Abstract
Inhibitors of histone deacetylases (HDACIs) are a new generation of anticancer agents that selectively kill tumor cells. However, the molecular basis for their tumor selectivity is not well understood. We investigated the effects of HDACIs on the oncogenic Rb-E2F1 pathway, which is frequently deregulated in human cancers. Here, we report that cancer cells with elevated E2F1 activity, caused either by enforced E2F1 expression, or by E1A oncogene expression, are highly susceptible to HDACI-induced cell death. This E2F1-mediated apoptosis is neither p53- nor p73-dependent but proceeds through selective induction of proapoptotic BH3-only protein Bim. We show that Bim is a direct target of E2F1 and that HDAC inhibition promotes the recruitment of E2F1 to the Bim promoter. Moreover, silencing of Bim by specific small interfering RNA (siRNA) effectively abolishes the E2F1-mediated cell death sensitization to HDACIs. These findings suggest that the oncogenic E2F1 pathway participates in HDACIs-induced apoptosis in cancer cells and underscore the importance of Bim as a key mediator of oncogene-induced apoptosis. Our study provides an important insight into the molecular mechanism of tumor selectivity of HDACIs and predicts that, clinically, HDACIs will be more effective in tumors with high E2F1 activity.
Keywords: histone deacetylase inhibitors, tumor selectivity, oncogene
Histone deacetylase inhibitors (HDACIs) have emerged recently as promising chemotherapeutic agents and can induce a range of antitumor activities, including induction of cell cycle arrest, stimulation of differentiation, and provocation of apoptosis (1–3). The efficacy of these agents, particularly trichostatin A (TSA) and suberoylanilide hydroxamic acid (SAHA), has been established by in vitro experiments and ongoing clinical trials (4–9). Unlike conventional chemotherapeutic agents that often cause DNA damage in both tumor and normal tissues, HDACIs display strong tumor selectivity and cause less toxicity to the normal tissues (2). However, the mechanism of this tumor selectivity is not understood, although recent studies show that HDACI sensitivity in tumor could be mediated by the activation of the death receptor pathway involving the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (10, 11) or preferential induction of oxidative injury in transformed cells (12).
The therapeutic effect of HDACIs might be mediated through modulation of chromatin structure and transcriptional activity by means of changes in the acetylation status of nucleosomal histones at gene promoters. In addition to chromatin remodeling, HDACI activity may also be linked with non-histone proteins important for growth and differentiation, such as tumor suppressor p53 (13). However, HDACIs induce histone hyperacetylation in both tumor and normal tissues. Thus, altered gene expression patterns through histone/chromatin modulation might not be the primary mechanism to confer cancer selectivity of HDACIs. Alternatively, the tumor selectivity of HDACIs could be related to the chromatin modifications that are associated with oncogenic transformation, which in turn activates an apoptosis program normally suppressed during oncogenesis, an innate tumor suppressive mechanism coupled to oncogenic signaling (14). As a result, cancer cells harboring oncogenic lesions are more susceptible to the cytotoxic effects of HDACIs.
One such oncogenic lesion lies in the Rb/E2F1 pathway. The loss of the Rb tumor suppressor gene has been reported in many human tumors (15). The Rb tumor suppressor regulates proliferation and survival by modulating the activity of E2F transcription factors. Hypophosphorylated Rb binds to and sequesters the transcription factor E2F, resulting in the repression of proliferation-associated genes. Inactivation of Rb results in increased E2F1 activity and subsequent transactivation of genes required for cell cycle progression, leading to aberrant cell proliferation (16). Although Rb disruption primarily occurs in retinoblastoma, Rb inactivation can be caused in many tumor types by alterations of other components in this regulatory machinery, such as loss of p16(INK4), or overexpression of cyclin D1 and Cdk4. In addition, increased E2F1 expression has also been observed in several types of human tumors including breast cancer, non-small cell lung cancer, and salivary gland tumor (17–19). Therefore, the activation of E2F1 activity through various mechanisms allows tumor cells to evade cell cycle regulation and proliferate uncontrollably. Accordingly, disruption of the normal Rb-E2F function is regarded as one of the most frequent alterations of malignant transformation (20). As a fail-safe mechanism to protect aberrant oncogenic transformation (14), E2F1 is also equipped with a tumor suppressor function by inducing apoptosis. Through this mechanism, cells with mutations in the Rb-E2F pathway will be predisposed to die and to be cleared. Indeed, deregulated E2F1 activity can trigger apoptosis through regulating the expression of proapoptotic genes (21, 22). These regulations include the induction of p19ARF (23, 24) or Chk2 (25) and subsequently activation of the p53-dependent apoptotic pathway. E2F1 also induces the expression of p73 (26, 27), caspases (28), and proapoptotic BH3-only proteins of the Bcl-2 family (29) and thus induces apoptosis through a p53-independent mechanism. To allow malignant outgrowth, the oncogene-coupled apoptosis function is either disrupted or inactivated. Therefore, therapeutic approaches for fully activating oncogene-induced apoptosis seem to be conceptually feasible to achieve tumor-specific intervention.
In this study, we demonstrate that HDACIs promote apoptosis through activation of the oncogenic Rb/E2F1 pathway and that cancer cells with increased E2F1 activity or Rb inactivation are highly susceptible to HDACI-induced cell death. We show that the proapoptotic Bcl-2 family member Bim is a key mediator of this apoptotic process. Our results provide a mechanistic explanation for the tumor selectivity of HDACIs and suggest that HDACIs might preferentially kill tumors with deregulated Rb-E2F1 pathway.
