Abstract
Interpretation of empirical results based on a taxa's lifetime distribution shows apparently conflicting results. Species' lifetime is reported to be exponentially distributed, whereas higher-order taxa, such as families or genera, follow a broader distribution, compatible with power-law decay. We show that both forms of evidence are consistent with a simple evolutionary model that does not require specific assumptions on species interaction. The model provides a zero-order description of the dynamics of ecological communities, and its species lifetime distribution can be computed exactly. Different behaviors are found as follows: an initial t-3/2 power law, emerging from a random walk type of dynamics, which crosses over to a steeper t-2 branching process-like regime and finally is cut off by an exponential decay that becomes weaker and weaker as the total population increases. Sampling effects also can be taken into account and shown to be relevant. If species in the fossil record were sampled according to the Fisher log-series distribution, lifetime should be distributed according to a t-1 power law. Such variability of behaviors in a simple model, combined with the scarcity of data available, casts serious doubt on the possibility of validating theories of evolution on the basis of species lifetime data.
Keywords: biodiversity, birth and death equations, branching processes, fossils analysis, stochastic processes
Ecosystems have become paradigmatic examples of complex systems, showing organization and collective dynamics across very different time and spatial scales (1). These features are captured by nontrivial relationships among measurable quantities, which take forms familiar to statistical physics. Well-known examples include the species-area scaling relationship (2, 3), allometric relations (4–7), and the occurrence of power laws in the distributions of species lifetime and size of extinction events (8–10). These statistical laws have been measured over many orders of magnitude and exhibit similar patterns across very different living ecosystems and also in different quantitative studies of fossil records (11). The ubiquity of these patterns (12) suggests that they may be amenable to be studied in a general and aspecific framework.
In this work, we will address the issue of species (or more general taxa) lifetime distribution. Although the analysis of fossil records has recently highlighted several patterns in the evolution of biodiversity, and motivated the proposition of different mechanisms that may have caused these patterns, the functional form of the species lifetime distribution remains a debated issue. According to several studies (13), species lifetime seems to be exponentially distributed. Others have found evidence of power-law behavior with exponent close to -2 if genera, and therefore longer time scales, are considered (ref. 12; see also ref. 11 and references therein). Keitt and Stanley (14) analyzed data sets from the North American Breeding Bird Survey (www.pwrc.usgs.gov/bbs) finding a power-law distribution for species lifetime (in their study defined as the time between colonization and local extinction) with an exponent close to -3/2. In fact, the detailed analysis of Newman and Sibani (15) of the data by Raup and Sepkoski (16) has shown how both these hypotheses consistently fit the data, and, when a power-law fit is applied, an exponent between -3/2 and -2 is estimated.
On the theoretical side, these different, not to say contrasting, findings have been invoked to support different macroecological theories. The power-law behavior with exponent -2 is to be expected when species dynamics can be regarded as a critical branching process (17) where two or more species can originate at a random moment from a common ancestor and, also randomly, become extinct. An exponential behavior in the lifetime distribution is often referred to as Van Valen's law (18). The mechanism proposed by Van Valen in support of this view is commonly known as the Red Queen effect: there may not be enough time for a species to gain evolutionary advantage over competing species before the rapidly changing environment completely redraws the fitness landscape. As a consequence, the extinction probability of any species does not depend on time, and an exponential behavior for lifetime distribution easily follows. Several data sets support these conclusions (refs. 19–22; see also ref. 23 for further analysis of the same data). More recently, the occurrence of power-law distributions with nontrivial exponents has attracted particular attention because of an ongoing debate on whether the observed patterns are caused by a self-organized critical dynamics (8–10) that would naturally lead to the notion of punctuated equilibrium (24). In this framework, an ecosystem is depicted as a system of interacting species whose dynamics converges spontaneously close to a critical point (12); the extinction of a given species may trigger a cascade of extinction events starting from the species that depend on, or directly interact with, the species just extinct and leading to fluctuations of any size in the number of extinction occurrences that may contiguously take place.
