Abstract
Animals under stress take adaptive actions that may lead to various types of behavioral disinhibition. Such behavioral disinhibition, when expressed excessively and impulsively, can result in harm in individuals and cause a problem in our society. We now show that, under social or environmental stress, mice deficient in prostaglandin E receptor subtype EP1 (Ptger1-/-) manifest behavioral disinhibition, including impulsive aggression with defective social interaction, impaired cliff avoidance, and an exaggerated acoustic startle response. This phenotype was reproduced in wild-type mice by administration of an EP1-selective antagonist, whereas administration of an EP1-selective agonist suppressed electric-shock-induced impulsive aggression. Dopamine turnover in the frontal cortex and striatum was increased in Ptger1-/- mice, and administration of dopaminergic antagonists corrected their behavioral phenotype. These results suggest that prostaglandin E2 acts through EP1 to control impulsive behavior under stress, a finding potentially exploitable for development of drugs that attenuate impulsive behavior in humans.
Keywords: dopamine, aggression, behavioral disinhibition
All organisms are repeatedly disturbed or threatened by changes in the internal or external environment. Such a state of perturbed homeostasis is referred to as “stress” (1, 2). Stress results not only from physical stimuli, such as heat, injury, or disease but also from psychological stimuli, such as exposure to a novel environment or a predator. Stress manifests itself in various characteristic responses that include emotions such as fear and aggression and activation of the neuroendocrine and sympathetic nervous systems (3). These stress responses are thought to be initiated and regulated in the CNS by biochemical processes that are evoked by threatening stimuli (“stressors”) and that determine the activity of several distinct but interacting neuronal pathways (4). However, the neural mechanisms responsible for initiation and regulation of stress responses have remained largely unknown. Neither is it known whether different forms of stressors (for example, physical vs. psychological stimuli) mobilize the same or distinct mechanisms to initiate and regulate their stress responses. Given that the stress responses are elicited to promote adaptation, severe dysfunction of either initiation or regulation mechanisms can not only be a threat to survival, but can also result in various physiological, psychological, or even psychiatric disorders (3, 5).
We have been studying the roles of prostaglandins (PGs) in various physiological and pathophysiological processes. PGs are arachidonic acid metabolites formed by the sequential actions of cyclooxygenase (COX) and respective synthases, and they include PGD2, PGE2, PGF2α, PGI2, and thromboxane A2. PGs interact with eight types or subtypes of G protein-coupled receptors, including the PGD receptor, four subtypes of PGE receptor (EP1, EP2, EP3, and EP4, which are encoded respectively by the genes Ptger1, Ptger2, Ptger3, and Ptger4), the PGF receptor, the PGI receptor, and the thromboxane receptor to exert their effects (6). Using mice deficient in each type or subtype of PG receptor individually, we examined the roles that PGs play in responses to physical stress stimuli. We used bacterial endotoxin to test this issue. Administration of bacterial endotoxin mimics the condition of systemic sickness, in which PGE2 is produced in brain microvessels as a result of the actions of blood-borne cytokines and acts on hypothalamic centers (7–9). We found that EP3 is important in the endotoxin-induced febrile response, and both EP1 and EP3 work critically in activation of the hypothalamic–pituitary–adrenal (HPA) axis (10, 11). However, PGs are also formed and released physiologically in the brain, and their production in distinct brain areas can be further enhanced by stress not related to sickness (12). These findings led us to speculate that PGs are produced in response to various psychological stimuli and regulate various forms of behavioral responses under stress. We now present evidence that, under conditions of social or environmental stress, PGE2 acts on EP1 to control impulsive behavior, including aggression.
Materials and Methods
Animals. EP1-, EP2-, and EP3-deficient mice (Ptger1-/-, Ptger2-/-, and Ptger3-/-, respectively) were back-crossed more than five generations to the C57BL/6 background and bred as described in refs. 10, 11, and 13. Progeny from N5 and N10 Ptger1-/- mice were used in experiments, without any apparent phenotypic difference. Because EP4-deficient (Ptger4-/-) mice could not survive in the C57BL/6 background because of the patent ductus arteriosus (14), we intercrossed survivors of F2 progeny and studied the resulting Ptger4+/+ and Ptger4-/- survivors on the mixed genetic background of 129/Ola × C57BL/6. All experiments were performed according to the guidelines for animal experimentation of the respective institutes. ONO-8713, dissolved as described in ref. 11, was injected at a dose of 10 mg/kg i.p. 60 min before behavioral tests. ONO-DI-004 was injected intracerebroventricularly (i.c.v.), as described in ref. 15, at a dose of 2 or 10 nmol in a volume of 3 μl (vehicle, 16.7% dimethyl sulfoxide) 30 min before the electric-shock-induced fighting test and 10 min before the open-field test. Lipopolysaccharide (LPS) (Escherichia coli 026:B6, Sigma Aldrich) was dissolved in PBS and injected i.p. at a dose of 0.1 mg/kg 60 min before animals were subjected to the resident–intruder test or measurement of the rectal temperature. SCH23390, haloperidol, and raclopride were obtained from Sigma Aldrich. SCH23390 (12.5 μg/kg i.p.), haloperidol (0.3 mg/kg per os), or raclopride (0.3 or 0.6 mg/kg i.p.) was administered 20–30, 60, or 20–30 min, respectively, before evaluation of the acoustic startle reflex or electric-shock-induced fighting and 0, 30, or 0 min, respectively, before recording of locomotor activity.