Materials and Methods
Cell Culture and Chemicals. p53-null HCT116 cells were kindly provided by B. Vogelstein (The Johns Hopkins University, Baltimore). IMR90, U2OS, and Saos-2 cells were from American Type Culture Collection. IMR90-E1A cells were kindly provided by C. Brancolini (University of Udine, Udine, Italy). ER-E2F1-expressing cells were generated as described (30). TSA was purchased from Cell Signaling Technology (Beverly, MA) and SAHA was from Alexis Biochemicals (San Diego).
Western Blotting. Western blotting was performed as described (31). Antibodies against the following proteins were used: E2F1, p73, poly(ADP-ribose) polymerase (PARP), cyclin E, α-tubulin, and β-actin (Santa Cruz Biotechnology); Bim (BD Pharmingen); and caspase-3 (Cell Signaling Technology). Signals were detected by enhanced chemiluminescence signal by x-ray film (Kodak).
Chromatin Immunoprecipitation (ChIP). ChIP assays were performed as described for E2F1 (32). Chromatin extracts containing a DNA fragment of an average size of 500 bp were immunoprecipitated by using anti-E2F1 polyclonal antibody (C20, Santa Cruz Biotechnology). PCR primers are available upon request.
RNA Interference. Bim-specific small interfering RNA (siRNA) and negative control siRNA were purchased from Cell Signaling Technology. SMARTpool E2F1 siRNA and negative control siRNA were purchased from Dharmacon Research (Lafayette, CO). Cells were transfected with Lipofectamine 2000 according to the manufacturer's protocol in the presence of siRNAs.
Further Details. Further details of the experimental procedures are provided in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Results
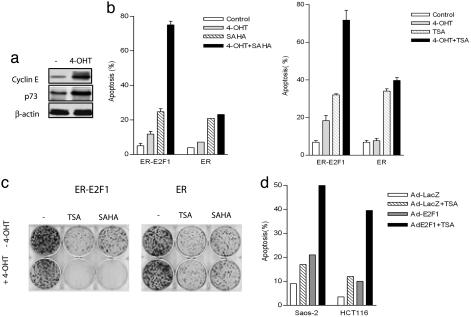
E2F1 Overexpression Facilitates HDACI-Induced Cell Death in Human Cancer Cells. Activation of E2F1 induces apoptosis through both p53-dependent and independent mechanisms. The former is primarily mediated through the p19ARF/Mdm2 pathway and has been well characterized (24, 25, 33, 34). To investigate the regulation of E2F1 apoptotic activity independent of p53, we used p53-null HCT116 cells to establish cell line stably expressing E2F1 fused to the 4-hydroxytamoxifen (4-OHT)-responsive ligand-binding domain of the estrogen receptor (ER) (30). ER-E2F1 fusion protein is maintained inactive in the cytoplasm in the absence of 4-OHT and becomes activated after addition of 4-OHT by allowing ER-E2F1 translocation to the nucleus (30, 35). As expected, addition of 4-OHT in ER-E2F1-expressing cells led to a strong increase of cyclin E and p73 expression (Fig. 1a), two bona fide E2F1 targets (26, 27, 36).
Fig. 1.
HDACIs SAHA and TSA promote E2F1-mediated cell death. (a) p53-null HCT116-ER-E2F1-expressing cells were treated with or without 4-OHT. Cyclin E and p73 expressions were evaluated by immunoblot analysis. (b) ER-E2F1-expressing or control ER-expressing cells were treated with 1 μM SAHA (Left) or 100 nM TSA (Right), in the presence or absence of 4-OHT. After 48 h, cells were harvested, and cell death was assessed by PI staining using FACS. Mean results of three independent experiments are shown with standard deviations. (c) Colony formation assay. One thousand ER-E2F1- or ER-expressing cells were plated per well in sixwell plates for 48 h and followed by the indicated treatment for 24 h. Cells were washed, fresh DMEM was added, and colonies were stained with crystal violet 12 days later. Representative plates are shown. (d) Saos-2 and HCT116 cells infected with adenovirus containing E2F1 or LacZ were treated with 100 nM TSA for 24 h, and cell death was analyzed by FACS.
To investigate whether E2F1 activation induces sensitization of cells to HDACI-induced cell death, we analyzed the cell cycle profiles by FACS in ER-E2F1-expressing HCT116 p53-null cells (ER-E2F1) and the control cells expressing ER-binding domain only (ER) after the treatment with HDACIs SAHA or TSA in the presence or absence of 4-OHT. In ER-E2F1-expressing cells, 4-OHT or SAHA alone induced ≈13% and 23% of sub-G1 cells indicative of cell death, respectively. Addition of both 4-OHT and SAHA resulted in a marked increase in sub-G1 cells (75%), indicating a strong synergistic cell death response (Fig. 1b Left). This synergistic effect was, however, not seen in the control ER-expressing cells. Similar results were obtained in TSA-treated cells (Fig. 1b Right). Moreover, E2F1 activation by 4-OHT greatly promoted TSA or SAHA-induced growth inhibition, as determined by colony formation assay (Fig. 1c). These results indicate that E2F1 overexpression resulted in enhanced cell death and growth inhibition in response to HDACIs.
To determine the effects of E2F1 overexpression on HDACI-induced apoptosis using a different system and an additional cell type, we infected p53-deficient osteosarcoma Saos-2 cells and p53 normal HCT116 cells with either E2F1-expressing adenovirus (Ad-E2F1) or control adenovirus (Ad-LacZ) and monitored the cell death response after TSA treatment. As shown in Fig. 1d, in each case, cell death after TSA treatment was significantly enhanced by Ad-E2F1 infection. In contrast, no such response was observed for cells infected with Ad-LacZ. These results are in agreement with the data from ER-E2F1-expressing cells and, together with Fig. 1 b and c, clearly indicate that E2F1 overexpression sensitizes HDACI-induced cell death and that this cell death potentiation is independent of p53 status.
