Abstract
The dual Rab11/Arf binding proteins, family of Rab11-interacting proteins FIP3 and FIP4 function in the delivery of recycling endosomes to the cleavage furrow and are, together with Rab11, essential for completion of abscission, the terminal step of cytokinesis. Here, we report that both FIP3 and FIP4 bind Arf6 in a nucleotide-dependent manner but exhibit differential affinities for Rab11 and Arf6. Both FIP3 and FIP4 can form ternary complexes with Rab11 and Arf6. Arf6 is localised to the furrow and midbody and we show that Arf6-GTP functions to localise FIP3 and FIP4 to midbodies during cytokinesis. Exo70p, a component of the Exocyst complex, also localises to the furrow of dividing cells and interacts with Arf6. We show that depletion of Exo70p leads to cytokinesis failure and an impairment of FIP3 and Rab11 localisation to the furrow and midbody. Moreover, Exo70p co-immunoprecipitates FIP3 and FIP4. Hence, we propose that FIP3 and FIP4 serve to couple Rab11-positive vesicle traffic from recycling endosomes to the cleavage furrow/midbody where they are tethered prior to fusion events via interactions with Arf6 and the Exocyst.
Keywords: cytokinesis, Exocyst, FIP3, FIP4, Rab11
Introduction
A crucial facet of mammalian cell division is the separation of two daughter cells by a process known as cytokinesis. An early event in cytokinesis is the formation of an acto-myosin contractile ring, which functions like a purse string in the constriction of the forming furrow between the two cells (Glotzer, 2001). Far less well characterised are the membrane trafficking steps, which deliver new membrane to the cell surface during the plasma membrane expansion known to accompany furrow formation (O'Halloran, 2000). It is now clearly established that the plasma membrane at the cleavage furrow of mammalian cells has a distinct lipid and protein composition to the rest of the plasma membrane (Emoto et al, 1996; Emoto and Umeda, 2000; O'Halloran, 2000; Finger and White, 2002). This may reflect a requirement both for increased surface area during furrowing and for the coordinated delivery of intracellular signalling or membrane remodelling activities to the correct spatial coordinates during cleavage. Understanding the molecular basis and regulation of these trafficking events is crucial to a full understanding of cell division.
We recently provided evidence showing that Rab11 is required for mammalian cell cytokinesis (Wilson et al, 2005), supporting and extending previous observations in both Drosophila melanogaster and Caenorhabditis elegans (Skop et al, 2001; Pelissier et al, 2003; Riggs et al, 2003). By cycling between GTP- and GDP-loaded conformations, Rab proteins function to recruit different effector proteins that regulate aspects of membrane function including trafficking and membrane fusion (Zerial and McBride, 2001). Our identification of a role for Rab11 in cytokinesis suggests that at least some of the new membrane incorporated into the plasma membrane during furrow expansion is derived from recycling endosomes. Recently, we and others have identified a novel family of Rab11-interacting proteins (FIPs) (Shin et al, 1999; Prekeris et al, 2000, 2001; Hales et al, 2001; Hickson et al, 2003), which all share a highly conserved, 20-amino-acid motif at the C-terminus of the protein, known as the Rab11-binding domain (RBD) (Prekeris et al, 2001; Meyers and Prekeris, 2002). Class II FIPs (Rab11-FIP3 and Rab11-FIP4) are characterised by the presence of EF hands and by a degree of homology with the Drosophila protein Nuclear Fallout, a protein required for cellularisation of Drosophila embryos (Rothwell et al, 1998; Riggs et al, 2003). Consistent with this, we observed that Rab11-FIP3/Arfophilin (hereafter FIP3) and Rab11-FIP4/Arfophilin-2 (hereafter FIP4) also play a role in cytokinesis (Wilson et al, 2005). Rab11 recruits FIP3 to endosomes that accumulate in the furrow region during telophase, and mutants of FIP3 that cannot bind Rab11 no longer associate with recycling endosomes. Such mutants, and also knock down of FIP3 expression with RNAi, result in defective cytokinesis (Wilson et al, 2005). We have shown that Rab11-/FIP3-positive vesicles derived from recycling endosomes traffic to the furrow via centrosome-anchored microtubules where they function to deliver membrane during furrow ingression and abscission as well as possibly to regulate actin remodelling (Wilson et al, 2005). Consistent with this model, both FIP3 and FIP4 are reported to bind Arf proteins (Hickson et al, 2003), monomeric GTPases that also regulate actin dynamics, and both FIP3 and FIP4 exhibit dynamic association with centrosomes (Wilson et al, 2005).
These data, although implicating FIP3 and FIP4 in cytokinesis, leave several questions unanswered. Firstly, why do the class II FIPs interact with different GTPases? Secondly, what are the relative affinities for binding of these GTPases? Thirdly, although Rab11 recruits FIP3 to endosomes, it is not involved in FIP3 and FIP4 recruitment to the midbody, so how do these proteins become localised in this region? Finally, how can class II FIPs be integrated into a model of membrane vesicle docking and fusion in the furrow and/or midbody? Here, we set out to answer these questions. We determined the relative affinity of FIP3 and FIP4 for Rab11, Arf5 and Arf6, and assayed their ability to form ternary complexes with Rab and Arf proteins using recombinant bacterially expressed proteins. We show that FIP3 exhibits preferential binding of Rab11>Arf6≫Arf5. By contrast, FIP4 binds Arf6≫Rab11≃Arf5. Interestingly, both FIP3 and FIP4 are capable of binding Rab11 and Arf6 simultaneously, suggesting that the binding sites for these small GTPases are distinct and nonoverlapping. Mutants of FIP3 and FIP4 that have lost the ability to bind Rab11 but are still capable of interaction with Arf6 support such an interpretation. Using fluorescence microscopy, we previously demonstrated FIP3 in vesicles around the cleavage furrow during late telophase/early cytokinesis, and a recruitment of FIP3 to the midbody during cytokinesis (Wilson et al, 2005). Here, we show that FIP4 also exhibits strong staining in the midbody and on the spindle microtubules. We further show that both FIP3 and FIP4 are localised to midbodies by interaction with Arf6: a GTP-restricted mutant of Arf6 (Arf6-Q67L) recruits FIP3 to midbodies, and the normal localisation of endogenous FIP3 and FIP4 to the midbody of dividing cells is blocked by the GDP-restricted Arf6 mutant, Arf6-T27N. Previous work has shown that Arf6 interacts with the mammalian Exocyst complex (Prigent et al, 2003). Such data offer an intriguing model whereby Rab11-dependent recruitment of FIPs to recycling endosomes may endow these vesicles with the means to interact, via Arf6, with a tethering factor upon arrival at the furrow or midbody. As active Arf6 localises to the furrow and midbody (Schweitzer and D'Souza-Schorey, 2002), we hypothesised that the FIP–Arf6–Exocyst interaction may serve to tether recycling endosomes in the furrow prior to fusion. Consistent with this, we find that Exo70p, a component of the Exocyst complex, co-immunoprecipitates FIP3 and FIP4 and that depletion of Exo70p using small interfering RNA results in a profound cytokinesis defect. Hence, we propose that FIP3 and FIP4 serve to couple Rab11-positive vesicle traffic from recycling endosomes to the cleavage furrow/midbody where they are tethered prior to fusion events via interactions with Arf6 and the Exocyst.