Behavioral Analyses. The light/dark box, open-field, elevated-plus-maze, and Y-maze tests were performed as described in refs. 16–18. Square and round test arenas were used for the open-field tests shown in Figs. 2B and 5B, respectively, because of their availability at the time of each of these experiments. The acoustic startle reflex was assessed with an SR-Lab startle-reflex system (San Diego Instruments, San Diego) (19). Cliff-avoidance and jumping were evaluated with the use of a round platform (an inverted glass container with a diameter of 13 cm and a height of 20 cm); mice were placed on the platform, and their behavior was video recorded for 7 min.
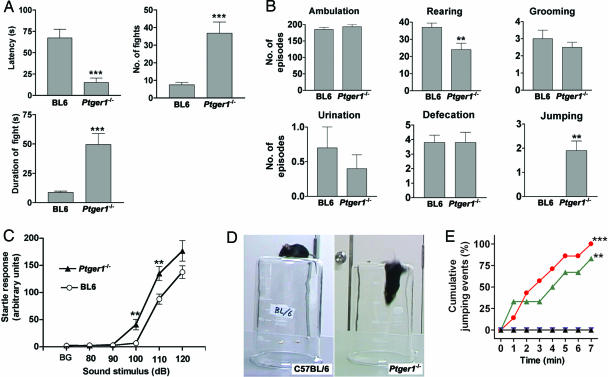
Fig. 2.
Behavioral analyses of EP1-deficient mice (A) Electric-shock-induced fighting behavior. Age-matched pairs of male wild-type or Ptger1-/- mice (n = 8 pairs for each) were placed in a transparent container and exposed to electric shock for 3 min. The latency to the first fighting episode, the number of fighting events, and the total duration of fighting were counted. **, P < 0.001 vs. wild type. (B) Open-field test. Individual wild-type (n = 12) or Ptger1-/- (n = 11) mice were allowed to explore freely the open field for 5 min. Ambulation was measured with an automatic infrared beam counter, and the occurrence of each typical behavior (rearing, grooming, urination, defecation, jumping) was scored from a video recording. **, P < 0.01 vs. wild type. (C) Acoustic startle reflex. Wild-type or Ptger1-/- mice (n = 16 for each) were placed in a startle chamber, and the startle reflex to audible stimuli of the indicated frequencies was measured. Background noise (BG) is 70 dB. **, P < 0.01 vs. corresponding value for wild type. (D) Impaired cliff avoidance in Ptger1-/- mice. Wild-type or Ptger1-/- mice were placed on the base of an inverted glass beaker, and their behavior was recorded with a video camera (see Movie 3). (E) The cumulative frequency of jumping for Ptger1-/- (red circles), Ptger3-/- (blue inverted triangles), or wild-type (black triangles) mice (n = 7 for each) and for wild-type mice injected with ONO-8713 (10 mg/kg i.p.) 1 h before the test (green triangles, n = 6) was determined over 7 min in the cliff-avoidance test. **, P < 0.01, ***, P < 0.001 vs. nontreated wild-type mice.
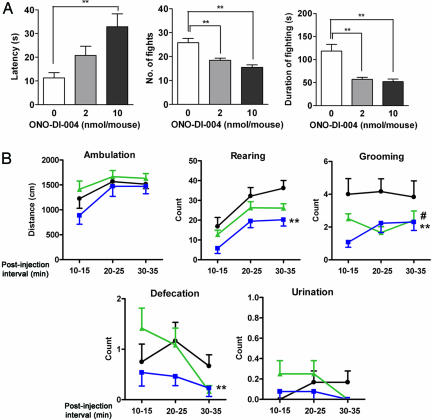
Fig. 5.
Effect of an EP1-selective agonist on behaviors. (A) Effect of ONO-DI-004 on electric-shock-induced fighting. Age-matched pairs of wild-type male mice were exposed to electric shock 30 min after i.c.v. injection of ONO-DI-004 (2 or 10 nmol per animal, n = 9 pairs per dose) or vehicle (n = 10 pairs). The amplitude and duration of the electric shock and the size of the arena were adjusted to increase the rate of fighting in wild-type animals. The latency to the first fighting episode and the total number and duration of fighting episodes during 5 min were measured. **, P < 0.01. (B) Effect of ONO-DI-004 on the open-field test. Wild-type male mice were put into a round open field 10 min after i.c.v. injection of ONO-DI-004 (2 or 10 nmol per animal, n = 12 and 13 pairs, green triangles and blue squares, respectively) or vehicle (n = 12 pairs, filled circles). Ambulation, rearing, grooming, defecation, and urination were scored during three separate 5-min intervals during 10–15, 20–25, and 30–35 min after the injection. #, P < 0.05 for the comparison between the group injected with 2 nmol of ONO-DI-004 vs. the one injected with vehicle. *, P < 0.05 and **, P < 0.01 for the comparison between the group injected with 10 nmol of ONO-DI-004 vs. the one injected with vehicle.