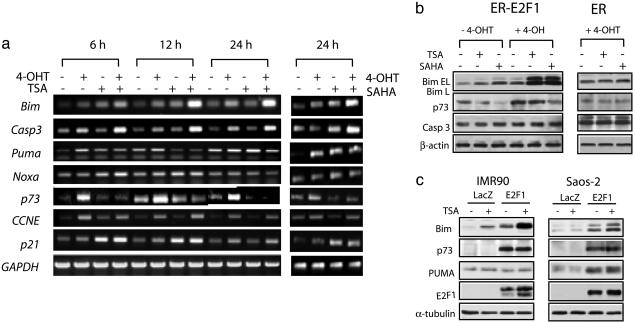
Selective Activation of E2F1 Proapoptotic Targets by HDACIs. We reasoned that HDACIs might promote E2F1-mediated cell death, which prompted us to search E2F1-regulated gene response to HDACIs. To achieve this aim, we used the microarray screening approach. BCL2L11, which encodes the proapoptotic BH3-only Bcl2 family member Bim (37, 38) and a recently identified E2F1 target (29), was found to be markedly induced by SAHA or TSA upon E2F1 activation (data not shown). To confirm the microarray data and to investigate whether other E2F1 proapoptotic targets are involved, we used RT-PCR to examine the expressions of a number of known E2F1 targets, including CCNE, p73, caspase-3, and the BH3-only proteins such as Puma, Noxa, and Bim, in ER-E2F1-expressing cells treated with TSA or SAHA in the presence or absence of 4-OHT (Fig. 2a). Time-course analysis showed that addition of 4-OHT to ER-E2F1-expressing cells led to strong inductions of CCNE and p73 transcripts, and to a lesser extent, caspase-3, Bim, Puma, and Noxa transcripts. Of these genes, 4-OHT-induced Bim and caspase-3 expressions were further up-regulated by TSA or SAHA treatment (Fig. 2a). By contrast, expression of CCNE and other closely related BH3-only transcripts Puma and Noxa were, however, not notably up-regulated. Intriguingly, the 4-OHT-induced p73 expression was paradoxically reduced by both TSA and SAHA. p21 activation, which is a typical response of HDACIs, was induced by TSA and SAHA as expected, and this activation was not further increased in the presence of 4-OHT. These findings suggest that HDAC inhibition alters the ability of E2F1 to activate transcription in a target gene-specific manner.
Fig. 2.
HDACIs selectively activate E2F1 target genes. (a) ER-E2F1-expressing cells were treated with 100 nM TSA for the indicated times or 1 μM SAHA for 24 h in the presence or absence of 4-OHT. mRNA levels of E2F1 target genes as well as p21 and GAPDH were detected with RT-PCR. (b) Cells were treated in a. The expressions of E2F1 target gene products were assessed by Western blotting with antibodies to Bim, Puma, caspase-3, and p73. (c) Saos-2 and IMR90 cells were infected with Ad-E2F1 or Ad-LacZ for 24 h before treatment with 100 nM TSA for an additional 24 h. Expressions of Bim, p73, Puma, and E2F-1 were assessed by Western blotting with corresponding antibodies.
To determine whether these transcriptional changes will result in alterations in protein expression, we performed immunoblot analysis of Bim, Puma, caspase 3, and p73. Fig. 2b shows that treatment of ER-E2F1-expressing cells with SAHA or TSA in the presence of 4-OHT dramatically increased Bim protein levels, compared with control ER-expressing cells or cells treated with TSA, SAHA, or 4-OHT alone. Caspase-3 protein levels, as opposed to the striking increase in mRNA levels, were not notably increased. Consistent with the mRNA analysis, Puma protein levels were not affected and the E2F1-dependent p73 induction was reduced by TSA and SAHA treatment. Thus, among the known E2F1 proapoptotic targets we have surveyed, Bim seems to be the primary E2F1 proapoptotic target whose expression is substantially up-regulated by HDACIs upon E2F1 activation in both mRNA and protein levels. This observation was further confirmed in normal human fibroblast IMR90 cells and osteosarcoma Saos-2 cells by using Ad-E2F1. In both cases, TSA treatment of cells infected with Ad-E2F1 resulted in a marked increase in Bim expression compared with cells infected with Ad-LacZ (Fig. 2c). In contrast, p73 and Puma were not affected. Collectively, these results suggest that HDACIs selectively activate the E2F1 proapoptotic target Bim. Interestingly, this observation is in contrast to E2F1 activation by DNA damage, which results in selective induction of p73 (32, 39). In agreement with the known role of Bim in mitochondria-mediated apoptosis, we observed increased activation of caspase-3 activity, increased PARP cleavage, and the disruption of mitochondria membrane potential after SAHA or TSA treatment upon E2F1 activation, and caspase 3 inhibitor partially blocked this apoptosis (Fig. 6, which is published as supporting information on the PNAS web site).