The aim of the present work is to show that all of the behaviors mentioned above for the lifetime distribution are captured by a simple model of noninteracting species. We conclude, therefore, that it may be problematic, if not inappropriate, to discriminate between existing macroecological theories on the basis of existent data sets. The framework we adopt here is inspired by the ecological neutral theory proposed by Hubbell (25) and thereafter extended and analytically studied in refs. 26–30. This class of models assumes that individuals in an ecological community are fully equivalent and that the population of a species is essentially subject to a birth and death process. Then, each species undergoes the same dynamics: the reproductive success of each individual depends only on the species population size and not on the particular species considered. Competition among species is taken into account explicitly only through a constraint on the total population of the community and implicitly through averages of birth and death rates.
From the point of view of evolutionary theory, the hypothesis of species equivalence may be still justified by a Red Queen effect (18), which is able to forbid the acquisition of a large evolutionary advantage (i.e., a significantly higher fitness level) of a species over its competitors. In the framework of population dynamics, this hypothesis implies that demographic stochasticity is the main driving force for the assembly of ecological communities, meaning that its effect is overwhelmingly strong compared with that of fitness differences among species, which, although present, may be neglected. It is worthwhile to stress that, in principle, complex ecological mechanisms acting on long time scales are not ruled out by these stochastic models, as far as they can be included in effective birth and death rates. This consideration opens the issue of determining whether these theories are able to assess realistic predictions on large time and geographical scales, such as those relevant for the fossil observations. It is widely believed (1) that statistical physics may provide the tools to bridge these very different scales. In this perspective, it is encouraging that Conette and Lieberman (31), based on the studies of the biodiversity time series compiled by Sepkoski and coworkers (32, 33), recently concluded that a random-walk-like model is not inconsistent with the observed biodiversity time patterns.
The model we consider here is analytically solvable and is introduced in the next section. The resulting lifetime distribution interpolates, through a scaling function, between the behaviors of two well-known stochastic processes: exit time problem for the one-dimensional random walk (34) and the critical Galton–Watson branching process (17). Our results show that, even in a simple model in which interactions among species are included only in an averaged way, a variety of different behaviors for the distribution of extinction time are possible. In particular, depending on the relevant time scales, we find an exponential, or a power-law, behavior. The latter can either occur with exponent -2, typical of branching processes (17), or, for shorter time scales, with a random-walk-like exponent -3/2. In addition, if we assume that the abundance of species is distributed according to a Fisher log-series (35), in the Galton–Watson case, we find a power-law distribution of extinction times with exponent -1.
As we will discuss in the conclusion, these results stress the importance of time scales and sampling effects in the analysis of lifetime distributions. This theory also can easily accommodate the contrasting empirical observations of refs. 11–13 and 15 by assuming that, whereas species lifetimes probe the exponential regime of the theory, genera lifetimes fall in the power-law range. The fact that power laws arise in an “effective” single-species theory, combined with the sparseness of available empirical data, suggests that it may not be possible to validate (or discard) ecological or evolutionary mechanisms like self-organized critical dynamics (12) on the basis of an observed nonexponential behavior in the lifetime distributions.
Description of the Model
According to the assumption of neutrality (25, 26), the dynamics of our model is uniquely specified by the effective birth and death rates b(n) and d(n) that depend exclusively on the population size n.