In the resident–intruder test, an 8-week-old male subject mouse was placed in a neutral cage for 30 min, after which a juvenile (4–5 weeks of age) conspecific male was introduced. The interaction between the subject and the juvenile was recorded with a video camera for 3 min. Social investigation, summarized as “sniffing,” was determined as the total amount of time that the subject spent on investigating (for example, anogenital licking, sniffing, and trailing) the juvenile. Aggression was defined as spontaneous fighting initiated by the subject, and the latency to the first attack was determined for each subject. Electric-shock-induced fighting (20) was examined by placing two 8- to 12-week-old male mice of the same genotype together in a transparent 3-liter glass container and subjecting them to electric foot shocks (1 Hz, 200 ms, 0.3 mA) for 3 min. For the experiments shown in Fig. 5A, we used a 0.5-liter glass container and subjected mice to stronger electric shocks (1 Hz, 200 ms, 0.8 mA) for 5 min to reproducibly elicit fighting behavior in wild-type animals. The latency to the first attack and the total number and duration of fighting episodes were measured.
Measurement of Adrenocorticotropic Hormone (ACTH). Plasma ACTH concentrations were measured as described in ref. 11. Briefly, after being individually housed and handled for 3 days, mice were subjected to the resident–intruder test for 3 min and killed by decapitation within 45 s after the test. The plasma concentration of ACTH was determined with an immunoradiometric assay kit (ACTH-IRMA, Mitsubishi Chemical, Tokyo). The basal ACTH concentration was determined in mice of the corresponding genotype that were not subjected to the behavioral test.
Analysis of Febrile Response After LPS Injection. The rectal temperatures of wild-type, Ptger1-/-, and Ptger3-/- mice were measured under mild restraint immediately before and 60 min after injection of LPS (0.1 mg/kg i.p.) as described in ref. 10. All of the mice were acclimated to the procedure for five consecutive days before the test day.
Analysis of Monoamine Metabolism in Vivo. Monoamine metabolism was analyzed as described in ref. 18.
Statistical Analysis. Data are presented as means ± SEM. Differences between two groups were analyzed by Student's t test. Differences among more than two groups with similar variances were assessed by one-way or two-way ANOVA, followed by Dunnett's, Tukey's, or Bonferroni's post hoc tests for evaluation of pairwise group differences. If the variance of the groups differed significantly, we used the Kruskal–Wallis test. The time-course data shown in Figs. 1 B and E and 2E (and in Figs. 6 and 7, which are published as supporting information on the PNAS web site) were analyzed with the log-rank test. All analyses were performed with the use of prism 4.0 software (GraphPad, San Diego). P < 0.05 was considered statistically significant.
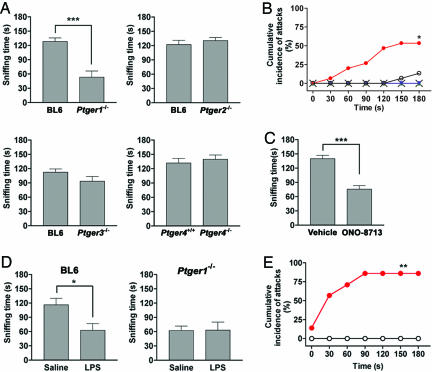
Fig. 1.
Increased aggressiveness and reduced social interaction in EP1-deficient mice. (A) Effects of EP deficiency on social interaction. Sniffing time of wild-type mice in the resident–intruder test was compared with that of Ptger1-/- (n = 7 for each group), Ptger2-/- (n = 9 for each group), Ptger3-/- (n = 13 for each group), or Ptger4-/- (n = 8 for each group) mice. In the case of aggressive encounters, the sniffing time until the fighting episode was determined. ***, P < 0.001. (B) Cumulative incidence of fighting events between the subject and the juvenile intruder. Data are from wild-type (open circles, n = 15), Ptger1-/- (filled circles, n = 15), Ptger2-/- (blue inverted triangles, n = 8), Ptger3-/- (green triangles, n = 7), and Ptger4-/- (crosses, n = 8) mice. *, P < 0.05 vs. wild-type mice. (C) Effect of the EP1 antagonist ONO-8713 on social interaction. Wild-type mice were treated with either vehicle (n = 6) or ONO-8713 (10 mg/kg i.p.) (n = 6) 1 h before the resident–intruder test. ***, P < 0.001. (D) Effect of LPS on social interaction. Wild-type (BL6) or Ptger1-/- mice (n = 4 per group) were treated with either saline or LPS (0.1 mg/kg i.p.) 1 h before the resident–intruder test. *, P < 0.05. (E) Cumulative incidence of fighting events between LPS-treated mice and a juvenile intruder. Wild-type (open circles, n = 7) or Ptger1-/- (red circles, n = 7) mice were treated with LPS and tested 1 h later (see Movie 2). **, P < 0.01 vs. wild type.