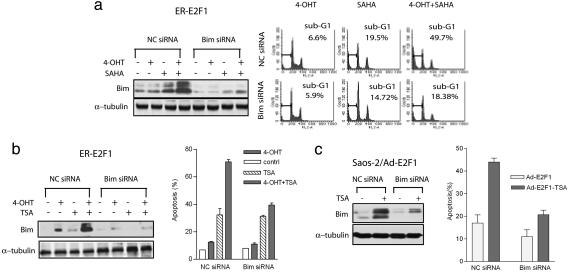
Bim Is Functionally Important in Conferring Sensitivity of HDACIs upon E2F1 Activation. To definitively establish the role of Bim in HDACI-induced apoptosis upon E2F1 activation, we used RNA interference to silence Bim expression and analyzed its biological effects. Treatment of ER-E2F1-expressing cells with Bim siRNA but not control siRNA resulted in the significant reduction in Bim expression and nearly completely abolished SAHA-induced Bim induction (Fig. 3a Left). As a result, ER-E2F1-expressing cells treated with Bim siRNA showed a marked decrease in SAHA-induced apoptosis in the presence of 4-OHT (18%), as compared with the control siRNA-treated cells (49%)(Fig. 3a Right). Similar results were also obtained in TSA-treated cells (Fig. 3b). Thus, silencing of Bim induction by siRNA transfection resulted in effective abrogation of apoptosis induced by HDACIs upon E2F1 activation. Likewise, silencing of Bim expression also resulted in the decreased cell death response to TSA in Saos-2 cells infected with Ad-E2F1 (Fig. 3c). These concordant results show that Bim induction mediates the apoptosis potentiation upon E2F1 activation in response to HDACIs.
Fig. 3.
The effect of Bim-specific siRNA on HDACI-induced apoptosis upon E2F1 activation. (a) ER-E2F1 were transfected with nonspecific control siRNA (NC siRNA) or Bim-specific siRNA for 48 h, and then either left untreated (-) or treated with 4-OHT, SAHA, or both for an additional 24 h. The expression of Bim was analyzed by Western blotting (Left). Cell death was analyzed by flow cytometry after propidium iodide staining (Right). The percentages of cells in sub-G1 are indicated. (b) ER-E2F1 cells were transfected with nonspecific control siRNA (NC siRNA) or Bim-specific siRNA for 48 h, and then either left untreated (-) or treated with 4-OHT, 100 nM TSA, or both for an additional 48 h. The expression of Bim was analyzed by Western blotting (Left). Cell death was assessed as in a, and the graph shows the mean results of three independent experiments with standard deviations (Right). (c) Saos-2 cells treated with Bim or control siRNA were infected with Ad-E2F1 for 24 h and followed by 100 nM TSA treatment for an additional 24 h. Bim expression was analyzed by Western blotting (Left), and cell death was assessed as in a; the graph shows the mean results of three independent experiments with standard deviations (Right).
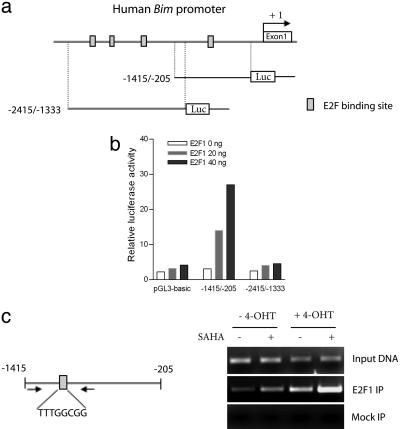
HDACIs Induce Bim Transcription Through Increased E2F1 Recruitment to the Bim Gene Promoter. BH3-only proteins including Bim have been proposed to be the E2F1 targets. However, functional E2F1-binding sites in the human Bim promoter have not been previously identified. Sequence analysis of a genomic sequence spanning 2.5 kb base pairs upstream of the transcription start site of the human Bim promoter revealed the presence of four putative E2F-binding sites [TTT(C/G)GCGC] at position -1270/-1263, -1734/-1727, -2112/-2105, and -2245/-2238 from the transcription start site (Fig. 4a). To determine whether the human Bim promoter was responsive to E2F1, we isolated two genomic DNA fragments spanning the Bim promoter region -1415/-205 and -2415/-1333 containing one and three putative E2F1-binding sites, respectively. The two fragments were cloned into the pGL3-luciferase reporter construct for analysis of promoter activity. The results indicate that E2F1 can induce strong induction in the promoter activity of the reporter construct that contains -1415/-205, suggesting that E2F1 is capable of activating the Bim -1415/-205 promoter. In contrast, Bim -2415/-1333 promoter had no response to E2F1 (Fig. 4b). Thus, the putative E2F1-binding site (TTTGGCGG) within -1415/-205 seemed to be a functional E2F1 responsive element, denoted as E2F1-RE, in the Bim promoter.
Fig. 4.
SAHA promotes E2F1 recruitment to the Bim promoter. (a) Schematic representation of human Bim promoter containing putative E2F-binding sites. The indicated regions were isolated and cloned into pGL3 reporter construct. (b) pGL3-basic, Bim -1415/-205 or -2415/-1333 luciferase construct and Renilla luciferase construct were transfected into HCT116 cells together with increasing amounts of E2F1-expressing vector (0, 20, and 40 ng). Relative luciferase activities were measured 48 h after transfection. Results are depicted as fold induction, after normalization to the Renilla luciferase activity. (c Left) Schematic representation of the human Bim promoter containing the E2F1-RE element. (Right) E2F1 binding to the Bim promoter was analyzed by ChIP. Crosslinked chromatin from ER-E2F1-expressing cells treated as indicated were immunoprecipitated with antibody to E2F1 and nonspecific IgG (mock IP), and the Bim promoter fragment was amplified by PCR by using primers flanking the E2F1-RE-containing region. Positive control (input DNA) amplifications are shown.