Results
Biochemical characterisation of FIP3 and FIP4 interactions with GTPases
We sought to determine the relative preference of Rab and ARF GTPases interactions with class II FIPs. To achieve this, we expressed the C-terminal domains of FIP3 (residues 300–759) and FIP4 (residues 227–557) as hexa–his fusion proteins in bacteria, and used these together with GST-Rab or GST-Arf proteins in a series of biochemical interaction studies (Figures 1 and 2). FIP3 was found to interact only with Rab11 (Figure 1A), exhibiting a roughly 100-fold greater affinity for Rab11 compared to the Rab family members tested (Rabs 3, 4, 5 (Figure 1A) 7, 9 and 22 (data not shown)). This is consistent with studies of other members of the FIP family of proteins (Junutula et al, 2004). We have previously shown that FIP3 can bind Arf6, and was originally identified in a yeast two-hybrid screen using Arf5-Q71L as bait (Hickson et al, 2003). Hence, we compared the ability of FIP3 to bind these different GTPases in vitro. Using a constant amount of GST-Arf or GST-Rab11, we performed a titration experiment to determine the relative affinities of FIP3 for these proteins. We found that FIP3 strongly interacted with Rab11, and also interacted with Arf6 (Figure 1B). The interaction of FIP3 with Arf5 was considerably less than that with Arf6. These data suggest a relative preference of interaction of FIP3 with Rab11>Arf6≫Arf5. Consistent with this, we found that FIP3 co-immunoprecipitated only Arf6 from transfected HeLa cells (Figure 1C). We then examined the ability of FIP3 to assemble ternary complexes with Rab11 and Arf6. As shown in Figure 1D and E, FIP3 forms a ternary complex with both these GTPases. Thus, Figure 1D shows that increasing concentrations of Rab11 are unable to displace GST-Arf6 from previously assembled FIP3 complexes, and Figure 1E shows that FIP3 in complex with Rab11 can associate with GST-Arf6. Collectively, these data argue that FIP3 can form a ternary complex with Arf6 and Rab11, at least in vitro. Figure 2 reveals a similar analysis for FIP4. This protein exhibited subtle differences in order of preference for the different GTPases, with Arf6 exhibiting the strongest binding, and Rab11 and Arf5 binding with similar affinities (Figure 2A). In data not shown here, we observed no interaction of FIP4 with Rab3, 4, 7, 9 or 22. FIP4 is also capable of forming a ternary complex with Rab11 and Arf6 in vitro (Figure 2B). Although we have previously shown a GTP-dependent interaction with Arf5 for both FIP3 and FIP4, the nucleotide dependence of Arf6 binding had not been addressed. To that end, Figure 2C shows that GTP-loaded wild-type Arf6-GST can pull down both FIP3 and FIP4, but that a dominant-negative mutant does not. As only Arf6 appears to interact with FIP3 and FIP4 in vivo, we have focused upon this interaction in the experiments below (see also Discussion).
Figure 1.
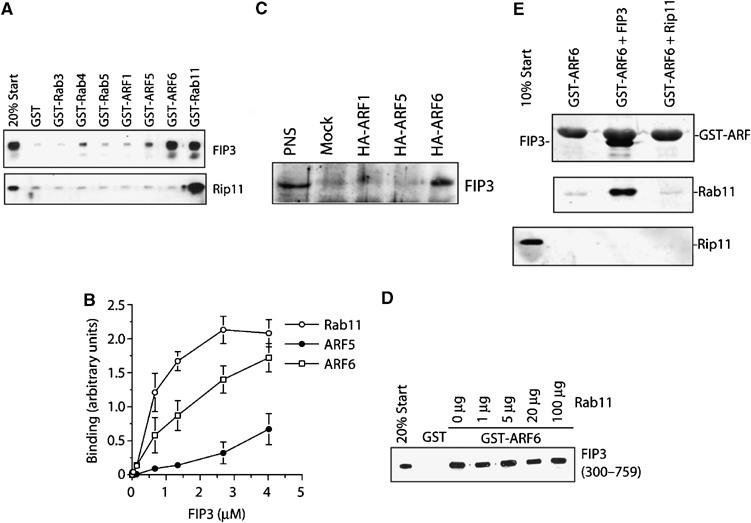
FIP3 binds Arf6 and Rab11. The binding characteristics of FIP3 were examined in a series of in vitro experiments. The C-terminus of FIP3 (residues 300–759) or Rip11 (residues 490–652) were expressed in bacteria as hexa–his fusions and incubated with 50 μl of beads loaded with 5 μg GST alone, or GST-Rabs/Arfs as indicated for 1 h. After washing, the beads were boiled in SDS–PAGE sample buffer and levels of associated FIP3 or Rip11 determined by immunoblotting. Data from a representative experiment is shown in (A). In the experiment shown in (B). increasing concentrations of FIP3-containing beads were incubated with 5 μg of Arf5, Arf6 or Rab11, and the binding determined by quantitative immunoblotting. The data shown is from three experiments of this type (mean±s.d.). (C) HeLa cells were transfected with HA-tagged Arf1, Arf5 or Arf6 as described. After 48 h, cells were lysed and an anti-HA immunoprecipitation performed. The immunoprecipitates were probed for the presence of endogenous FIP3. The result of a typical experiment is shown. In the experiment shown in (D), 50 μl of beads loaded with 5 μg of GST or GST-Arf6 were incubated with recombinant FIP3 in the presence of increasing amounts of Rab11. The ability of Rab11 to displace FIP3 from the Arf6-loaded beads was determined by immunoblotting. The experiment shown is typical of three experiments of this type. In order to determine whether FIP3 can form a ternary complex with Arf6 and Rab11, we performed the experiment shown in (E). GST-Arf6 was incubated with recombinant FIP3 (residues 300–759) or Rip11 (residues 490–652). The upper panel shows a Coomassie stain, revealing the interaction of FIP3 with Arf6, but not Rip11. These beads were incubated with recombinant Rab11, washed and then boiled in SDS–PAGE buffer and immunoblotted for Rab11 (middle panel) or Rip11 (lower panel).
Figure 2.