Results
Impulsive Aggression and Defective Social Interaction in EP1-Deficient Mice. We subjected mice lacking EP1, EP2, EP3, and EP4 individually (Ptger1-/-, Ptger2-/-, Ptger3-/-, and Ptger4-/-, respectively) to a resident–intruder test. We placed an 8-week-old male in a neutral cage and introduced a younger conspecific male shortly thereafter. We examined the behavior of wild-type mice and mice lacking each EP subtype by video recording the interaction between the subject and the young intruder (see Movie 1, which is published as supporting information on the PNAS web site). The wild-type subjects exhibited immediate and prolonged social investigative behaviors, such as sniffing, anal licking, and grooming of the younger animal (Fig. 1A). Only 2 of 15 wild-type subjects underwent an aggressive encounter with the intruder (Fig. 1B). Among the mice deficient in each EP subtype, only Ptger1-/- mice exhibited abnormality in this test. Ptger1-/- males exhibited a significant decrease in sniffing time (Fig. 1A) and a significant increase in the frequency of aggressive encounters (8 of 15 animals) compared with the wild type (Fig. 1B). This aggression with defective social interaction was specific to Ptger1-/- mice among the various EP-deficient animals (Fig. 1 A and B) and depended on gene dosage in littermates generated by mating of Ptger1+/- heterozygotes (see Fig. 6). To examine whether the mice subjected to this test experienced stress, we measured the plasma concentration of ACTH. The ACTH concentration in resident wild-type mice subjected to this test for 3 min was increased markedly, relative to the basal level (782.8 ± 85.6 vs. 190.5 ± 29.4 pg/ml, n = 7, P < 0.001). Ptger1-/- mice subjected to the resident–intruder test showed a similar increase in plasma ACTH level (713.2 ± 97.2 vs. 177.3 ± 40.2 pg/ml, n = 7, P < 0.01). These results indicate that wild-type and Ptger1-/- mice experience similar levels of stress during this behavioral test and that, in contrast to our findings on the response to endotoxin (11), EP1 deficiency did not affect activation of the HPA axis in response to the stress associated with this test. We next pharmacologically confirmed the role of EP1 in social interaction with the use of a specific EP1 antagonist, ONO-8713 (11). Treatment of wild-type mice with ONO-8713 reproduced defective social interaction (Fig. 1C) and induced aggression in animals housed in a home cage (data not shown). These results thus revealed a role for EP1 in regulation of impulsive aggressiveness and social interaction under social stress.
Given that sickness stress reduces locomotion and social contact in animals (21), we next examined the effect of the administration of bacterial endotoxin (LPS) to wild-type or Ptger1-/- mice on behavior in the resident–intruder test. LPS-treated wild-type mice showed a reduced level of social interaction compared with saline-treated animals, and they underwent no aggressive encounters (Fig. 1 D and E; see also Movie 2, which is published as supporting information on the PNAS web site). In contrast, LPS-treated Ptger1-/- mice manifested extensive aggression with little social contact. Aggression occurred as rapidly and as frequently in LPS-treated Ptger1-/- mice as it did in nontreated Ptger1-/- animals, with six of seven mice attacking the intruder. Importantly, Ptger1-/- mice showed LPS-induced febrile response to the same level as did wild-type mice (37.6 ± 0.2°C and 37.6 ± 0.2°C before and 38.3 ± 0.1°C and 38.2 ± 0.2°C at 60 min after LPS injection, n = 7 and n = 6, respectively), whereas no febrile response to LPS was observed with Ptger3-/- mice (37.4 ± 0.1°C before and 37.4 ± 0.2°C at 60 min after LPS injection, respectively). These results suggest that EP1 signaling works also to suppress aggressive behavior of mice in the febrile state.
We next characterized the aggressive phenotype of Ptger1-/- mice by evaluation of electric-shock-induced fighting (20). We placed two male mice of the same genotype (either Ptger1-/- or wild type) together in a transparent glass container, subjected them to foot shock, and measured the latency to the first attack and the number and duration of fighting episodes. Ptger1-/- mice showed a shorter latency before the onset of fighting, a greater number of fighting episodes, and a longer total fighting time than did wild-type mice (Fig. 2A). The sensitivity to electric shock itself, determined as the minimal current intensity that elicited vocalization, was similar in the wild-type and Ptger1-/- animals (0.20 ± 0.03 mV and 0.18 ± 0.03 mV, respectively, n = 5 for each). This behavioral paradigm thus also revealed a significant increase in impulsive aggressiveness of Ptger1-/- mice.