To confirm that E2F1 binds to the E2F1RE in vivo, we performed ChIP assays using an anti-E2F1 antibody and PCR primers encompassing the E2F1-RE (Fig. 4c Left). The results show that E2F1 occupancy of the Bim E2F1-RE is readily detectable in ER-E2F1-expressing cells in the absence of 4-OHT, indicating endogenous binding of E2F1 to the Bim promoter. SAHA treatment promoted recruitment of endogenous E2F1 to the Bim promoter. In the presence of 4-OHT, E2F1 binding to the Bim promoter was significantly increased, and this binding was further markedly increased by SAHA (Fig. 4c Right). No signal above background was seen with nonspecific IgG. Thus, consistent with Bim being a direct target of E2F1, our study identified the functional E2F1-binding site in the human Bim promoter and further demonstrated that the increase in E2F1 occupancy onto the Bim promoter after HDAC inhibition is associated with the up-regulation of Bim expression.
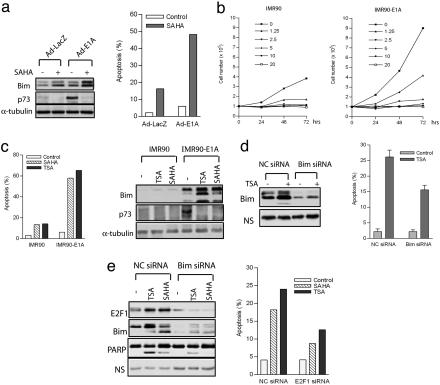
The Role of Endogenous E2F1 in Bim Induction and Apoptosis Sensitization to HDACIs. We next examined the apoptosis response to HDAC inhibition resulting from deregulation of endogenous E2F activity. E1A oncoprotein protein binds to and inactivates Rb family members (40, 41), resulting in the activation of the endogenous E2F1 (42, 43). To determine the effect of Rb inactivation on Bim expression and cellular response to HDACIs, we infected Rb wild-type U2OS cells with adenovirus-expressing E1A or the control adenovirus. Fig. 5a shows that U2OS cells infected with Ad-E1A express higher E2F1 targets Bim and p73 as expected, and, after SAHA treatment, Bim but not p73 was further increased. Consistently, U2OS cells expressing E1A were much more sensitive to SAHA treatment compared with the U2OS cells infected with Ad-LacZ (Fig. 5a Right).
Fig. 5.
The role of endogenous E2F1 in Bim and apoptosis induction after HDACI treatment. (a) U2OS cells were infected with Ad-E1A or Ad-LacZ for 24 h and treated with SAHA (1 μM) for an additional 48 h. Levels of Bim and p73 were assessed by immunoblotting by using antibodies against the indicated proteins (Left). Cell death was determined by FACS analysis (Right). (b) Normal IMR90 and E1A-transformed IMR90 cells were treated with SAHA at indicated concentrations, and cell proliferation was evaluated for the indicated times. (c) Expression of E1A results in up-regulation of Bim and apoptosis potentiation in response to SAHA and TSA. IMR90 and IMR90-E1A cells were treated with SAHA (2.5 μM) and TSA (1 μM) for 48 h. Cell death was determined by FACS analysis (Left), and the levels of Bim and p73 were assessed by immunoblotting by using antibodies against the indicated proteins (Right). (d) IMR90-E1A cells transfected with Bim siRNA or control siRNA (NC siRNA) were treated with TSA (1 μM) for 24 h. The expressions of Bim and β-actin were assessed by immunoblotting by using antibodies against the indicated proteins. Apoptosis was evaluated by FACS analysis. (e) Saos-2 cells were transfected with E2F1 siRNA or negative control siRNA (NC siRNA) and treated with TSA (100 nM) or SAHA (1 μM). The expressions of E2F1, Bim, and PARP were assessed by immunoblotting by using antibodies against the indicated proteins. NS, nonspecific band as the loading control. Apoptosis was evaluated by FACS analysis.
To determine the tumor selectivity of E2F1-Bim activation, we compared the impacts of HDACIs on both proliferation and apoptosis in normal diploid human lung fibroblast IMR90 cells and E1A-transformed IMR90 cells. A dose-response study showed that SAHA substantially inhibited proliferation of both normal and transformed IMR90 cells (Fig. 5b). However, normal IMR90 cells were basically not responsive to SAHA and TSA in inducing apoptosis, whereas the same treatments in E1A-transformed IMR90 cells triggered a strong apoptosis (Fig. 5c Left). This observation is consistent with the previous report (12) using different cell lines and suggests that the tumor selectivity of HDACIs might primarily occur in the pathway(s) that regulates apoptosis rather than cell proliferation. Consistent with the higher E2F1 activity in IMR90-E1A cells, Bim and p73 were up-regulated in these cells, and, after SAHA and TSA treatments, Bim levels were further dramatically increased. In contrast, Bim induction was barely induced by TSA and SAHA in normal IMR90 cells (Fig. 5c Right). p73 expression, again, was reduced by SAHA, an observation consistent with E2F1-overexpresing cells. In addition, siRNA-mediated knockdown of Bim in IMR90-E1A cells effectively inhibited apoptosis induction by TSA (Fig. 5d). Thus, oncogene E1A expression in normal IMR90 cells leading to the increased endogenous E2F1 activity sensitized these cells to HDACI-induced apoptosis at least in part through the induction of Bim.
We further used Rb-deficient Saos-2 cells to examine the role of endogenous E2F1 in apoptotic response and Bim induction after TSA or SAHA treatment. Fig. 5e shows that Saos-2 cells treated with E2F1 siRNA had substantially reduced Bim induction, inhibited PARP cleavage, and reduced apoptosis after TSA or SAHA treatment. These results clearly demonstrate that endogenous E2F1 is required for the robust induction of Bim and participates in the apoptotic response to HDACIs.