We refer to the functions b(n) and d(n) as effective because they may embody, in a cumulative way, a variety of ecological causes that may, in principle, influence the increase/decrease over time of the number of individuals in a species or, more generally, in a given taxon. The framework is therefore ample enough to describe a population dynamics that is not simply dominated by demographic stochasticity, but also, for example, by immigration, emigration, or niche assembly. We can safely assume that b(n)/n and d(n)/n, the birth and death rates per individuals, can be expanded in a power series in 1/n around their asymptotic values b1 and d1 (28)
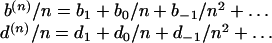 |
[1] |
The nonzero coefficient in this Taylor series can be generally related to various kind of ecological effects giving advantages (or disadvantages) to a less abundant species with respect to a more abundant one. In Hubbell's theory (25, 26), the terms b0 and d0 may be interpreted as the result of an immigration/emigration mechanism that couples the community to a metacommunity living on a larger geographical scale. The mechanisms described by higher power in 1/n in Eq. 1 are relevant only for small population sizes, and they are unable, reasonably, to affect properties observed on large spatial scales and long time scales. In the following, therefore, we will study the dynamics described only by the first two terms in the expansion of Eq. 1:
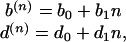 |
[2] |
for all n ≥ 1. Despite the simple form of the birth and death rates, and the simplicity of the assumptions, this class of models is able to provide very good fits of species abundance relations (26–28), which can be related to the probability Pn of having species with population size n. This probability, Pn, evolves with time according to a birth and death master equation
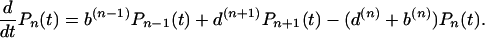 |
[3] |
We impose b1 < d1, ensuring that the average number of individuals is finite and there is no “demographic explosion.” The ratio α = b1/d1 fixes, in fact, the average population per species (27, 28).
To study the lifetime distribution, we consider an absorbing barrier at n = 0, imposing b(0) = d(0) = 0. The initial condition is that the new species at time t = 0 has just one individual
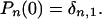 |
[4] |
Making these assumptions, P0(t) represents the probability of being already extinct at time t, and the lifetime probability distribution function (or exit time distribution), p(t), is just the time derivative of P0(t)
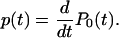 |
[5] |
We will first examine the two limit cases b1 = d1 = 0 and b0 = d0 = 0 and then move to the general case.
Results
When b1 = d1 = 0, the number of individuals belonging to a given species undergoes a random walk in n space where b0 (d0) is the probability per unit time to jump one step to the right (left). A species lifetime therefore would correspond to the time it takes for the random walk to reach n = 0, i.e., to exit the positive axis. The problem of exit time distribution for a random-walk process has been widely studied in the literature (see, for example, ref. 34). In particular, it is well known that in the critical case b0 = d0 the lifetime follows a distribution of the form p(t) ∼ t-3/2. Indeed, it is easy to verify that the solution of Eq. 3, in the present case, is
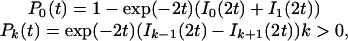 |
[6] |
where 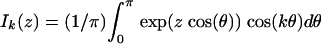 are modified Bessel functions of integer order, and the unit of time has been chosen such that b0 = d0 = 1. Since for large
are modified Bessel functions of integer order, and the unit of time has been chosen such that b0 = d0 = 1. Since for large 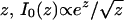 , from Eqs. 5 and 6, it follows that p(t) ∼ t-3/2 asymptotically.
, from Eqs. 5 and 6, it follows that p(t) ∼ t-3/2 asymptotically.
Let us now analyze the case b0 = d0 = 0. This limiting case is interesting from an ecological point of view because the d0 and b0 terms happen to be small when one looks on a very large scale (such as a continental scale). The dynamics is equivalent to a Galton–Watson process in continuous time (17): the asymptotic behavior of the lifetime distribution is a classic result of the theory of critical branching processes (36).