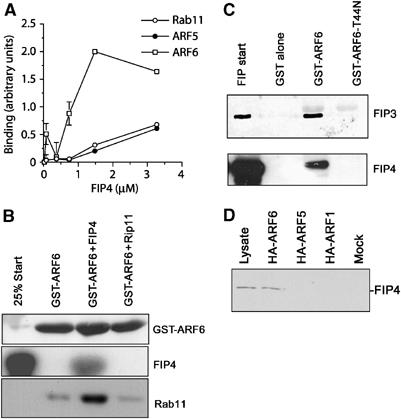
FIP4 has distinct GTPase preferences. Increasing concentrations of FIP4-containing beads (residues 227–557) were incubated with 5 μg of Arf5, Arf6 or Rab11, and the binding determined by quantitative immunoblotting. The data shown in (A) is from three experiments of this type, with the mean±s.d. shown. In order to determine whether FIP4 can form a ternary complex with Arf6 and Rab11, we performed the experiment shown in (B). GST-Arf6 was incubated with bacterially expressed FIP4 (residues 227–557) or Rip11 (residues 490–652). The upper panel shows a Coomassie stain demonstrating equivalent loads of Arf6 in each of the three conditions; the presence of FIP4 was demonstrated by immunoblotting (middle panel). These beads were incubated with bacterially expressed Rab11, washed and then boiled in SDS–PAGE buffer and immunoblotted for Rab 11 (lower panel). As shown, Rab11 was only recovered in the GST-Arf6 beads in the presence of FIP4. (C) GST, GST-Arf6 or GST-Arf6-T44N expressed in bacteria were loaded on glutathione–sepharose and incubated with either bacterially expressed FIP3 (upper panel) or FIP4 expressed in CHO cells (lower panel). The beads were washed and boiled in SDS–PAGE buffer and immunoblotted for FIP3 or FIP4 as indicated. Start refers to the starting material (100 ng of FIP3, or 50% of the start volume of cell lysate for FIP4). Data are representative of three experiments of this kind. (D) HeLa cells were transfected with HA-tagged Arf1, Arf5 or Arf6 as described. After 48 h, cells were lysed and an anti-HA immunoprecipitation performed. The immunoprecipitates were probed for the presence of endogenous FIP4. The result of a typical experiment is shown.
The Arf6 and Rab11 binding sites are distinct
We have recently identified and characterised a domain present in class I FIPs that mediate interaction with Rab11 (Meyers and Prekeris, 2002; Junutula et al, 2004). Based on this work, we created a mutation in FIP3 (I737E) that also displays an inability to bind Rab11 (Wilson et al, 2005). Here, we show that this mutant retains the ability to interact with Arf6, suggesting that the binding sites for these two small GTPases are distinct (Figure 3). Similarly, a mutation in the Rab11 binding site of FIP4 (D538A) abrogates Rab11 binding but not Arf6 binding (Figure 3). When expressed in interphase cells, a GFP fusion of this protein was unable to induce the collapse of recycling endosomes observed with wild-type FIP4, consistent with a loss of its ability to bind Rab11 (data not shown). These data, together with the experiments shown in Figures 1D, E and 2C, strongly suggest that the binding sites for Arf and Rab proteins on FIP3 and FIP4 are distinct.
Figure 3.
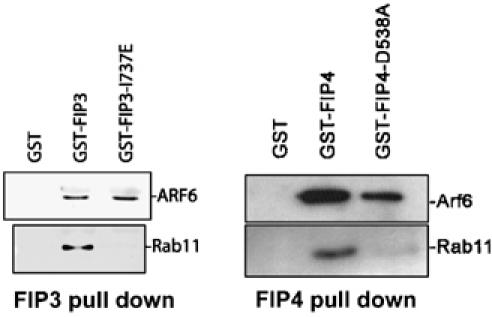
The Rab11 and Arf6 binding sites are spatially distinct. Beads loaded with GST, GST-FIP3 or GST-FIP3-I737E were incubated with either Rab11 or Arf6 as described. After incubation, the beads were washed and boiled in SDS–PAGE buffer and subjected to immunoblot analysis with anti-Arf6 (middle panel) or anti-Rab11 (lower panel). Similar data using GST-FIP4 constructs are shown in the adjacent panel.
FIP3 and FIP4 localisation is controlled by GTPase interactions
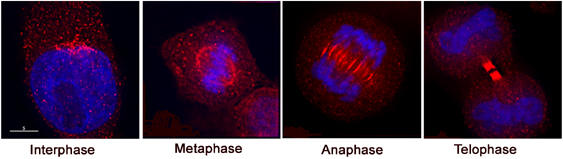
We next sought to determine whether these different GTPase interactions contribute to compartmentalisation of the proteins in dividing cells. First, we examined the localisation of endogenous Arf6 during mitosis (Figure 4). In agreement with previous studies (Schweitzer and D'Souza-Schorey, 2002), Arf6 was localised to the plasma membrane and intracellular vesicles in interphase cells (Figure 4). By late telophase/cytokinesis, strong Arf6 staining in the midbody was clearly evident. This pattern of Arf6 localisation is similar to that reported elsewhere (Schweitzer and D'Souza-Schorey, 2002).
Figure 4.
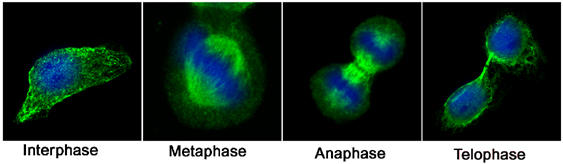
The distribution of Arf6 during the cell cycle. HeLa cells were plated onto glass coverslips and 12 h later fixed and stained with anti-Arf6 antibodies. Shown are representative images at different stages of the cell cycle. Arf6 staining is pseudo-coloured green, DNA is pseudo-coloured blue.