Discussion
HDACIs are considered to be promising chemotherapeutic agents due to their selective activity toward cancer cells. However, the basis for tumor selectivity of these compounds is one of the unsolved questions (2). The results described here establish the oncogenic Rb/E2F1 pathway as a target for HDACIs and demonstrate that the E2F1 pathway participates in HDACI-induced apoptosis. That Rb/E2F pathway is frequently deregulated in many types of cancers and that HDACIs preferentially kill tumor cells carrying enhanced E2F1 activity define a set of molecular conditions for selectivity of anti-tumor effects of HDACIs. Taken together, we posit that tumors with defective pRb and confirmed up-regulation of the E2F1 pathway would be specifically sensitive to HDAC inhibitors.
Importantly, we identified Bim as a key mediator of E2F1-induced apoptosis provoked by HDACIs. Bim is a proapoptotic BH3 domain-only member of the Bcl-2 family and can trigger intrinsic apoptosis pathway through activation of Bax (37, 44, 45). Among previously identified E2F1 proapoptotic targets that have been served by using both microarray and RT-PCR, Bim was found to be dramatically increased in both mRNA and protein levels by HDACIs upon E2F1 activation. Moreover, silencing of Bim expression by RNA interference (RNAi) efficiently abrogated the cell death enhancement induced by HDACIs in E2F1-overexpressing cells, indicating that the sole up-regulation of Bim is sufficient to drive these cells into strong apoptosis. However, we do not exclude the possibility that induction of other previously unidentified E2F1 targets might also contribute to the sensitization of apoptosis. Nevertheless, the nearly complete abrogation of apoptosis sensitization by Bim siRNA indeed indicates that Bim plays a central role in this process.
An intriguing observation is that the other BH3-only proteins such as Puma and Noxa are not affected by HDAC inhibition, although they are also previously identified E2F1 targets (29). The molecular basis that dictates the selective activation of E2F1 target genes by HDACIs is not known. One possibility for this target selection is due to E2F1 acetylation, because acetylation of transcription factors p53 and p73 were known to lead to the selective activation of proapoptoic targets (46, 47). However, we found that ER-E2F1 mutated in three acetylation sites had no reduced ability to transactivate Bim as well as the apoptotic response to HDACI (data not shown). Alternatively, the selected activation of target genes could be due to the increased acetylation of proteins physically associated with E2F1 or increased recruitment of histone acetyltransferases (HATs) and subsequent acetylating of histones of affected promoters.
Previously, studies show that DNA damage promotes E2F1-mediated apoptosis through selective induction of p73 (32, 39) or activation of the Chk2-p53 network (25). In striking contrast to that induced by DNA damage, we demonstrate that activation of E2F1 apoptotic function by HDACI does not require either p73 or p53, but proceeds through robust activation of Bim. Unlike other BH-3-only members, such as Puma, Noxa, and Bid, that are p53 targets and participate in DNA damage-induced apoptosis (48–51), there is no evidence that Bim is a p53 target. Instead, its expression is tightly regulated by growth factor signals through phosphatidylinositol 3-OH kinase (PI3K)/protein kinase B (PKB)/Akt (52, 53) or ERK/mitogen-activated protein kinase (MAPK) pathway (54–56) and participate in apoptosis induced by cytokine withdraw. Thus, Bim seems to be separated out from other members of BH-3-only proteins and seems to have emerged as a key mediator of oncogene-induced apoptosis, whereas Puma, Noxa, and Bid are primarily involved in DNA damage and p53-mediated apoptotic response. Indeed, it was recently reported that loss of Bim facilitates oncogene Myc-induced tumorigenesis (57, 58), although it is not clear whether Bim is a direct target of Myc. Taken together, Bim seems to be a key mediator of oncogene-induced apoptosis. It is thus expected that, in tumors where p53 is lost, Bim-mediated apoptotic pathway might be key to couple oncogenic lesions to achieve the fail-safe homeostatic mechanism. Thus, using HDACIs to promote E2F1-mediated but p53-independent apoptosis provides the proof of concept that restoration of oncogene-induced apoptosis without causing DNA damage is a feasible strategy for cancer-specific therapy.
Supplementary Material
Acknowledgments
We thank Dr. Kristian Helin (European Institute of Oncology, Milan) for the ER-E2F1 plasmids, Dr. Joseph Nevins (Duke University, Durham, NC) for the adenoviral E2F1, Dr. Andrew Turnell (University of Birmingham, Birmingham, U.K.) for adenoviral E1A, Dr. Bert Vogelstein for p53-null HCT116 cells, and Dr. Claudio Brancolini for IMR90-E1A cells. This work was supported by the Agency for Science, Technology and Research of Singapore.
Author contributions: Y.Z., J.T., L.Z., and X.J. performed research; Y.Z., J.T., L.Z., X.J., E.T.L., and Q.Y. analyzed data; Q.Y. designed research; and Q.Y. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HDACI, histone deacetylase inhibitor; TSA, trichostatin A; SAHA, suberoylanilide hydroxamic acid; ChIP, chromatin immunoprecipitation; siRNA, small interfering RNA; ER, estrogen receptor; 4-OHT, 4-hydroxytamoxifen; PARP, poly(ADP-ribose) polymerase.