Also in this case, the birth and death equation can be analytically solved; defining the characteristic function 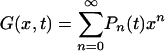 , the birth and death equation can be transformed into a first-order partial differential equation for the function G, which can be integrated by the characteristics method. In the following, without loss of generality, we set d1 = 1, and the initial condition in Eq. 4 translates in G(x, 0) = x. As shown in detail in Supporting Text, which is published as supporting information on the PNAS web site, the exact solution is
, the birth and death equation can be transformed into a first-order partial differential equation for the function G, which can be integrated by the characteristics method. In the following, without loss of generality, we set d1 = 1, and the initial condition in Eq. 4 translates in G(x, 0) = x. As shown in detail in Supporting Text, which is published as supporting information on the PNAS web site, the exact solution is
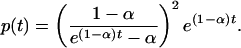 |
[7] |
This distribution has an exponential-like shape when (d1 - b1) (or 1 - α) is not too small. On the other hand, when b1 approaches d1, the distribution has a power-law behavior with exponent -2 and a characteristic time scale t* = 1/(1 - α). The distribution p(t) can be cast in a more appealing form by using the language of critical phenomena in statistical mechanics. For large t and t/t* fixed, it follows, from Eq. 7, that
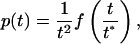 |
[8] |
where f(x) = [x/(1 - e-x)]2e-x. Thus, plotting t2p(t) vs. t/t*, one gets, in the scaling region, a universal curve where all of the model details are absorbed in the characteristic time scale, t*. When dealing with observational data, an estimate of t* can be obtained by the ratio of two consecutive moments, 〈tk 〉(k ≥1), of lifetime probability distribution function.
It also is interesting to investigate the role of the initial condition on the lifetime p.d.f. Taking into account an effective speciation rate, one can show, for the particular case at hand, that the resulting stationary distribution (26) is the celebrated Fisher log series (35)
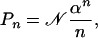 |
[9] |
where n > 0 and 𝒩 is a normalization constant. By using the result above, it is therefore possible to calculate the expected extinction time of a species that is chosen at random in the ecosystem. Setting as initial conditions the characteristic function associated to the distribution in Eq. 9, G(x, 0) = log(1 - xα)/log (1 - α), one finds
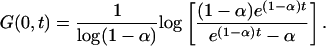 |
[10] |
In this case, again p(t) = ∂G(0, t)/∂t ∼ e-t/t* when t >> t*, whereas p(t) ∼ t-1 when t << t*, which means that the critical exponent for the lifetime p.d.f is now -1.
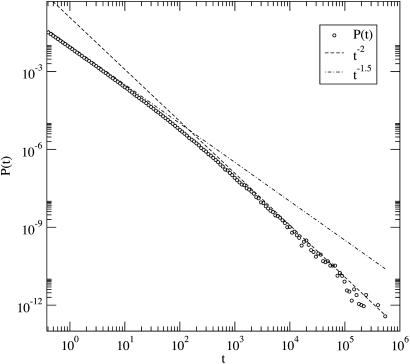
We now discuss, qualitatively first, the solution in the general case when all of the coefficients are different from zero and b0 ∼ d0, b1 ∼ d1. Heuristically, long-living species typically have a large number of individuals. For such species, the b0 and d0 terms can be reasonably neglected. Thus, one expects a crossover from the t-3/2 to the t-2 behavior at a certain characteristic time and finally an exponential decay beyond another characteristic time scale. Numerical simulations do support this hypothesis, as shown in Fig. 1, and suggest that the crossover time is proportional to the ratio b1/b0.
Fig. 1.
Numerical sample of the lifetime probability distribution function with parameters d1 = 1, b1 = 1 - 5 × 10-5, b0 = d0 = 10. Notice the crossover between the two power laws (shown; notice the log–log scale) and the beginning of the exponential regime.
In Supporting Text, we provide the analytical solution of the general case, proving rigorously both the asymptotic critical behaviors and the scaling of the solution with the ratio b0/b1. In the following, we discuss the main results and their consequences. In terms of the Laplace transform of 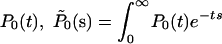 , the exact solution for the critical case b0 = d0 = r and b1 = d1 = 1, is given by
, the exact solution for the critical case b0 = d0 = r and b1 = d1 = 1, is given by
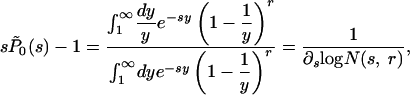 |
[11] |
where we have defined 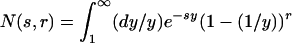 . For small s the function N(s, r) diverges as -c log s, where c depends only on r; this divergence implies that P̃0(s) behaves as P̃0(s) ∼ (1/s) + c log s. The Tauberian theorem ensures in this case that P0(t) behaves like 1 - ct-1 for large t, implying that the lifetime distribution has a t-2 power-law tail.