We have previously shown that the accumulation of FIP3-positive vesicles in the furrow of dividing cells required Rab11 (Wilson et al, 2005). However, we also observed that a population of FIP3 localised to the midbody independently of Rab11 (Wilson et al, 2005). The strong localisation of Arf6 staining in the plasma membrane of the furrow and midbody, together with the ability of FIP3 and FIP4 to bind Arf6 revealed in Figures 1 and 2, suggested that Arf6 may be involved in the localisation of FIPs to the furrow or midbody. The data in Figure 5 support this hypothesis. Overexpression of the GTP restricted form of Arf6 (Arf6-Q67L) recruits FIP3 to the midbody (Figure 5A and B), and increased the amount of FIP4 present in this region (Figure 5D). Quantification of this data revealed that in cells expressing wild-type Arf6, FIP3 was present in the furrow of 92% (n=62) of cells; expression of Arf6-Q67L increased this to 96% (n=53). Similarly, FIP4 was present in the furrow or midbody of 91% (n=50) of cells expressing Arf6 wild type and 98.6% of cells expressing Arf6-Q67L (n=41). More strikingly however, Figure 5A shows that the association of FIP3 with the furrow or midbody is blocked by the expression of GDP-restricted Arf6-T27N mutant; similarly, localisation of FIP4 to the furrow/midbody was blocked by this mutant (Figure 5D). Analysis of three separate experiments of this type revealed no furrow or midbody FIP3 staining in 79% of cells expressing Arf6-T27N and no furrow or midbody FIP4 staining in 86% of such cells. We also examined the localisation of endogenous Rab11 in cells expressing these different Arf6 mutants (Figure 5D) as we have previously reported that Rab11-positive structures are localised within the furrow and midbodies of dividing cells (Figure 5C and Wilson et al, 2005). We consistently observed a reduction in the intensity of Rab11 staining in midbody region of cells overexpressing Arf6-T27N; however, overexpression of wild-type or constitutively active Arf6 had little effect. Finally, we used real-time image analysis to examine the trafficking of YFP-FIP3 in cells coexpressing either CFP-Rab11 or CFP-Arf6 (Figure 6; Supplementary movies 1 and 2). These tagged proteins have been shown by other studies to faithfully represent the distribution of the endogenous proteins (Aalto et al, 1993; Ashery et al, 1999; Aikawa and Martin, 2003). These data further support our hypothesis that Rab11 controls recruitment of FIP3 to endosomes, and further reveal that Arf6 appears at the midbody at a later stage of cytokinesis. Note that in the absence of overexpression of active Arf6, the fraction of total cellular FIP3 in the midbody is low, and cannot be clearly observed in the image series presented. However, the data in Figure 5A and B clearly reveal that FIP3 is present in the furrow and midbody, and is recruited to the midbody by active Arf6. Finally, we note that FIP4 exhibits similar spatial dynamics to FIP3, exhibiting clear recruitment to centrosomes prior to movement into the furrow and midbody (see Supplementary Figure S1).
Figure 5.
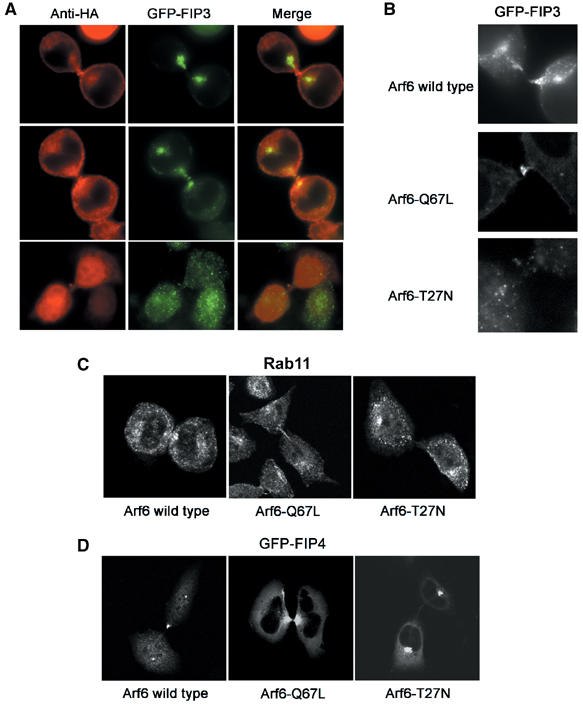
Arf6 controls midbody localisation of FIP3 and FIP4. (A) HeLa cell were cotransfected with GFP-FIP3 (green) and HA-Arf6, HA-Arf6-Q67L or HA-Arf6-T27N (red). Cells were fixed and imaged. Yellow represents the degree of the overlap. Note that expression of Arf6-Q67L results in a marked accumulation of FIP3 at the midbody, and that 79% of cells expressing Arf6-T27N exhibited no FIP3 in the midbody. (B) HeLa cells stably expressing GFP-FIP3 were transfected with either wild-type Arf6 or Arf6-Q67L. Shown are representative images of the FIP3 localisation in the furrow/midbody regions of such cells. Note that for clarity, the HA staining is not shown in panels B–D. (C) As panel A, except cells were stained for endogenous Rab11 distribution. (D) As panel A, except GFP-FIP4 was used. Note that FIP4 accumulation in the midbodies was abrogated by overexpression of Arf6-T27N. In all, >80% of cells expressing Arf6-T27N exhibited little or no FIP4 staining in the midbody.
Figure 6.
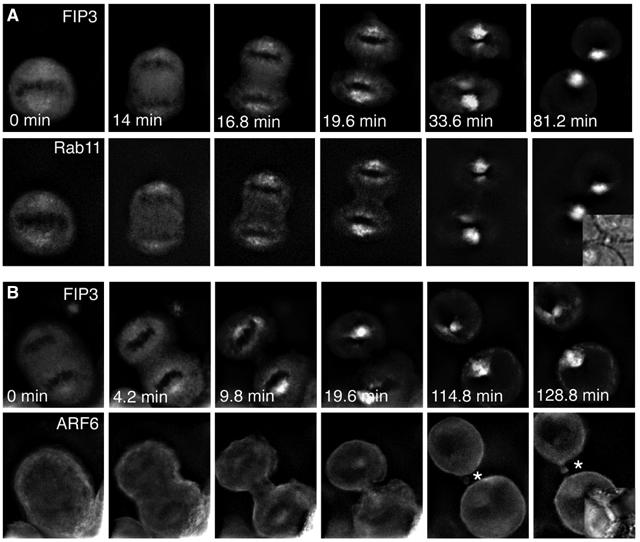
Real-time analysis of Rab11, Arf6 and FIP3 movement to the furrow and midbody. HeLa cells stably expressing either FIP3-YFP/CFP-Rab11 (A) or FIP3-YFP/Arf6-CFP (B) were plated on collagen-coated coverslips and imaged using time-lapse microscopy (see Supplementary movies 1 and 2). Images were collected every 1.4 min for 2.5 h. Note that Rab11 always colocalizes with FIP3, while Arf6 appears to be recruited directly to the midbody (asterisk). FIP3 at the midbody is a small fraction of the total cellular FIP3, and is not clearly evident in these nonconfocal images.
FIP4 and Arf6 exhibit similar patterns of immunofluorescence staining at all stages of the cell cycle (Figure 7). This suggests that the ability of both FIP3 and FIP4 to bind Arf6 is mirrored by their localisation to midbodies. Such data suggest the hypothesis that Arf6 is involved in a late stage of vesicle docking at the midbody. Consistent with this interpretation, we and others have found a cytokinesis defect in cells expressing Arf6 mutants (data not shown; Schweitzer and D'Souza-Schorey, 2002).
Figure 7.