Other Behavioral Abnormalities of EP1-Deficient Mice. Given that anxiety is thought to be a behavioral response to stress (22), we analyzed the level of anxiety in Ptger1-/- mice with the use of the light/dark-box test and the elevated-plus-maze test. No behavioral abnormalities of Ptger1-/- mice were apparent in these tests, however (see Fig. 8 A and B, which is published as supporting information on the PNAS web site). The behavior of Ptger1-/- mice also did not differ from that of wild-type animals in a conventional Y-maze (Fig. 8C), suggesting that Ptger1-/- mice are not deficient in either exploratory motivation or short-term memory.
In the open-field test, no difference was found in ambulation, grooming, urination, or defecation between wild-type and Ptger1-/- mice. The incidence of rearing was significantly reduced, and the frequency of spontaneous jumping was significantly increased in Ptger1-/- mice (Fig. 2B). Notably, examination of the acoustic startle reflex of Ptger1-/- mice revealed an exaggerated response to stimuli throughout the dynamic range of audible sound frequencies (Fig. 2C). In contrast, prepulse inhibition was unaltered in Ptger1-/- mice (data not shown), indicating that sensorimotor gating itself was likely intact.
In the cliff-avoidance test, wild-type mice placed on an elevated transparent platform (the base of an inverted glass beaker with a height more than twice the animal's body length) avoided the edge and did not jump (Fig. 2 D and E). In contrast, seven of seven Ptger1-/- mice jumped off the platform within 7 min (Fig. 2 D and E; see also Movie 3, which is published as supporting information on the PNAS web site), whereas none of seven wild-type or Ptger3-/- mice jumped within this period (Fig. 2E). The jumping behavior of Ptger1-/- mice depended on gene dosage in littermates generated by mating of Ptger1+/- heterozygotes (see Fig. 7). Jumping behavior was also reproduced in wild-type animals treated with ONO-8713, with five of six mice jumping (Fig. 2E).
Increased Dopamine (DA) Turnover in the Frontal Cortex and Striatum of EP1-Deficient Mice. We thus detected behavioral disinhibition in Ptger1-/- mice under stress in multiple paradigms. Previous studies have indicated that drugs or manipulations that affect dopaminergic activity alter both the acoustic startle reflex (23) and intermale aggression (24–27). We therefore measured the content of monoamines and their metabolites in various regions of the brain of Ptger1-/- and wild-type mice. A significant decrease in DA content and increase in the content of the DA metabolites dihydroxyphenylacetic acid and homovanillic acid were apparent in the frontal cortex and striatum of Ptger1-/- mice (see Table 1, which is published as supporting information on the PNAS web site). These changes resulted in an increased ratio of DA metabolites to DA in these brain regions (Fig. 3). The increase in this ratio was reproduced in the striatum of wild-type mice by injection of ONO-8713 (data not shown). In contrast, metabolism of serotonin was not altered in either of these brain regions of Ptger1-/- mice (Fig. 3 and Table 1). These data indicate that DA turnover is specifically increased in the frontal cortex and striatum of Ptger1-/- mice compared with wild-type mice.
Fig. 3.
Increased DA turnover in the frontal cortex and the striatum of EP1-deficient mice. Contents of DA and serotonin and of their metabolites in the frontal cortex and the striatum of wild-type and Ptger1-/- mice (n = 6–7) were determined (see Table 1). The ratios of the content of dihydroxyphenylacetic acid (DOPAC) to DA, homovanillic acid (HVA) to DA, (DOPAC plus HVA) to DA, and 5-hydroxyindole acetic acid (5-HIAA) to serotonin (5-HT) of Ptger1-/- mice (filled bars) were normalized, relative to the average of the corresponding measures of wild-type animals (open bars). **, P < 0.01; ***, P < 0.001 vs. the corresponding wild-type value.
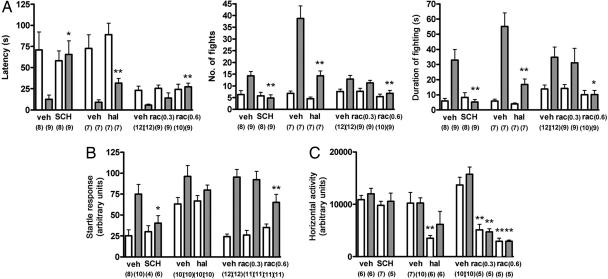
Effects of Dopaminergic Antagonists on Behavioral Abnormalities of EP1-Deficient Mice. We next investigated whether the increased dopaminergic activity in Ptger1-/- mice was responsible for their abnormal behaviors. We first examined the effects of DA-receptor antagonists on electric-shock-induced aggression and the acoustic startle reflex in wild-type and Ptger1-/- mice. A D1-receptor antagonist, SCH23390, restored the latency to the first fight and the number and duration of fights induced by electric shock in Ptger1-/- mice to values similar to those for wild-type mice (Fig. 4A). SCH23390 also normalized the acoustic startle reflex of Ptger1-/- mice (Fig. 4B) but had no effect on locomotor activity at the dose used (Fig. 4C). Treatment with raclopride, a D2-receptor-specific antagonist, also normalized the responses of Ptger1-/- mice in these tests (Fig. 4 A and B). Haloperidol, which is relatively selective for the D2 receptor, also attenuated the electric-shock-induced fighting (Fig. 4A). However, concomitant suppression of general locomotor activity by the latter two drugs (Fig. 4C) made it difficult to assess the role of D2 receptors in the phenotype of Ptger1-/- mice. Indeed, both the electric-shock-induced fighting and the acoustic startle response were less sensitive to the lower dose of raclopride tested (0.3 mg/kg) than was the locomotor activity. Thus, inhibition of the D1 receptor apparently suppresses the behavioral abnormalities of Ptger1-/- mice selectively. However, how the dopaminergic signaling is involved in elicitation of their behavioral phenotype should be rigorously examined, given that DA antagonists may be rather nonspecific in their inhibition of many goal-directed behaviors.