. For small s the function N(s, r) diverges as -c log s, where c depends only on r; this divergence implies that P̃0(s) behaves as P̃0(s) ∼ (1/s) + c log s. The Tauberian theorem ensures in this case that P0(t) behaves like 1 - ct-1 for large t, implying that the lifetime distribution has a t-2 power-law tail.
To derive the crossover to the t-3/2 behavior, we need to focus on time scales t << 1/r for r << 1. This scale is related to the limit s → 0 with rs fixed in the solution, for which one obtains
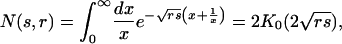 |
[12] |
where K0 is a modified Bessel function. By using this result and Eq. 11 one gets that the lifetime distribution obeys the following scaling form:
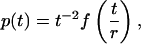 |
[13] |
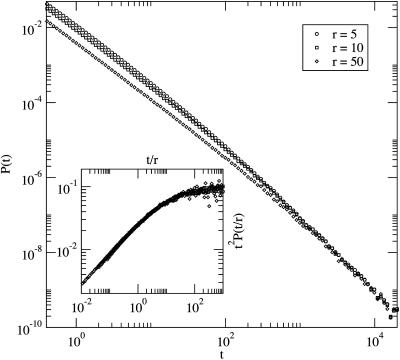
where f(x) → const when x → ∞, leading to the t-2 scaling at large t, and 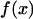 when x → 0, corresponding to the random-walk scaling t-3/2 at intermediate t. The validity of this scaling law is numerically confirmed (see Fig. 2).
when x → 0, corresponding to the random-walk scaling t-3/2 at intermediate t. The validity of this scaling law is numerically confirmed (see Fig. 2).
Fig. 2.
Plot of curves with different values of b0 = d0 = r, shown in the legend. The other parameters are d1 = 1, b1 = 1 - 10-5. Inset shows collapse of the curves according to the scaling law (13).
Discussion
As sketched in the Introduction, the fact that species lifetimes are usually exponentially distributed is often referred to as Van Valen's law (18): Under the assumption that the fitness level is correlated in some way to the extinction probability, Van Valen states that an observed exponential lifetime distribution is the fingerprint of an acting Red Queen mechanism. Later, more detailed analysis (and data sets) (15) indicated power-law behaviors in genera lifetimes, while species exponential lifetime distributions have been generally confirmed. This difference is, to a certain degree, counterintuitive, because one would expect to see a deviation from criticality as a finite size effect when looking at long time scales. A possible explanation, proposed in refs. 11 and 12, is that at longer time scales, like those relevant for genera extinction, collective events like mass extinctions play a more important role. The interdependence of generic taxa in an ecosystem generates stronger correlations in their probability to survive, and these correlations, in turn, may originate a power-law behavior in the lifetime distribution (11, 12).
We also have shown, in a simple model, in which every species undergoes an effective independent dynamics, that a critical behavior for the lifetimes may occur, with an exponent that is compatible with the observed value. This critical behavior is generated only by demographic stochasticity, which is known to be a very important factor in causing species extinction (37). Interestingly enough, the hypotheses underlying this model are not so different to those of Van Valen for an explanation of the exponential species lifetime. Our results clearly indicate that the presence of a Red Queen effect, i.e., the fitness equivalence of all species, does not ensure an exponential lifetime distribution, as far as one takes into account the population sizes in an explicit way. In some sense, in these models the population size acts as a simple “memory” of the evolutionary history of the species.
