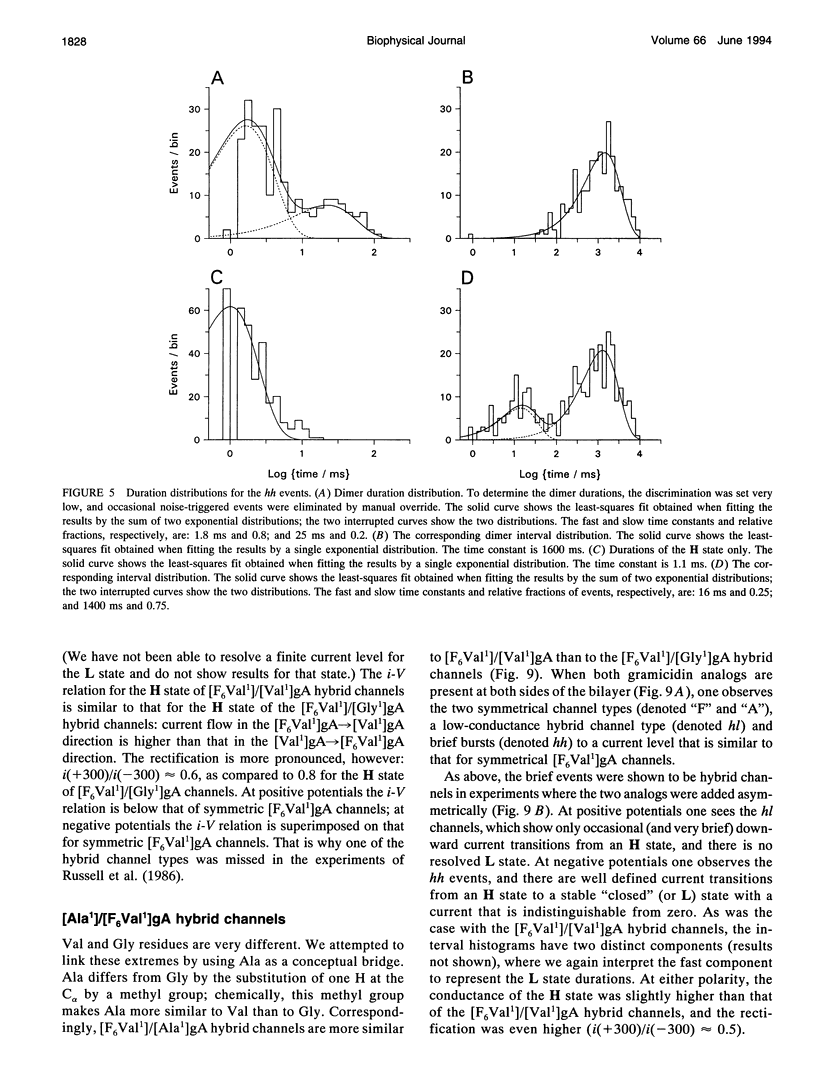
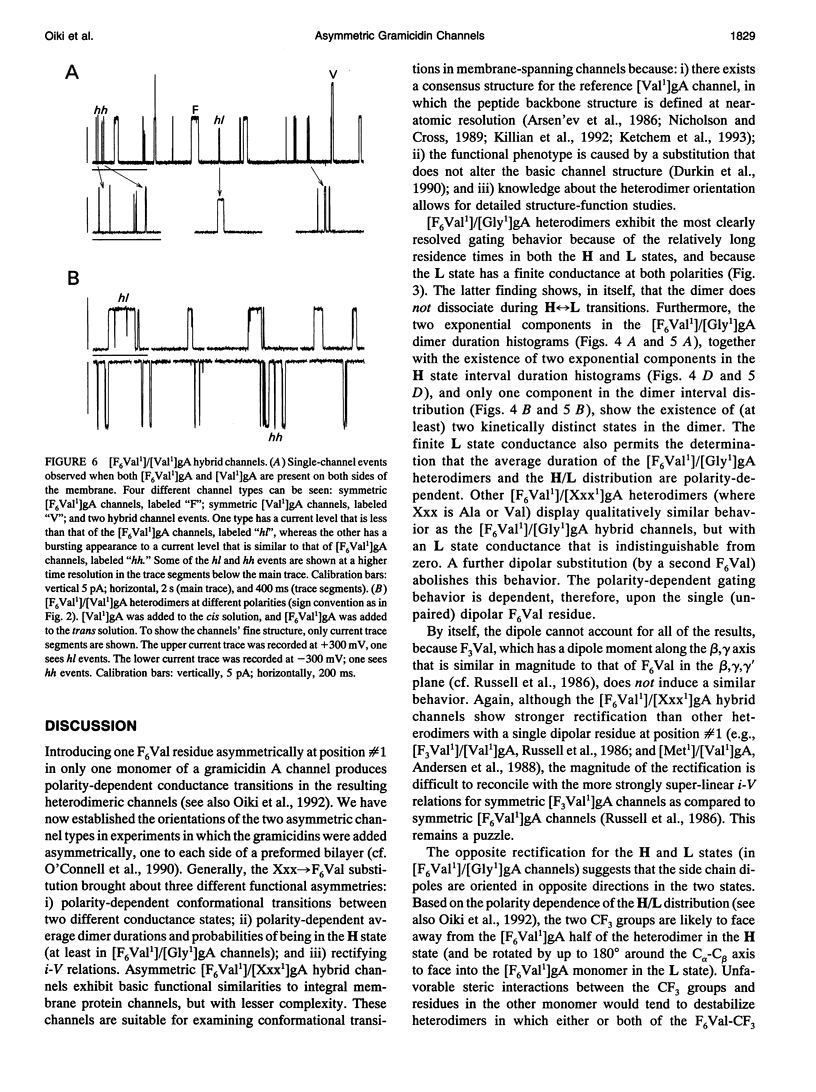
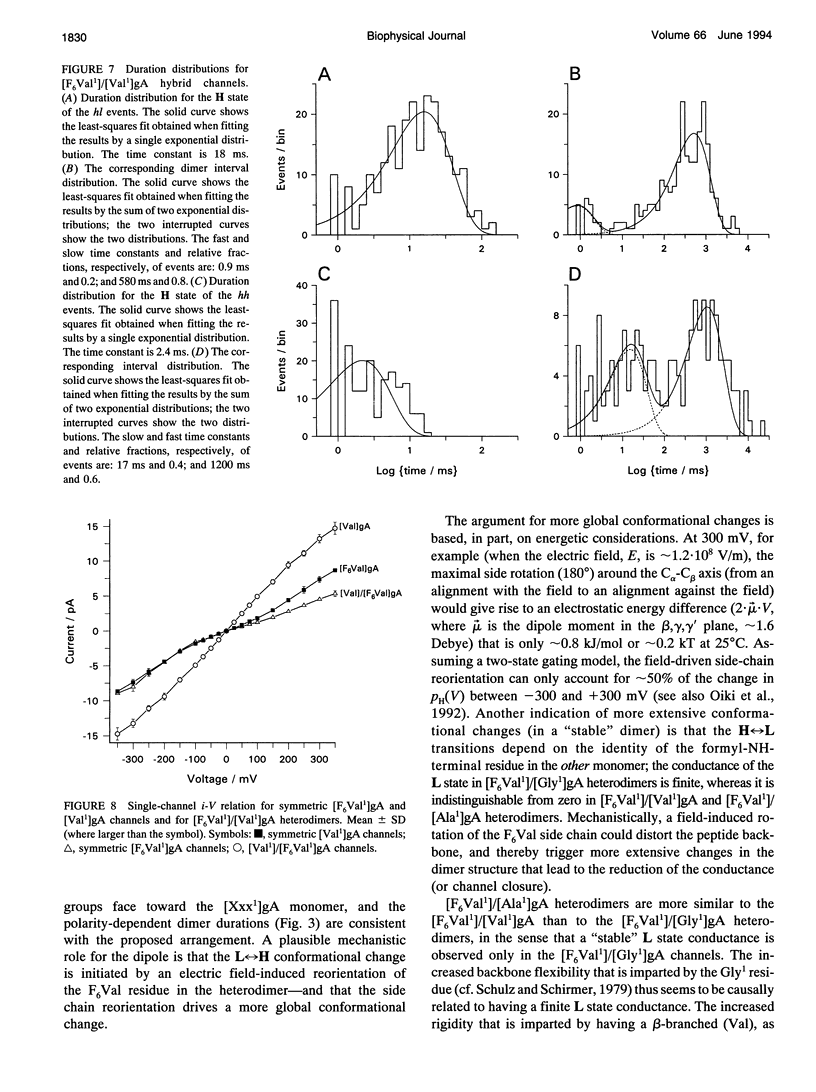
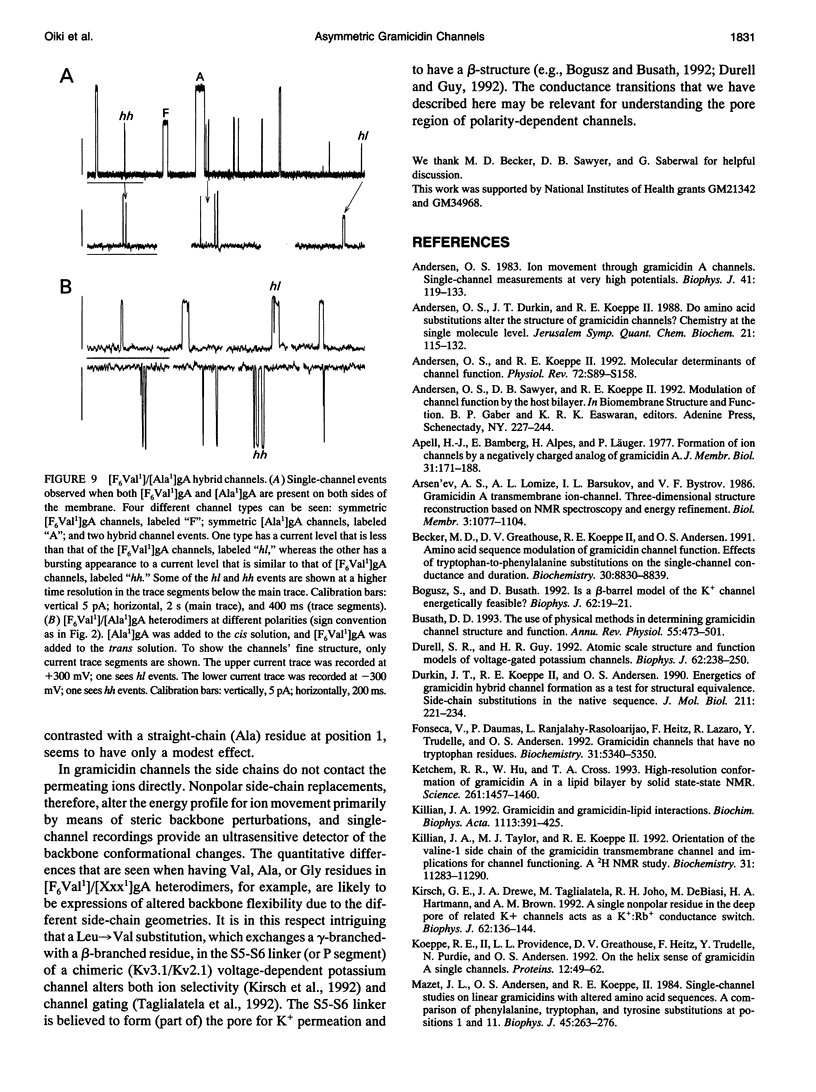
Substitution of Val1 by 4,4,4,4',4',4'-F6Val in [Val1]gramicidin A ([Val1]gA) produces channels in which the effects of amino acid replacements on dimer stability and ion permeation are nonadditive. If only one Val1 (in a symmetric [Val1]gA channel) is substituted by F6Val, the resulting heterodimeric channels are destabilized relative to both homodimeric parent channels and the single-channel conductance of the heterodimeric channels is reduced relative to the parent channels (Russell, E. W. B., L. B. Weiss, F. I. Navetta, R. E. Koeppe II, and O. S. Andersen. 1986. Single-channel studies on linear gramicidins with altered amino acid side chains. Effects of altering the polarity of the side chain at position #1 in gramicidin A. Biophys. J. 49:673; Durkin, J. T., R. E. Koeppe II, and O. S. Andersen. 1990. Energetics of gramicidin hybrid channel formation as a test for structural equivalence. Side-chain substitutions in the native sequence. J. Mol. Biol. 211:221-234). To understand the basis for this destabilization, we have examined further the characteristics of [F6Val1]/[Xxx1]gA heterodimers, where Xxx = Gly, Val, and Ala. These heterodimeric channels show rapid current transitions between (at least) two current levels and display asymmetric i-V characteristics. The orientation of the heterodimers relative to the applied potential was determined by asymmetric addition of the gramicidin analogs, one to each side of a preformed bilayer. The current transitions are most clearly illustrated for [F6Val1]/[Gly1]gA