FIP4 exhibits a similar distribution to Arf6 during the cell cycle. Cells plated as outlined in Figure 5 were stained for FIP4 (red) or DNA (blue). The images shown are DeltaVision reconstructions and are typical of many images of this type. Scale bar 5 μm.
FIP3 and FIP4 interact with the Exocyst
Previous work has shown that Arf6 interacts with the mammalian Exocyst complex (Prigent et al, 2003). Such data offer an intriguing model whereby Rab11-dependent recruitment of FIPs to recycling endosomes may endow these vesicles with the means to interact, via Arf6, with a tethering factor upon arrival at the furrow or midbody. Consistent with an important role of the Exocyst complex in cytokinesis, immunostaining of dividing cells revealed localisation of Exo70p, a component of the Exocyst, to the cleavage furrow (Figure 8A). In order to ascertain whether Exo70p was important for cytokinesis, we utilised siRNA to knock down levels of this protein. Figure 8B shows that siRNA targeted to Exo70p effectively knocked down expression levels of this protein, but were without effect on levels of either Rab11 or FIP3. Knockdown of Exo70p resulted in a significant fraction of cells exhibiting a binuclear phenotype compared to controls (39.4±4.2 versus 8.1±2.9%, respectively; Figure 8C and D), consistent with an important role for Exo70p in cytokinesis. To test directly for an interaction of FIP3 or FIP4 with this protein, we performed co-immunoprecipitation experiments. GFP-tagged FIP3 or FIP4 were overexpressed in CHO cells, and antibodies against Exo70p were used to immunoprecipitate any potential complexes of these proteins. As shown in Figure 8E, Exo70p consistently pulled down GFP-FIP3 from cell lysates; Rab11 was also present in the pull-downs. Similar data were also observed using GFP-FIP4 lysate. Interestingly, the Exo70p:FIP3 interaction was found to not require Rab11, since this interaction was also observed in cells depleted of Rab11 using siRNA (Supplementary Figure 2). Based on these observations, we hypothesised that depletion of Exo70p should result in reduced levels of FIP3 and Rab11 immunoreactivity in the furrow/midbody region of dividing cells. Figure 9 clearly shows that this is the case for FIP3: depletion of Exo70p results in most of the cellular FIP3 observed in structures away from the cleavage furrow (although some FIP3 in the cleavage furrow was still observed), compared to control cells that exhibit strong FIP3 staining in the furrow region. Similarly, Rab11 staining in the furrow is not evident upon depletion of Exo70p (Figure 9, compare control cell at left). The decreased staining of FIP3 and Rab11 in the furrow was not due to the failure to form the midbody consistent with our previous data suggesting that FIP3 and ARF6 regulate the late cytokinesis step, possibly abscission.
Figure 8.
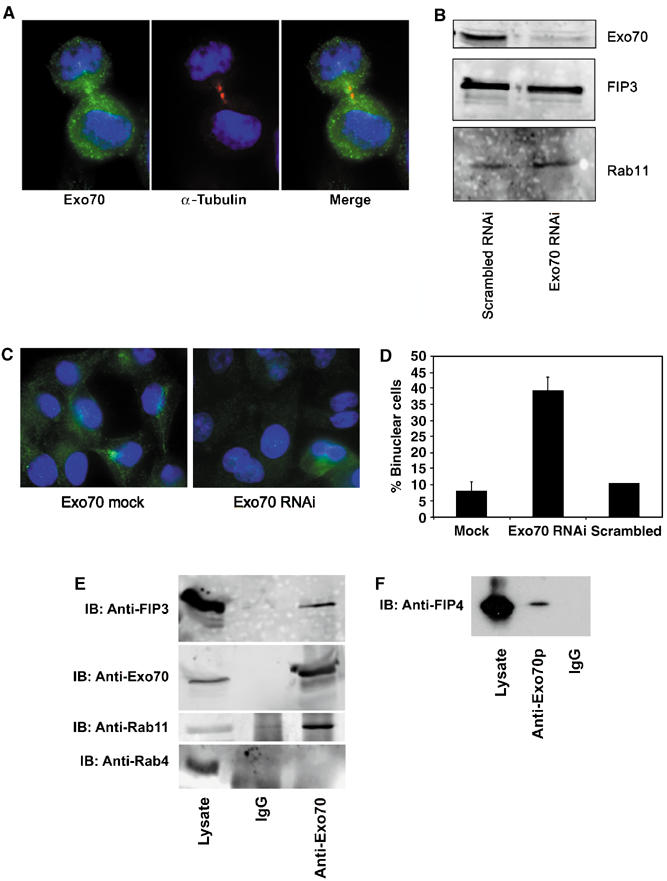
Exo70p interacts with FIPs and is required for cytokinesis. (A) Representative fields of HeLa cells stained with antibodies against Exo70 (green) and α-tubulin (red). DNA is stained using DAPI (blue). (B) HeLa cells were transfected with either siRNA designed to knock down Exo70p or a scrambled siRNA, and lysates prepared after 48 h. Lysates were immunoblotted with antibodies specific for Exo70p, FIP3 or Rab11, and a representative experiment is shown. (C) Representative fields of HeLa cells transfected with either mock or Exo70 siRNA. At 48 h after transfection, cells were immunostained with anti-Exo70 antibodies (green) and DAPI (blue). (D) Quantification of the effect of Exo70 RNAi on levels of binucleate cells. n refers to the numbers of cell counted and compares cells transfected with Exo70p siRNA, scrambled siRNA or mock-transfected cells. The difference between Exo70p siRNA-treated cells and both control groups is significant (P<0.05). (E–F) Lysates of CHO cells expressing either GFP-FIP3 (E) or GFP-FIP4 (F) were used in an experiment to determine whether antibodies specific for Exo70 or random IgG can co-immunoprecipitate FIP3 or FIP4. Shown are representative experiments in which an aliquot of cell lysate, and the immunoprecipitated material were immunoblotted for FIP3 or FIP4. Data shown are representative of three experiments of this type.
Figure 9.
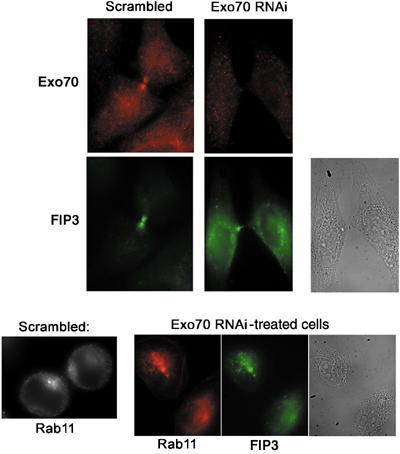
Exo70p depletion results in mislocalisation of FIP3 and Rab11 in the furrow. (Top panels) Representative fields of HeLa cells transfected with either scrambled or Exo70 siRNA. At 48 h after transfection, cells were immunostained with anti-FIP3 (green) or anti-Exo70p (red) antibodies. The bright field image at the right shows the presence of the furrow and midbody. (Bottom panels) Representative field of HeLa cells transfected with Exo70p siRNA and immunostained for FIP3 (green) or Rab11 (red) (cf. bright field image at right). The asterisk indicates the position of the midbody. For comparison, a cell transfected with a scrambled siRNA and stained for Rab11 is shown at the left.