References
- 1.Gabrielli, B. G., Johnstone, R. W. & Saunders, N. A. (2002) Curr. Cancer Drug Targets 2, 337-353. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone, R. W. & Licht, J. D. (2003) Cancer Cell 4, 13-18. [DOI] [PubMed] [Google Scholar]
- 3.Marks, P. A., Richon, V. M., Breslow, R. & Rifkind, R. A. (2001) Curr. Opin. Oncol. 13, 477-483. [DOI] [PubMed] [Google Scholar]
- 4.Melnick, A. & Licht, J. D. (2002) Curr. Opin. Hematol. 9, 322-332. [DOI] [PubMed] [Google Scholar]
- 5.He, L. Z., Tolentino, T., Grayson, P., Zhong, S., Warrell, R. P., Jr., Rifkind, R. A., Marks, P. A., Richon, V. M. & Pandolfi, P. P. (2001) J. Clin. Invest. 108, 1321-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warrell, R. P., Jr. (1999) Haematologica 84, Suppl. EHA-4, 75-77. [PubMed] [Google Scholar]
- 7.Kelly, W. K., O'Connor, O. A., Krug, L. M., Chiao, J. H., Heaney, M., Curley, T., MacGregore-Cortelli, B., Tong, W., Secrist, J. P., Schwartz, L., et al. (2005) J. Clin. Oncol. 23, 3923-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida, M., Kijima, M., Akita, M. & Beppu, T. (1990) J. Biol. Chem. 265, 17174-17179. [PubMed] [Google Scholar]
- 9.Richon, V. M., Emiliani, S., Verdin, E., Webb, Y., Breslow, R., Rifkind, R. A. & Marks, P. A. (1998) Proc. Natl. Acad. Sci. USA 95, 3003-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nebbioso, A., Clarke, N., Voltz, E., Germain, E., Ambrosino, C., Bontempo, P., Alvarez, R., Schiavone, E. M., Ferrara, F., Bresciani, F., et al. (2005) Nat. Med. 11, 77-84. [DOI] [PubMed] [Google Scholar]
- 11.Insinga, A., Monestiroli, S., Ronzoni, S., Gelmetti, V., Marchesi, F., Viale, A., Altucci, L., Nervi, C., Minucci, S. & Pelicci, P. G. (2005) Nat. Med. 11, 71-76. [DOI] [PubMed] [Google Scholar]
- 12.Ungerstedt, J. S., Sowa, Y., Xu, W. S., Shao, Y., Dokmanovic, M., Perez, G., Ngo, L., Holmgren, A., Jiang, X. & Marks, P. A. (2005) Proc. Natl. Acad. Sci. USA 102, 673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy, S., Packman, K., Jeffrey, R. & Tenniswood, M. (2005) Cell Death Differ. 12, 482-491. [DOI] [PubMed] [Google Scholar]
- 14.Lowe, S. W., Cepero, E. & Evan, G. (2004) Nature 432, 307-315. [DOI] [PubMed] [Google Scholar]
- 15.Sherr, C. J. & McCormick, F. (2002) Cancer Cell 2, 103-112. [DOI] [PubMed] [Google Scholar]
- 16.Sherr, C. J. (1996) Science 274, 1672-1677. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, S. Y., Liu, S. C., Al-Saleem, L. F., Holloran, D., Babb, J., Guo, X. & Klein-Szanto, A. J. (2000) Cancer Epidemiol. Biomarkers Prev. 9, 395-401. [PubMed] [Google Scholar]
- 18.Gorgoulis, V. G., Zacharatos, P., Mariatos, G., Kotsinas, A., Bouda, M., Kletsas, D., Asimacopoulos, P. J., Agnantis, N., Kittas, C. & Papavassiliou, A. G. (2002) J. Pathol. 198, 142-156. [DOI] [PubMed] [Google Scholar]
- 19.Etges, A., Nunes, F. D., Ribeiro, K. C. & Araujo, V. C. (2004) Oral Oncol. 40, 326-331. [DOI] [PubMed] [Google Scholar]
- 20.Cam, H. & Dynlacht, B. D. (2003) Cancer Cell 3, 311-316. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg, D. (2002) FEBS Lett. 529, 122-125. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura, I., Tanaka, H. & Kanakura, Y. (2003) Cell Cycle 2, 333-338. [PubMed] [Google Scholar]
- 23.Zhu, J. W., DeRyckere, D., Li, F. X., Wan, Y. Y. & DeGregori, J. (1999) Cell Growth Differ. 10, 829-838. [PubMed] [Google Scholar]
- 24.Bates, S., Phillips, A. C., Clark, P. A., Stott, F., Peters, G., Ludwig, R. L. & Vousden, K. H. (1998) Nature 395, 124-125. [DOI] [PubMed] [Google Scholar]
- 25.Rogoff, H. A., Pickering, M. T., Frame, F. M., Debatis, M. E., Sanchez, Y., Jones, S. & Kowalik, T. F. (2004) Mol. Cell. Biol. 24, 2968-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiewe, T. & Putzer, B. M. (2000) Nat. Genet. 26, 464-469. [DOI] [PubMed] [Google Scholar]
- 27.Irwin, M., Marin, M. C., Phillips, A. C., Seelan, R. S., Smith, D. I., Liu, W., Flores, E. R., Tsai, K. Y., Jacks, T., Vousden, K. H. & Kaelin, W. G., Jr. (2000) Nature 407, 645-648. [DOI] [PubMed] [Google Scholar]
- 28.Nahle, Z., Polakoff, J., Davuluri, R. V., McCurrach, M. E., Jacobson, M. D., Narita, M., Zhang, M. Q., Lazebnik, Y., Bar-Sagi, D. & Lowe, S. W. (2002) Nat. Cell Biol. 4, 859-864. [DOI] [PubMed] [Google Scholar]
- 29.Hershko, T. & Ginsberg, D. (2004) J. Biol. Chem. 279, 8627-8634. [DOI] [PubMed] [Google Scholar]