heterodimers, which possess two finite and well defined current levels. Based on the existence of these two conductance states and the analysis of duration and interval distributions, we conclude that the transitions between the two current levels correspond to conformational transitions in "stable" heterodimers. In the case of [F6Val1]/[Val1]gA and [F6Val1]/[Ala1]gA heterodimers, the low-conductance state is indistinguishable from zero. The two (or more) conductance states presumably correspond to different orientations of the dipolar F6Val1 side chain. The distribution between the high- and the low-conductance states varies as a function of potential in [F6Val1]/[Gly1]gA channels. These characteristics cause the [F6Val1]/nonpolar (Val, Ala, Gly)gA hybrid channels to serve as a "simple" model for understanding gating transitions in membrane-spanning channels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S. Ion movement through gramicidin A channels. Single-channel measurements at very high potentials. Biophys J. 1983 Feb;41(2):119–133. doi: 10.1016/S0006-3495(83)84414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Koeppe R. E., 2nd Molecular determinants of channel function. Physiol Rev. 1992 Oct;72(4 Suppl):S89–158. doi: 10.1152/physrev.1992.72.suppl_4.S89. [DOI] [PubMed] [Google Scholar]
- Apell H. J., Bamberg E., Alpes H., Läuger P. Formation of ion channels by a negatively charged analog of gramicidin A. J Membr Biol. 1977 Feb 24;31(1-2):171–188. doi: 10.1007/BF01869403. [DOI] [PubMed] [Google Scholar]
- Becker M. D., Greathouse D. V., Koeppe R. E., 2nd, Andersen O. S. Amino acid sequence modulation of gramicidin channel function: effects of tryptophan-to-phenylalanine substitutions on the single-channel conductance and duration. Biochemistry. 1991 Sep 10;30(36):8830–8839. doi: 10.1021/bi00100a015. [DOI] [PubMed] [Google Scholar]