Discussion
We have previously reported that Rab11 is an essential component of cytokinesis in mammalian cells. The role of Rab11 likely involves the delivery of membrane, presumably derived from recycling endosomes, to the furrow, where it is required either for the expansion of the plasma membrane associated with furrow ingress or during the abscission and daughter cell separation (Wilson et al, 2005). Furthermore, we have shown that two Rab11 effectors, FIP3 and FIP4, appear to play a key role in the membrane trafficking events associated with cytokinesis. Depletion of FIP3 using siRNA or perturbation of the Rab11–FIP3 complex both result in defective cytokinesis, consistent with these proteins functioning as a complex to regulate membrane traffic to the furrow and midbody of cells in cytokinesis (Wilson et al, 2005). These data, although implicating FIP3 and FIP4 in cytokinesis, leave several questions unanswered. Firstly, why do the class II FIPs interact with two different classes of GTPases (Arfs and Rab11)? Secondly, what are the relative affinities for binding of these GTPases? Thirdly, although Rab11 recruits FIP3 to endosomes, we have clearly demonstrated that it is not involved in FIP3 and FIP4 recruitment to the midbody, so how do these proteins become localised in this region? Finally, how can class II FIPs be integrated into a model of membrane vesicle docking and fusion in the furrow and/or midbody? Here, we set out to answer these questions.
We expressed the C-terminal domains of FIP3 and FIP4 proteins in bacteria to study the relative affinity of interaction with different GTPases. The data in Figures 1 and 2 clearly show that FIP3 and FIP4 exhibit subtly different preferences for association with GTPases. FIP3 was found to interact strongest with Rab11 and with Arf6. Although interaction with Arf5 was clearly evident in these studies, this was the weakest of the interactions studied and could not be detected in vivo. FIP4 by contrast interacted strongly with Arf6, and the interaction with Rab11 and Arf5 were similar. These in vitro binding data are subtly distinct from studies using yeast two-hybrid systems both in our laboratory (Hickson et al, 2003) and others (Shin et al, 1999, 2001), or from previous studies using GTPase pull-down's of FIP3 or FIP4 expressed in CHO cells (Hickson et al, 2003; Wilson et al, 2005). These previous studies suggested that FIP3 bound Arf6 only weakly, and FIP4 did not interact with Arf6 at all. However, the data presented here clearly reveal this not the case. Potential explanations for these differences are that FIP3 or FIP4 expressed in CHO cells may be already tightly bound to Rab11 or the respective Arf proteins, or that a post-translational modification of the FIPs may contribute to subtle variations in binding specificity. A further possibility is that domains within the amino-terminus of FIP3 or FIP4 may contribute to the binding specificity. However, in our hands expression of the full-length proteins in bacteria has proven difficult and has to date precluded further investigation of this point. Finally, previous studies used yeast two-hybrid or mammalian cell overexpression assays to test FIP3 and FIP4 interaction with Arfs. The levels of protein expression in these assays are difficult to control, thus the differences in interactions could be due to the differences in protein levels and/or stability. In this work, for the first time, we used recombinant proteins to perform a quantitative analysis of FIP3 and Arf interactions. In summary, all our data clearly show that FIP3 and FIP4 both bind Rab11 preferentially among Rab family members tested, consistent with previous studies of other FIPs (Junutula et al, 2004). Although both FIP3 and FIP4 exhibit a degree of interaction with Arf5, the interaction with Arf6 is stronger for both proteins, and Arf6 is the only Arf that appears to associate with these FIPs in vivo (Figures 1C and 2D) and in nucleotide-dependent manner (Figure 2C).
Interestingly, we have found that both FIP3 and FIP4 are capable of binding both Arf6 and Rab11 simultaneously (Figures 1 and 2). Thus, Figure 1D shows that increasing concentrations of Rab11 are not able to displace GST-Arf6 from previously assembled FIP3 complexes, and Figure 1E shows that FIP3 in complex with Rab11 can associate with GST-Arf6. Similar results were obtained using FIP4 (Figure 2B). These data argue that FIP3 and FIP4 can form ternary complexes with Arf6 and Rab11 and implies that the binding sites of these two GTPases are spatially distinct within the 3D structures of FIP3 and FIP4. The Rab11 binding site of FIP3 and FIP4 has been localised to a short C-terminal stretch of amino acids (Prekeris et al, 2001; Meyers and Prekeris, 2002; Junutula et al, 2004). We therefore mutated residues within this sequence in both FIP3 (I737E) and FIP4 (D538A) and studied their interaction with Rab11 and Arf6. As shown in Figure 3, both these mutant FIP's lost the ability to bind Rab11 in vitro, but were clearly both capable of binding Arf6. Such data, together with the in vitro studies (Figures 1D, E and 2B), strongly suggest that the binding sites of for these different GTPases are distinct, or at least involve critically distinct residues. We believe that this underpins a crucial facet of class II FIP function (see below).
We next sought to determine whether these differential interactions may account for the subcellular localisation of FIP3 and FIP4 during the cell cycle. We have previously shown that FIP3 associated with recycling endosomes in the region of the furrow, and that this association required Rab11. By contrast, both FIP3 and FIP4 localised to the midbody of dividing cells independently of Rab11 (Wilson et al, 2005). We therefore sought to test the hypothesis that interaction of FIP3 and/or FIP4 with Arf6 may regulate localisation to the midbody. Several lines of investigation support this suggestion. Firstly, expression of a GTP-restricted mutant of Arf6 (Q67L) recruited FIP3 to the furrow and midbody (Figure 5A and B). More strikingly, expression of the GDP-restricted mutant Arf6-T27N blocked the delivery of FIP3 to the plasma membrane of the midbody (Figure 5A). Similarly, localisation of Rab11 and FIP4 to the midbody was also blocked by overexpression of Arf6-T27N (Figure 5C and D). Real-time imaging also supports the contention that Rab11 is involved in recruitment of FIP3 to endosomes and Arf6 is involved in the localisation of these FIP3-positive vesicles in the midbody (Figure 6). Inspection of the movies (Supplementary movie 1 and 2) highlights the Rab11 and FIP3 association on endosomes prior to traffic to the furrow/midbody, but Arf6 appears at the midbody independently of traffic via Rab11-containing endosomes; the data from Figure 5D further indicate that the distribution of Rab11 is not modulated by expression of GTP- or GDP-restricted mutants of Arf6. These data support the hypothesis that Arf6 regulates FIP3 and FIP4 localisation to the midbody. Such observations are interesting since the regulated delivery of membrane vesicles to the furrow/midbody during cytokinesis is most likely accompanied by alterations in actin dynamics (Field et al, 1999; Glotzer, 2001; Pelham and Chang, 2002; Robinson and Spudich, 2004), and Arf6 has previously been reported to control actin dynamics in mammalian cells (Zhang et al, 1999; Schafer et al, 2000; Donaldson, 2003). In support of such a contention, it is interesting to note that there is a well-established dynamic interplay between the mitotic spindle and the acto-myosin cortex; FIP3 and FIP4 may provide a focus for this interaction, regulating membrane delivery and actin dynamics through the same molecule.