It is worthwhile to connect our approach with a model proposed by Raup (38) as a null model for the survivorship curves of Phanerozoic genera (the lifetime distribution can be thought of as the derivative of the survivorship curve). This model, fitting rather well the fossil data, assumes that species constituting the genera have a constant speciation and extinction rate. Obviously, the resulting lifetimes distribution is the same as we recover in a limiting case of our model in Eq. 7. The only difference is that, in Raup's case, the branching-like dynamics is applied at the level of species (not at the level of individuals). This feature implies that our model is compatible with the data from the fossil record, with the advantage of being grounded on more realistic (and testable) hypotheses than the assumption of constant species immigration and speciation rates.
In our framework, it is also possible to explain why the critical behavior in the lifetimes is generally observed when studying higher taxonomic levels. Let us assume that we can neglect the terms b0 and d0, as far as we are interested in the tail of the lifetime distribution. By taking the mean value of the distribution in Eq. 9, the typical population size can be expressed as
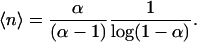 |
[14] |
The right-hand side of Eq. 14 diverges when α → 1-; thus, a choice of the parameters closer to criticality implies a larger population size. Since genera lump together the individuals of many species, the effective value of α for a genera should be closer to 1 than it is for a species. Therefore, it may not be possible to observe the power law in the species lifetime because of the experimental error bars and the presence of the exponential cutoff occurring at t ∼ (1 - α)-1 according to Eq. 7, which, depending on its value, might mask both scaling regimes, i.e., t-3/2 and t-2 or only the latter.
Finally, we demonstrated that, although “local” birth and death terms, i.e., terms that are negligible in the large population size limit, are known to modify the mean species extinction time (39, 40), they are unable to affect the long time-scale behavior of the lifetime distribution. The critical behavior of the distribution, in this class of models, is uniquely determined by the Galton–Watson part of the dynamics. Given the robustness of this “criticality” with respect to modification of the dynamics on a a small scale, we suggest the hypothesis that the observed power law could be simply a consequence of the branching-like structure of single population dynamics rather than an effect of the interactions among different species.
Supplementary Material
Acknowledgments
We thank Jayanth Banavar, Igor Volkov, and Tommaso Zillio for collaboration on the subject. This work was supported by National Science Foundation Grant DEB-0346488.
Author contributions: S.P., A.F., M.M., and A.M. designed research, performed research, and wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Chave, J. & Levin, S. (2003) Environ. Resour. Econ. 26, 527-557. [Google Scholar]
- 2.Harte, J., Kinzig, A. & Green, J. (1986) Science 231, 1528-1533.11542058 [Google Scholar]
- 3.Durrett, R. & Levin, S. (1996) J. Theor. Biol. 179, 119-127. [Google Scholar]
- 4.Kleiber, M. (1947) Physiol. Rev. 27, 511-541. [DOI] [PubMed] [Google Scholar]
- 5.Damuth, J. (1998) Nature 395, 115-116. [Google Scholar]
- 6.Banavar, J. R., Maritan, A. & Rinaldo, A. (1999) Nature 399, 130-131. [DOI] [PubMed] [Google Scholar]
- 7.Banavar, J. R., Damuth, J., Maritan, A. & Rinaldo, A. (2002) Proc. Natl. Acad. Sci. USA 99, 10506-10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sneppen, K., Bak, P., Flyvbjerg, H. & Jensen, M. H. (1995) Proc. Natl. Acad. Sci. USA 92, 5209-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman, M. E. J. (1996) Proc. R. Soc. London Ser. B 263, 1605-1610. [Google Scholar]
- 10.Sole, R. V. & Bascompte, J. (1996) Proc. Biol. Sci. 263, 161-168. [DOI] [PubMed] [Google Scholar]
- 11.Drossel, B. (2001) Adv. Phys. 50, 209-295. [Google Scholar]
- 12.Bak, P. (1997) How Nature Works: The Science of Self-Organized Criticality (Oxford Univ. Press, Oxford).