- Bogusz S., Busath D. Is a beta-barrel model of the K+ channel energetically feasible? Biophys J. 1992 Apr;62(1):19–21. doi: 10.1016/S0006-3495(92)81765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busath D. D. The use of physical methods in determining gramicidin channel structure and function. Annu Rev Physiol. 1993;55:473–501. doi: 10.1146/annurev.ph.55.030193.002353. [DOI] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R. Atomic scale structure and functional models of voltage-gated potassium channels. Biophys J. 1992 Apr;62(1):238–250. doi: 10.1016/S0006-3495(92)81809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin J. T., Koeppe R. E., 2nd, Andersen O. S. Energetics of gramicidin hybrid channel formation as a test for structural equivalence. Side-chain substitutions in the native sequence. J Mol Biol. 1990 Jan 5;211(1):221–234. doi: 10.1016/0022-2836(90)90022-E. [DOI] [PubMed] [Google Scholar]
- Fonseca V., Daumas P., Ranjalahy-Rasoloarijao L., Heitz F., Lazaro R., Trudelle Y., Andersen O. S. Gramicidin channels that have no tryptophan residues. Biochemistry. 1992 Jun 16;31(23):5340–5350. doi: 10.1021/bi00138a014. [DOI] [PubMed] [Google Scholar]
- Ketchem R. R., Hu W., Cross T. A. High-resolution conformation of gramicidin A in a lipid bilayer by solid-state NMR. Science. 1993 Sep 10;261(5127):1457–1460. doi: 10.1126/science.7690158. [DOI] [PubMed] [Google Scholar]
- Killian J. A. Gramicidin and gramicidin-lipid interactions. Biochim Biophys Acta. 1992 Dec 11;1113(3-4):391–425. doi: 10.1016/0304-4157(92)90008-x. [DOI] [PubMed] [Google Scholar]
