Abstract
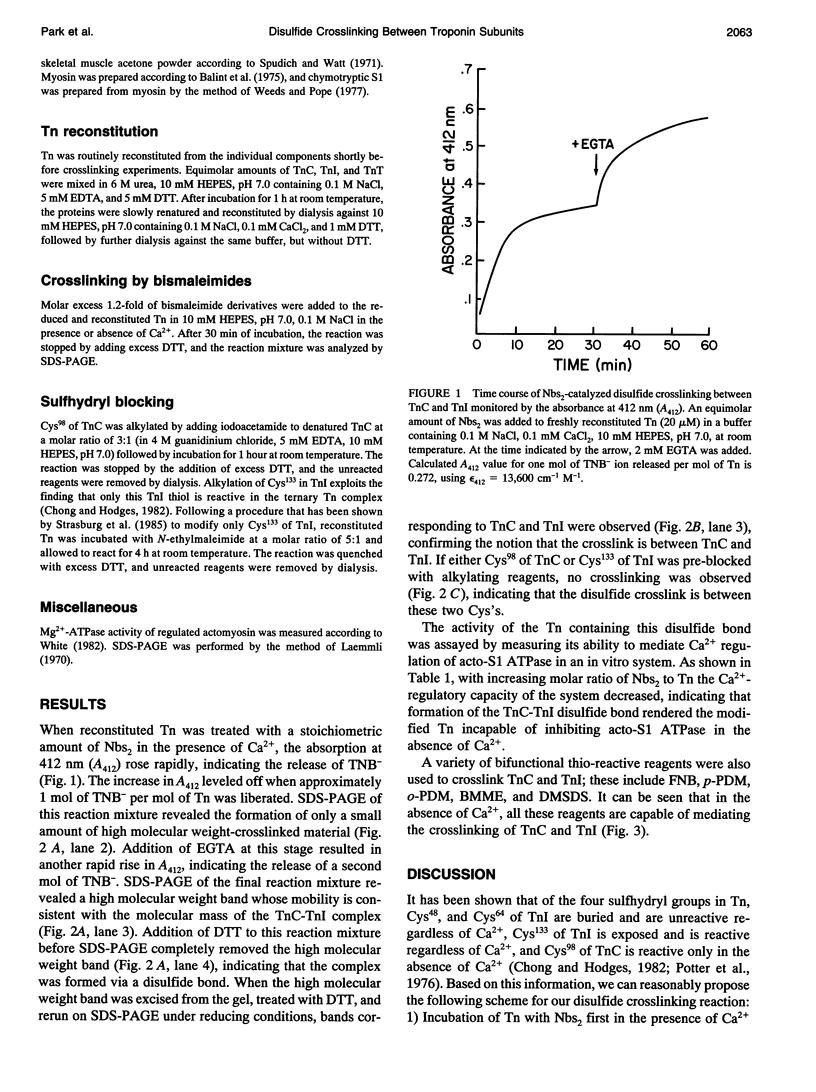
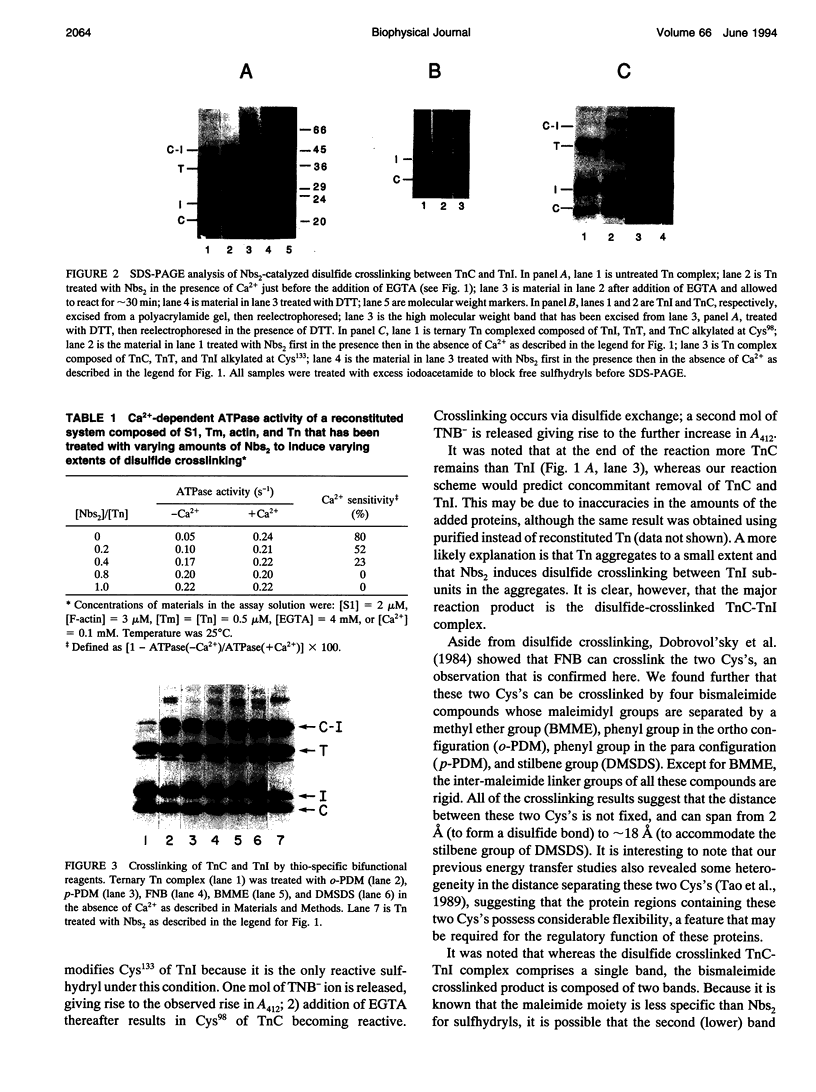
Various thio-reactive bifunctional crosslinkers as well as 5,5'-dithiobis(2-nitrobenzoate)-mediated disulfide bond formation were used to crosslink troponin-C and troponin-I, the Ca(2+)-binding and inhibitory subunits of troponin, respectively. In all cases, substantial crosslinking was obtained when the reactions were carried out in the absence of Ca2+. No disulfide crosslinking occurred if either Cys98 of TnC, or Cys133 of TnI were blocked, indicating that these thiols are involved in the crosslinking. Troponin containing the disulfide crosslink is no longer capable of regulating actomyosin ATPase activity in a Ca(2+)-dependent manner. Our results suggest that the relative movement between the Cys98 region of TnC and the Cys133 region of TnI is required for the Ca(2+)-regulatory process in skeletal muscle.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bálint M., Sréter F. A., Wolf I., Nagy B., Gergely J. The substructure of heavy meromyosin. The effect of Ca2+ and Mg2+ on the tryptic fragmentation of heavy meromyosin. J Biol Chem. 1975 Aug 10;250(15):6168–6177. [PubMed] [Google Scholar]
- Chong P. C., Hodges R. S. Proximity of sulfhydryl groups to the sites of interaction between components of the troponin complex from rabbit skeletal muscle. J Biol Chem. 1982 Mar 10;257(5):2549–2555. [PubMed] [Google Scholar]
- Dobrovol'sky A. B., Gusev N. B., Friedrich P. Crosslinking of troponin complex with 1,3-difluoro-4,6-dinitrobenzene. Identification of the crosslink formed between troponin C and troponin I in the absence of Ca2+. Biochim Biophys Acta. 1984 Sep 11;789(2):144–151. doi: 10.1016/0167-4838(84)90198-5. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Grabarek Z., Drabikowski W., Leavis P. C., Rosenfeld S. S., Gergely J. Proteolytic fragments of troponin C. Interactions with the other troponin subunits and biological activity. J Biol Chem. 1981 Dec 25;256(24):13121–13127. [PubMed] [Google Scholar]