- 30.Vigo, E., Muller, H., Prosperini, E., Hateboer, G., Cartwright, P., Moroni, M. C. & Helin, K. (1999) Mol. Cell. Biol. 19, 6379-6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kho, P. S., Wang, Z., Zhuang, L., Li, Y., Chew, J. L., Ng, H. H., Liu, E. T. & Yu, Q. (2004) J. Biol. Chem. 279, 21183-21192. [DOI] [PubMed] [Google Scholar]
- 32.Urist, M., Tanaka, T., Poyurovsky, M. V. & Prives, C. (2004) Genes Dev. 18, 3041-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue, K., Roussel, M. F. & Sherr, C. J. (1999) Proc. Natl. Acad. Sci. USA 96, 3993-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson, K. D. & Jones, P. A. (1998) Mol. Cell. Biol. 18, 6457-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanelle, J., Stiewe, T., Theseling, C. C., Peter, M. & Putzer, B. M. (2002) Nucleic Acids Res. 30, 1859-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohtani, K., DeGregori, J. & Nevins, J. R. (1995) Proc. Natl. Acad. Sci. USA 92, 12146-12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouillet, P., Purton, J. F., Godfrey, D. I., Zhang, L. C., Coultas, L., Puthalakath, H., Pellegrini, M., Cory, S., Adams, J. M. & Strasser, A. (2002) Nature 415, 922-926. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor, L., Strasser, A., O'Reilly, L. A., Hausmann, G., Adams, J. M., Cory, S. & Huang, D. C. (1998) EMBO J. 17, 384-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pediconi, N., Ianari, A., Costanzo, A., Belloni, L., Gallo, R., Cimino, L., Porcellini, A., Screpanti, I., Balsano, C., Alesse, E., et al. (2003) Nat. Cell Biol. 5, 552-558. [DOI] [PubMed] [Google Scholar]
- 40.Sherr, C. J. (2001) Nat. Rev. Mol. Cell Biol. 2, 731-737. [DOI] [PubMed] [Google Scholar]
- 41.Dyson, N. (1998) Genes Dev. 12, 2245-2262. [DOI] [PubMed] [Google Scholar]
- 42.Macleod, K. F., Hu, Y. & Jacks, T. (1996) EMBO J. 15, 6178-6188. [PMC free article] [PubMed] [Google Scholar]
- 43.Hurford, R. K., Jr., Cobrinik, D., Lee, M. H. & Dyson, N. (1997) Genes Dev. 11, 1447-1463. [DOI] [PubMed] [Google Scholar]
- 44.Gilley, J., Coffer, P. J. & Ham, J. (2003) J. Cell Biol. 162, 613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Essafi, A., Fernandez de Mattos, S., Hassen, Y. A., Soeiro, I., Mufti, G. J., Thomas, N. S., Medema, R. H. & Lam, E. W. (2005) Oncogene 24, 2317-2329. [DOI] [PubMed] [Google Scholar]
- 46.Costanzo, A., Merlo, P., Pediconi, N., Fulco, M., Sartorelli, V., Cole, P. A., Fontemaggi, G., Fanciulli, M., Schiltz, L., Blandino, G., et al. (2002) Mol. Cell 9, 175-186. [DOI] [PubMed] [Google Scholar]
- 47.Terui, T., Murakami, K., Takimoto, R., Takahashi, M., Takada, K., Murakami, T., Minami, S., Matsunaga, T., Takayama, T., Kato, J. & Niitsu, Y. (2003) Cancer Res. 63, 8948-8954. [PubMed] [Google Scholar]
- 48.Villunger, A., Michalak, E. M., Coultas, L., Mullauer, F., Bock, G., Ausserlechner, M. J., Adams, J. M. & Strasser, A. (2003) Science 302, 1036-1038. [DOI] [PubMed] [Google Scholar]
- 49.Nakano, K. & Vousden, K. H. (2001) Mol. Cell 7, 683-694. [DOI] [PubMed] [Google Scholar]
- 50.Yu, J., Wang, Z., Kinzler, K. W., Vogelstein, B. & Zhang, L. (2003) Proc. Natl. Acad. Sci. USA 100, 1931-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sax, J. K., Fei, P., Murphy, M. E., Bernhard, E., Korsmeyer, S. J. & El-Deiry, W. S. (2002) Nat. Cell Biol. 4, 842-849. [DOI] [PubMed] [Google Scholar]
- 52.Dijkers, P. F., Birkenkamp, K. U., Lam, E. W., Thomas, N. S., Lammers, J. W., Koenderman, L. & Coffer, P. J. (2002) J. Cell Biol. 156, 531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dijkers, P. F., Medema, R. H., Lammers, J. W., Koenderman, L. & Coffer, P. J. (2000) Curr. Biol. 10, 1201-1204. [DOI] [PubMed] [Google Scholar]
- 54.Reginato, M. J., Mills, K. R., Paulus, J. K., Lynch, D. K., Sgroi, D. C., Debnath, J., Muthuswamy, S. K. & Brugge, J. S. (2003) Nat. Cell Biol. 5, 733-740. [DOI] [PubMed] [Google Scholar]
- 55.Shinjyo, T., Kuribara, R., Inukai, T., Hosoi, H., Kinoshita, T., Miyajima, A., Houghton, P. J., Look, A. T., Ozawa, K. & Inaba, T. (2001) Mol. Cell. Biol. 21, 854-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weston, C. R., Balmanno, K., Chalmers, C., Hadfield, K., Molton, S. A., Ley, R., Wagner, E. F. & Cook, S. J. (2003) Oncogene 22, 1281-1293. [DOI] [PubMed] [Google Scholar]
- 57.Egle, A., Harris, A. W., Bouillet, P. & Cory, S. (2004) Proc. Natl. Acad. Sci. USA 101, 6164-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hemann, M. T., Bric, A., Teruya-Feldstein, J., Herbst, A., Nilsson, J. A., Cordon-Cardo, C., Cleveland, J. L., Tansey, W. P. & Lowe, S. W. (2005) Nature 436, 807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.