- Killian J. A., Taylor M. J., Koeppe R. E., 2nd Orientation of the valine-1 side chain of the gramicidin transmembrane channel and implications for channel functioning. A 2H NMR study. Biochemistry. 1992 Nov 24;31(46):11283–11290. doi: 10.1021/bi00161a004. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Drewe J. A., Taglialatela M., Joho R. H., DeBiasi M., Hartmann H. A., Brown A. M. A single nonpolar residue in the deep pore of related K+ channels acts as a K+:Rb+ conductance switch. Biophys J. 1992 Apr;62(1):136–144. doi: 10.1016/S0006-3495(92)81800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Providence L. L., Greathouse D. V., Heitz F., Trudelle Y., Purdie N., Andersen O. S. On the helix sense of gramicidin A single channels. Proteins. 1992 Jan;12(1):49–62. doi: 10.1002/prot.340120107. [DOI] [PubMed] [Google Scholar]
- Mazet J. L., Andersen O. S., Koeppe R. E., 2nd Single-channel studies on linear gramicidins with altered amino acid sequences. A comparison of phenylalanine, tryptophane, and tyrosine substitutions at positions 1 and 11. Biophys J. 1984 Jan;45(1):263–276. doi: 10.1016/S0006-3495(84)84153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson L. K., Cross T. A. Gramicidin cation channel: an experimental determination of the right-handed helix sense and verification of beta-type hydrogen bonding. Biochemistry. 1989 Nov 28;28(24):9379–9385. doi: 10.1021/bi00450a019. [DOI] [PubMed] [Google Scholar]
- O'Connell A. M., Koeppe R. E., 2nd, Andersen O. S. Kinetics of gramicidin channel formation in lipid bilayers: transmembrane monomer association. Science. 1990 Nov 30;250(4985):1256–1259. doi: 10.1126/science.1700867. [DOI] [PubMed] [Google Scholar]