Fig. 4.
Effects of dopaminergic antagonists on the behavioral abnormalities of EP1-deficient mice. Wild-type (open bars) or Ptger1-/- (filled bars) male mice were treated with SCH23390 (SCH) (12.5 μg/kg i.p.), haloperidol (hal) (0.3 mg/kg per os), or raclopride (rac) (0.3 or 0.6 mg/kg i.p.) and were subsequently examined for electric-shock-induced fighting (A), the acoustic startle reflex to a 110-dB stimulus (B), and spontaneous locomotor activity (C). All of the tests were run simultaneously with corresponding control groups with proper vehicle (veh) over multiple days. The numbers of mice for each group are shown below each column. *, P < 0.05; **, P < 0.01 compared with vehicle-treated mice of the same genotype.
Suppression of Stress-Induced Impulsive Behavior by an EP1 Agonist. Finally, we used electric-shock-induced fighting and examined the effect of pharmacological activation of EP1 on impulsive behavior of wild-type animals. In this experiment, the amplitude of electric shock and its duration and the size of the arena were modified to increase the level of fighting in wild-type mice. Wild-type males were injected i.c.v. with an EP1-selective agonist, ONO-DI-004, and subjected to electric shock 30 min later. Treatment with ONO-DI-004 significantly prolonged the latency to the first fighting episode in a dose-dependent manner (Fig. 5A). ONO-DI-004 also reduced the total number and duration of fighting events. On the other hand, the sensitivity of electric shock, determined as the minimal current intensity that elicited vocalization, was similar in animals treated with ONO-DI-004 and vehicle (0.30 ± 0.01 mV (n = 8) and 0.27 ± 0.01 mV (n = 7), respectively). In the open-field test, ONO-DI-004 did not significantly affect ambulation, although it suppressed the rate of rearing and grooming (Fig. 5B). These data suggested that pharmacological activation of EP1 in the brain suppresses electric-shock-induced fighting behavior while sparing general sensory and motor responses.
Discussion
Here, we have shown that EP1-deficient mice manifest a variety of abnormal behaviors, including impulsive aggression with defective social interaction, an enhanced acoustic startle response, and impaired cliff avoidance under social or environmental stress. This phenotype is reproduced in wild-type mice by administration of an EP1-specific antagonist. Given that all of these abnormal behaviors can be attributed to a lack of proper inhibition of impulsive behaviors, we conclude that the behavioral phenotype of Ptger1-/- mice represents behavioral disinhibition and suggest that EP1 serves to suppress impulsive behavior under stress. Stress evokes various behavioral responses, such as fight, flight, freezing, and emotional responses, including fear and anxiety. However, both light/dark-box and elevated-plus-maze tests indicate a normal level of anxiety in Ptger1-/- mice. These results suggest that PGE2–EP1 signaling is specifically involved in the mechanism that regulates impulsive behavior under stress and that the activity of this signaling pathway determines behavioral predisposition.
Historically, PGE2 and its receptors in the brain have been studied for their involvement in neuroendocrine and/or febrile responses under sickness stress. We had reported that EP1 is necessary for the maximal activation of the HPA axis after injection of bacterial endotoxin. Given the EP1 involvement in behavioral control under psychological stress, PGE2–EP1 signaling in the brain is involved in both physiological and pathological processes. These two mechanisms are likely to be distinct, because Ptger1-/- mice did not show impairment in HPA-axis activation but did show aggressive behavior in the resident–intruder test. Under sickness stress, however, these two mechanisms are simultaneously activated, because Ptger1-/- mice injected with LPS showed both reduced HPA-axis response (11) and enhanced aggressiveness. Thus, animals can adapt flexibly to various forms of stress by using, in a context-dependent manner, distinct mechanisms, either alone or in combination, that share PGE2 as a common mediator. Given that PG acts on its receptor in the vicinity of its synthesis (6), the site and distribution of PGE2-release in a context may critically determine what EP1-medited mechanism is activated. Thus, under sickness stress, blood-borne cytokines induce coexpression of COX-2 and PGE synthase in brain microvessels, so that PGE2 can be released and distributed to the large brain area. Under psychological stress, on the other hand, PGE2 is likely to be derived from brain-cell populations expressing either COX isoform constitutively, namely, pyramidal neurons in the cerebral cortex and the hippocampus that express COX-2 (12, 28) and microglia-like cells expressing COX-1 (28). Presumably, PGE2 is produced by these cell populations upon neural activation and works locally. Although the comprehensive analysis of EP1 expression in the brain is currently underway, we reported that EP1 is expressed in areas such as the amygdala and the paraventricular nucleus of the hypothalamus and is present at presynaptic terminals there (11). We can, therefore, hypothesize that PGE2 is released from some population of neurons activated under psychological stress and modulates synaptic transmission through EP1 at presynaptic terminals to work for behavioral control.