- 13.Stenseth, N. C. & Maynard Smith, J. (1984) J. Evol. 38, 870-880. [DOI] [PubMed] [Google Scholar]
- 14.Keitt, T. H. & Stanley, H. E. (1998) Nature 393, 257-260. [Google Scholar]
- 15.Newman, M. E. J. & Sibani, P. (1999) Proc. R. Soc. London Ser. B 266, 1593-1599. [Google Scholar]
- 16.Raup, D. M. & Sepkoski, J. J. (1982) Science 215, 1501-1503. [DOI] [PubMed] [Google Scholar]
- 17.Watson, H. W. & Galton, F. (1874) J. Anthropol. Inst. Great Britain Ireland 4, 138-144. [Google Scholar]
- 18.Van Valen, L. M. (1973) Evol. Theory 1, 1-30. [Google Scholar]
- 19.Blow, W. H. (1979) The Cainozoic globigerinida (Brill, Leiden, The Netherlands).
- 20.Boersma, A., Premoli Silva, I. & Shackleton, N. J. (1987) Paleoceanography 2, 287-331. [Google Scholar]
- 21.Saunders, J. B., Beaudry, F. M., Bolli, H. M., Roegl, F., Riedel, W. R., Sanfilippo, A. & Premoli Silva, I. (1973) Initial Reports of the Deep Sea Drilling Project 15, 769-773. [Google Scholar]
- 22.Toumarkine, M. & Luterbacher, H. (1985) in Plankton Stratigraphy, eds. Bolli, H. M., Saunders, J. B. & Perch-Nielsen, K. (Cambridge Univ. Press, Cambridge, U.K.).
- 23.Pearson, P. N. (1992) Paleobiology 18, 115-131. [Google Scholar]
- 24.Gould, S. J. & Eldredge, N. (1977) Paleobiology, 3, 115-151. [Google Scholar]
- 25.Hubbell, S. P. (2001) The Unified Neutral Theory of Biodiversity and Biogeography (Princeton Univ. Press, Princeton).
- 26.Volkov, I., Banavar, J. R, Hubbell, S. P. & Maritan, A. (2003) Nature 424, 1035-1037. [DOI] [PubMed] [Google Scholar]
- 27.Pigolotti, S., Flammini, A. & Maritan, A. (2004) Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 70, 011916. [DOI] [PubMed] [Google Scholar]
- 28.Volkov, I., Banavar, J. R., He, F., Hubbell, S. P. & Maritan, A., Nature, in press.
- 29.Alonso, D. & McKane, A. (2004) J. Ecol. Lett. 7, 911-914. [Google Scholar]
- 30.Vallade, M. & Houchmandzadeh, B. (2003) Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 68, 061902. [DOI] [PubMed] [Google Scholar]
- 31.Conette, J. L. & Lieberman, S. L. (2004) Proc. Natl. Acad. Sci. USA 101, 187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sepkoski, J. J. (1998) Philos. Trans. R. Soc. London B 353, 315-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plotnik, R. E. & Sepkoski, J. J. (2001) Paleobiology 27, 126-139. [Google Scholar]
- 34.Chandrasekhar, S. (1943) Rev. Mod. Phys. 15, 1-89. [Google Scholar]
- 35.Fisher, R. A., Corbet, S. A. & Williams, C. B. (1943) J. Animal Ecol. 12, 42-58. [Google Scholar]
- 36.Harris, T.E. (1989) The Theory of Branching Processes (Dover Univ. Press, New York).
- 37.MacArthur, R. & Wilson, E. O. (1967) The Theory of Island Biogeography (Princeton Univ. Press, Princeton).
- 38.Raup, D. M. (1991) Paleobiology 17, 37-48. [DOI] [PubMed] [Google Scholar]
- 39.Shaffer, M. L. (1987) BioScience 31, 131-134. [Google Scholar]
- 40.Lande, R. (1993) Am. Nat 142, 911-927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.