- Grabarek Z., Tao T., Gergely J. Molecular mechanism of troponin-C function. J Muscle Res Cell Motil. 1992 Aug;13(4):383–393. doi: 10.1007/BF01738034. [DOI] [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Purification and properties of the components from troponin. J Biol Chem. 1973 Mar 25;248(6):2125–2133. [PubMed] [Google Scholar]
- Herzberg O., James M. N. Structure of the calcium regulatory muscle protein troponin-C at 2.8 A resolution. Nature. 1985 Feb 21;313(6004):653–659. doi: 10.1038/313653a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leavis P. C., Gergely J. Thin filament proteins and thin filament-linked regulation of vertebrate muscle contraction. CRC Crit Rev Biochem. 1984;16(3):235–305. doi: 10.3109/10409238409108717. [DOI] [PubMed] [Google Scholar]
- Leszyk J., Collins J. H., Leavis P. C., Tao T. Cross-linking of rabbit skeletal muscle troponin subunits: labeling of cysteine-98 of troponin C with 4-maleimidobenzophenone and analysis of products formed in the binary complex with troponin T and the ternary complex with troponins I and T. Biochemistry. 1988 Sep 6;27(18):6983–6987. doi: 10.1021/bi00418a047. [DOI] [PubMed] [Google Scholar]
- Leszyk J., Collins J. H., Leavis P. C., Tao T. Cross-linking of rabbit skeletal muscle troponin with the photoactive reagent 4-maleimidobenzophenone: identification of residues in troponin I that are close to cysteine-98 of troponin C. Biochemistry. 1987 Nov 3;26(22):7042–7047. doi: 10.1021/bi00396a028. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Seidel J. C., Leavis P., Lehrer S. S., Gergely J. Effect of Ca2+ binding on troponin C. Changes in spin label mobility, extrinsic fluorescence, and sulfhydryl reactivity. J Biol Chem. 1976 Dec 10;251(23):7551–7556. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Strasburg G. M., Leavis P. C., Gergely J. Troponin-C-mediated calcium-sensitive changes in the conformation of troponin I detected by pyrene excimer fluorescence. J Biol Chem. 1985 Jan 10;260(1):366–370. [PubMed] [Google Scholar]
- Sundaralingam M., Bergstrom R., Strasburg G., Rao S. T., Roychowdhury P., Greaser M., Wang B. C. Molecular structure of troponin C from chicken skeletal muscle at 3-angstrom resolution. Science. 1985 Feb 22;227(4689):945–948. doi: 10.1126/science.3969570. [DOI] [PubMed] [Google Scholar]
- Syska H., Wilkinson J. M., Grand R. J., Perry S. V. The relationship between biological activity and primary structure of troponin I from white skeletal muscle of the rabbit. Biochem J. 1976 Feb 1;153(2):375–387. doi: 10.1042/bj1530375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T., Gowell E., Strasburg G. M., Gergely J., Leavis P. C. Ca2+ dependence of the distance between Cys-98 of troponin C and Cys-133 of troponin I in the ternary troponin complex. Resonance energy transfer measurements. Biochemistry. 1989 Jul 11;28(14):5902–5908. doi: 10.1021/bi00440a029. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- White H. D. Special instrumentation and techniques for kinetic studies of contractile systems. Methods Enzymol. 1982;85(Pt B):698–708. doi: 10.1016/0076-6879(82)85057-x. [DOI] [PubMed] [Google Scholar]
- Zot A. S., Potter J. D. Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Annu Rev Biophys Biophys Chem. 1987;16:535–559. doi: 10.1146/annurev.bb.16.060187.002535. [DOI] [PubMed] [Google Scholar]
- el-Saleh S. C., Warber K. D., Potter J. D. The role of tropomyosin-troponin in the regulation of skeletal muscle contraction. J Muscle Res Cell Motil. 1986 Oct;7(5):387–404. doi: 10.1007/BF01753582. [DOI] [PubMed] [Google Scholar]