Most animals exposed to stress exhibit changes in behavior that persist for hours to days. Given that the PG production that accompanies induction of COX-2 occurs with various time courses and continues for hours to days (29), PGs are good candidates for long-term mediators of impulsivity control after exposure to stress. However, administration of COX inhibitors does not usually induce the behavioral phenotype observed in Ptger1-/- mice or in wild-type mice treated with an EP1-specific antagonist. Different PGs or PG receptors have been shown to exert opposite actions in other organs, as exemplified by effects on thrombogenesis and smooth-muscle contraction (6). Given that COX inhibitors block the formation of all types of PGs, these observations suggest that another, unidentified PG-mediated pathway may oppose EP1 action in the brain. The balance between opposite PG actions may set a point for impulsivity control during exposure to various types of stressful stimuli.
Impulsive behavior associated with psychiatric illness presents a serious problem in modern society. We found that Ptger1-/- mice showed increased impulsivity in multiple stress paradigms, whereas most other behavioral measures, such as general sensory and motor activities, short-term memory, and the level of anxiety were not impaired. These findings indicate that EP1 is involved in a specific subset of neural processes that control impulsive behavior under stress. Consistently, pharmacological activation of EP1 in the brain suppresses impulsive aggression in the face of an intense stressful stimulus, whereas it did not affect general sensory and motor activities. Given that dopaminergic antagonists, one of the mainstays for controlling impulsive behavior, easily cause general behavioral suppression because of the extra-pyramidal effect, the above specificity of action of an EP1-selective agonist may be advantageous for therapeutic applications to manipulate the impulsive behavior of psychiatric patients or to control impulsive behavior under stress.
Supplementary Material
Acknowledgments
We thank Yukihiko Sugimoto, Eri Segi, and Toshiyuki Matsuoka for discussion; Chikuma Hamada for advice on statistical analyses; Teruhisa Fujiwara for animal care and breeding; Kimiko Nonomura for technical assistance; Tae Arai and Yurie Kitagawa for secretarial assistance; and Ono Pharmaceuticals, Osaka, for ONO-8713 and ONO-DI-004. This work was supported, in part, by Grants-in-Aid for Scientific Research and Special Coordination Funds for Promoting Science and Technology for the Target-Oriented Brain Science Research Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a grant from the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation of Japan; and a grant from Ono Research Foundation.
Author contributions: Y.M., T.F., K.Y., T. Nagai, H.B., F.U., T. Nabeshima, and S.N. designed research; Y.M., T.F., K.Y., T. Nagai, H.B., Y.T., and S.K. performed research; Y.M., T.F., K.Y., T. Nagai, H.B., Y.T., S.K., T. Nabeshima, and S.N. analyzed data; and Y.M., T.F., H.B., and S.N. wrote the paper.
Conflict of interest statement: Our work has been partially supported by Ono Pharmaceuticals, and Ono Pharmaceuticals has applied for a patent on our findings of potential use of EP1-agonists as therapeutic agents for post-traumatic stress disorder.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACTH, adrenocorticotropic hormone; COX, cyclooxygenase; DA, dopamine; HPA, hypothalamo–pituitary–adrenal; i.c.v., intracerebroventricularly; PG, prostaglandin.