However, we believe that a further potential explanation for the Arf6 interaction may be to facilitate docking of vesicles at the furrow and midbody prior to fusion. This hypothesis is based upon the fact that Exo70p, a component of the mammalian Exocyst complex, was recently reported to interact with Arf6. The Exocyst is involved in budding events in yeast (TerBush et al, 1996; Wang et al, 2002), is thought to control membrane vesicle docking during exocytosis (Hsu et al, 1999; Yeaman et al, 2001; Boyd et al, 2004) and may also play a key role in cytokinesis, since components of the Exocyst have been identified in midbodies (Gromley et al, 2004). Moreover, Arf6 localises to the furrow and midbody and is required for abscission. Hence, we propose the following model for membrane trafficking during cytokinesis (Figure 10): Rab11 recruits FIP3 or FIP4 to recycling endosome-derived vesicles for traffic along microtubules into the cleavage furrow or midbody. Perturbation of the function of either Rab11 or FIP3 results in defective abscission (Wilson et al, 2005). We propose that an interaction of FIP3 or FIP4 with active Arf6 at the cleavage furrow or in the midbody serves to tether these vesicles in this region, via interaction with Exo70p, prior to membrane fusion. In support of this model, we show that (i) Arf6 binds both FIP3 and FIP4 in a GTP-dependent manner (Figure 2C), (ii) that Arf6 is localised to the furrow and midbody (Figure 4) and (iii) that Arf6-GTP recruits FIP3/4 to the midbody of dividing cells (Figure 5). Furthermore, we have shown that Exo70p is localised to the furrow of dividing cells and that depletion of Exo70p resulted in a profound cytokinesis defect (Figure 8). These data clearly implicate Exo70p as an important component of the cytokinesis machinery. Strikingly, we show that antibodies specific for Exo70p co-immunoprecipitate GFP-FIP3 and GFP-FIP4 from CHO cells (Figure 8E) and that Rab11 is not required for this interaction (Supplementary Figure 2). Consistent with the model described above, Rab11 was also observed in these immunoprecipitates. The model we propose suggests that the interaction of FIP3 or FIP4 with active Arf6 at the cleavage furrow/midbody serves to regulate a docking event involving Exo70p. Hence, depletion of Exo70p should result in a loss of localisation of FIP3 or FIP4 and Rab11 from the cleavage furrow and midbody. The data shown in Figure 9 clearly support this hypothesis. Our in vitro binding data support the notion that Rab11 and Arf6 bind distinct sites on FIP3 and FIP4. Whether these three proteins make a ternary complex in vivo remains to be determined; we have been unable to demonstrate the existence of this complex by immunoprecipitation. However, it is likely that the amount of FIP3/Rab11 interacting with Arf6 at any given stage will represent a small fraction of the total FIP3/Rab11. However, the fact that Arf6 (Prigent et al, 2003) and Rab11/FIP3 or FIP4 (this study) can interact with the Exocyst supports our hypothesis.
Figure 10.
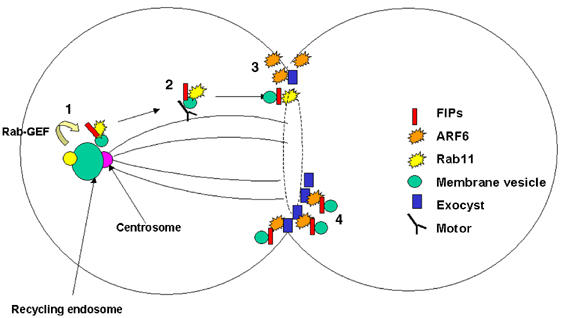
A model for FIP function in cytokinesis. (1) Rab11 is activated by GTP loading, which serves to recruit FIP3 to vesicles derived from recycling endosomes in the region of the centrosome. (2) These vesicles move along microtubules into the furrow (or later midbody) via interaction with a motor protein. (3) In the furrow, the FIP3/Rab11/vesicle encounters active Arf6, perhaps localised within microdomains on the plasma membrane; the interaction between Rab11/FIP3/Arf6 may serve to tether the vesicle to the plasma membrane via interaction with the Exocyst complex. (4) In the midbody at late stages of cytokinesis (represented here by a dashed line), we suggest that clusters of FIP3 vesicles may become tethered at the midbody ring just prior to abscission. See text for details.
In summary, during cytokinesis vesicles derived from recycling endosomes and identified by the presence of Rab11/FIP3 and Rab11/FIP4 protein complexes traffic to the furrow and midbody along microtubules. The recruitment of these complexes to the midbody is controlled by active Arf6 and the ability of FIP3/4 to bind Arf6-GTP, perhaps in a ternary complex with Rab11. In the midbody, the interaction of FIP3/4 Arf6 may facilitate tethering via Exo70p, perhaps prior to homotypic compound membrane fusion, and thus implicate FIP3 and FIP4 in the abscission event.
Materials and methods
Materials
Antibodies against FIP3 and FIP4 were as described (Hickson et al, 2003; Wilson et al, 2005). Anti-Arf6 was from Julie Donaldson (NIH, Bethesda, MD). Anti-Rab11 was from Zymed (San Francisco). Secondary antibodies were from Jackson Labs Inc. Sec8 antibodies were from Calbiochem (Darmstadt, Germany). Antibodies against mouse Exo70 were raised in sheep. The coding region was amplified from IMAGE clone ID 2136726, sub-loned into pET28b and expressed in bacteria as a hexa–his fusion. The resultant fusion protein was purified by sequential affinity chromatography on Ni-NTA agarose and gel permeation chromatography on Superdex 200. Antibodies were purified from the antiserum by chromatography on a column of the fusion protein, immobilized on CNBr-activated sepharose CL-4B. All other reagents were as described in Hickson et al (2003) and Wilson et al (2005).