- Oiki S., Koeppe R. E., 2nd, Andersen O. S. A dipolar amino acid substitution induces voltage-dependent transitions between two stable conductance states in gramicidin channels. Biophys J. 1992 Apr;62(1):28–30. doi: 10.1016/S0006-3495(92)81768-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. W., Weiss L. B., Navetta F. I., Koeppe R. E., 2nd, Andersen O. S. Single-channel studies on linear gramicidins with altered amino acid side chains. Effects of altering the polarity of the side chain at position 1 in gramicidin A. Biophys J. 1986 Mar;49(3):673–686. doi: 10.1016/S0006-3495(86)83694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARGES R., WITKOP B. GRAMICIDIN A. V. THE STRUCTURE OF VALINE- AND ISOLEUCINE-GRAMICIDIN A. J Am Chem Soc. 1965 May 5;87:2011–2020. doi: 10.1021/ja01087a027. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela M., Kirsch G. E., VanDongen A. M., Drewe J. A., Hartmann H. A., Joho R. H., Stefani E., Brown A. M. Gating currents from a delayed rectifier K+ channel with altered pore structure and function. Biophys J. 1992 Apr;62(1):34–36. doi: 10.1016/S0006-3495(92)81770-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch W., Stryer L. The dimeric nature of the gramicidin A transmembrane channel: conductance and fluorescence energy transfer studies of hybrid channels. J Mol Biol. 1977 Jun 15;113(1):89–102. doi: 10.1016/0022-2836(77)90042-0. [DOI] [PubMed] [Google Scholar]
- Weiss L. B., Koeppe R. E., 2nd Semisynthesis of linear gramicidins using diphenyl phosphorazidate (DPPA). Int J Pept Protein Res. 1985 Sep;26(3):305–310. doi: 10.1111/j.1399-3011.1985.tb03209.x. [DOI] [PubMed] [Google Scholar]