References
- 1.Chrousos, G. P. & Gold, P. W. (1992) J. Am. Med. Assoc. 267, 1244-1252. [PubMed] [Google Scholar]
- 2.Kopin, I. J. (1995) Ann. N.Y. Acad. Sci. 771, 19-30. [DOI] [PubMed] [Google Scholar]
- 3.Johnson, E. O., Kamilaris, T. C., Chrousos, G. P. & Gold, P. W. (1992) Neurosci. Biobehav. Rev. 16, 115-130. [DOI] [PubMed] [Google Scholar]
- 4.Van de Kar, L. D. & Blair, M. L. (1999) Front. Neuroendocrinol. 20, 1-48. [DOI] [PubMed] [Google Scholar]
- 5.McEwen, B. S. (2000) Neuropsychopharmacology 22, 108-124. [DOI] [PubMed] [Google Scholar]
- 6.Narumiya, S., Sugimoto, Y. & Ushikubi, F. (1999) Physiol. Rev. 79, 1193-1226. [DOI] [PubMed] [Google Scholar]
- 7.Elmquist, J. K., Scammell, T. E. & Saper, C. B. (1997) Trends Neurosci. 20, 565-570. [DOI] [PubMed] [Google Scholar]
- 8.Turnbull, A. V. & Rivier C. L. (1999) Physiol. Rev. 79, 1-71. [DOI] [PubMed] [Google Scholar]
- 9.Yamagata, K., Matsumura, K., Inoue, W., Shiraki, T., Suzuki, K., Yasuda, S., Sugiura, H., Cao, C., Watanabe, Y. & Kobayashi, S. (2001) J. Neurosci. 21, 2669-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ushikubi, F., Segi, E., Sugimoto, Y., Murata, T., Matsuoka, T., Kobayashi, T., Hizaki, H., Tuboi, K., Katsuyama, M., Ichikawa, A., et al. (1998) Nature 395, 281-284. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka, Y., Furuyashiki, T., Bito, H., Ushikubi, F., Tanaka, Y., Kobayashi, T., Muro, S., Satoh, N., Kayahara, T., Higashi, M., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 4132-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamagata, K., Andreasson, K. I., Kaufmann, W. E., Barnes, C. A. & Worley, P. F. (1993) Neuron 11, 371-386. [DOI] [PubMed] [Google Scholar]
- 13.Hizaki, H., Segi, E., Sugimoto, Y., Hirose, M., Saji, T., Ushikubi, F., Matsuoka, T., Noda, Y., Tanaka, T., Yoshida, N., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 10501-10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segi, E., Sugimoto, Y., Yamasaki, A., Aze, Y., Oida, H., Nishimura, T., Murata, T., Matsuoka, T., Ushikubi, F., Hirose, M., et al. (1998) Biochem. Biophys. Res. Commun. 246, 7-12. [DOI] [PubMed] [Google Scholar]
- 15.Oka, T., Oka, K. & Saper, C. B. (2003) Brain Res. 968, 256-262. [DOI] [PubMed] [Google Scholar]
- 16.Yamada, K., Hiramatsu M., Noda, Y., Mamiya, T., Murai, M., Kameyama, T., Komori, Y., Nikai, T., Sugihara, H. & Nabeshima, T. (1996) Neuroscience 74, 365-374. [DOI] [PubMed] [Google Scholar]
- 17.Yamada, K., Iida, R., Miyamoto, Y., Saito, K., Sekikawa, K., Seishima, M. & Nabeshima, T. (2000) J. Neuroimmunol. 111, 131-138. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto, Y., Yamada, K., Noda, Y., Mori, H., Mishina, M. & Nabeshima, T. (2002) J. Neurosci. 22, 2335-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura, K., Manabe, T., Watanabe, M., Mamiya, T., Ichikawa, R., Kiyama, Y., Sanbo, M., Yagi, T., Inoue, Y., Nabeshima, T., et al. (2001) Eur. J. Neurosci. 13, 179-189. [DOI] [PubMed] [Google Scholar]
- 20.Tedeschi, R. E., Tedeschi, D. H., Mucha, A., Cook, L., Mattis, P. A. & Fellows, E. J. (1959) J. Pharmacol. Exp. Ther. 125, 28-34. [PubMed] [Google Scholar]
- 21.Kent, S., Bluthé, R.-M., Kelley, K. W. & Dantzer, R. (1992) Trends Pharmacol. Sci. 13, 24-28. [DOI] [PubMed] [Google Scholar]
- 22.Mayer, E. A. & Fanselow, M. S. (2003) Nat. Neurosci. 6, 1011-1012. [DOI] [PubMed] [Google Scholar]
- 23.Meloni, E. G. & Davis, M. (2000) J. Neurosci. 20, 5374-5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguilar, M. A., Minarro, J., Perez-Iranzo, N. & Simon, V. M. (1994) Pharmacol. Biochem. Behav. 47, 753-756. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Arias, M., Minarro, J., Aguilar, M. A., Pinazo, J. & Simon, V. M. (1998) Eur. Neuropsychopharmacol. 8, 95-103. [DOI] [PubMed] [Google Scholar]
- 26.Kudryavtseva, N. N., Lipina, T. V. & Koryakina, L. A. (1999) Pharmacol. Biochem. Behav. 63, 229-236. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguiz, R. M., Chu, R., Caron, M. G. & Wetsel, W. C. (2004) Behav. Brain Res. 148, 185-198. [DOI] [PubMed] [Google Scholar]
- 28.Li, S., Wang, Y., Matsumura, K., Ballou, L. R., Morham, S. G. & Blatteis, C. M. (1999) Brain Res. 825, 86-94. [DOI] [PubMed] [Google Scholar]
- 29.Smith, W. L., DeWitt, D. L. & Garavito, R. M. (2000) Annu. Rev. Biochem. 69, 145-182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.