Constructs
All FIP3 or FIP4 constructs were as described in Hickson et al (2003) and Wilson et al (2005). The virus encoding myc-tagged Rab11-S25N and the Arf5 and Arf6 constructs were as described in Hickson et al (2003). cDNAs encoding FIP3 were cloned in pEGFP-N1 or pEYFP-N1. cDNA encoding CFP-Rab11a was a generous gift from Dr Alexander Sorkin (UCHSC). Arf6-T44N was constructed by site-directed mutagenesis using a QuickChange kit according to the manufacturer's instructions and fully sequenced on both strands to confirm the mutation.
Cell culture and adenoviral infection
HeLa cells and KE37 leukaemic human cell lines were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum, 100 IU/ml penicillin and 100 mg/ml streptomycin, at 37°C in a 5% CO2 atmosphere. For transient expression experiments, HeLa cells were transfected using SuperFect (Invitrogen, UK) according to the manufacturer's instructions. Adenoviruses were amplified by ViraQuest Inc. (North Liberty, IA) and infections were performed as outlined in Hickson et al (2003).
RNAi
Exo70 was depleted with siRNA designed based on human sequence 5′-ggagaatgttgagaagac-3′. The siRNA sequences for Rab11a and Rab11b were published previously (Junutula et al, 2004; Wilson et al, 2005). Exo70 or Rab11a and Rab11b siRNAs were transfected into HeLa cells using Lipofectamine2000 (Gibco BRL). Transfected cells were incubated for 74 h and analyzed for Exo70 or Rab11a/b expression by immunoblotting. Remaining cells were used for fluorescence microscopy studies.
Recombinant protein expression and binding analysis
The carboxy-terminal domains of FIP3 (residues 300–759), FIP4 (residues 227–557) or Rip11 (residues 490–652) were expressed in Escherichia coli strain BL21(DE3) either as GST or as hexahistidine fusion proteins and purified as described previously (Prekeris et al, 2001; Hickson et al, 2003; Peden et al, 2004). GST-Arf5, GST-Arf6 and GST-Rab11 were expressed, purified and, when appropriate, thrombin cleaved to remove the GST. Briefly, 75 ml terrific broth was inoculated and grown for 16 h at 37°C with shaking. This culture was added to 1 l terrific broth plus antibiotic. When the OD600 reached 0.6, IPTG was added to 1 mM and cultures were incubated for a further 2 h at 37°C. Bacteria were harvested by centrifugation at 4000 r.p.m. for 15 min at 4°C, the supernatant removed and pellets resuspended in 20 ml/l of breaking buffer (PBS plus 0.1% Tween, 1 mM EDTA, 1 mM 2-mercaptoethanol, 4 mM PMSF and 1 mM pepstatin). Samples were then French pressed twice at 950 psi and subsequently spun at 17 000 r.p.m., for 25 min at 4°C. The supernatant was added to 0.5 ml/l original culture of glutathione beads (Amersham Biosciences, Cardiff, UK) and rotated at 4°C for 1 h. The beads were washed four times with PBS containing 0.1% Tween, and then resuspended in an equal volume of PBS and stored at 4°C. If soluble protein was required, thrombin (Amersham Biosciences, Cardiff, UK) was added, the beads incubated overnight at 4°C, reapplied to a glutathione bead column and the flow-through collected and stored at −80°C.
For in vitro binding, 5 μg of GST-Arf/Rab or GST control beads were made up to 50 μl with carrier beads. A measure of 100 μl of reaction buffer (PBS containing 200 mM NaCl, 1 mM MgCl2, 0.2% Triton X-100) was added to each tube. BSA was added to a final concentration of 0.2 mg/ml, PMSF to 4 mM and GMP-PMP (Roche Molecular Biochemicals) to 0.2 mM. In all, 20 μg (unless otherwise stated) of FIP3 or FIP4 was then added to each tube and the total volume brought to 500 μl with reaction buffer. Tubes were then incubated at 4°C for 1 h with rotation, washed four times with reaction buffer and then eluted with 50 μl of 1 × SDS–PAGE sample buffer.
Co-immunoprecipitation of Exo70p and FIPs
CHO cells transfected with GFP alone, GFP-FIP3 or GFP-FIP were lysed 48 h after transfection as described (Hickson et al, 2003). A measure of 5 μl washed Protein G agarose was added to 600 μg of CHO lysate and rotated at 4°C for 2 h. The samples were spun and the beads removed. Anti-Exo70 (5 μg) antibody or random rabbit IgG was added to 300 μg of precleared CHO lysate and rotated at 4°C overnight. A measure of 15 μl washed Protein A agarose was added to each sample and then rotated at 4°C for a further 4 h, after which time the beads were washed three times in buffer (50 mM HEPES, 100 mM KCl, 5 mM NaCl, 1 mM MgCl2, 0.5 mM EGTA, 1 mM EDTA, 0.1% Triton X-100, 1 mM DTT). Beads were collected by centrifugation, and then boiled in 50 μl SDS–PAGE loading buffer for 5 min.
Supplementary Material
Supplemental Movie 1
Supplemental Movie 2
Legend to Supplemental Movies 1 and 2
Supplemental Figure 1
Supplemental Figure 2
Acknowledgments
We thank Scottie Robinson, Dick Pagano and Julie Donaldson for provision of cDNAs, Paul Andrews for assistance with the DeltaVision analysis and Jean Ingram for assistance in antibody production. We are most grateful to Nia J Bryant for critical reading of the manuscript. This work was supported by grants from the Biotechnology and Biological Sciences Research Council (17/C13723, 17/C12621 and REI17/18423 to GWG; 24/B17173 to SAB), Diabetes UK (PhD studentship to GRXH), The Wellcome Trust (Research Leave Award to GWG; PhD studentships to ABF and XY), National Institute of Health (NIH-NIDDK Grant RO1-DK064380 to RP) and Howard Hughes Medical Institute Junior Faculty Award (RP). SS was supported by Scholarships from the Overseas Research Students Award Scheme (UK) and the Faculty of Biological Sciences, University of Leeds, UK.
References
- Aalto MK, Ronne H, Keranen S (1993) Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J 12: 4095–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa Y, Martin TFJ (2003) ARF6 regulates a plasma membrane pool of phosphatidylinositol (4,5)bisphosphate required for regulated exocytosis. J Cell Biol 162: 647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashery U, Koch H, Scheuss V, Brose N, Rettig J (1999) A pre-synaptic role for the ADP ribosylation factor (ARF)-specific GDP/GTP exchange factor msec7-1. Proc Natl Acad Sci (USA) 96: 1094–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C, Hughes T, Pypaert M, Novick P (2004) Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J Cell Biol 167: 889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG (2003) Multiple roles for Arf6: sorting, structuring, and signalling at the plasma membrane. J Biol Chem 278: 41573–41576 [DOI] [PubMed] [Google Scholar]
- Emoto K, Kobayashi T, Yamaji A, Aizawa H, Yahara I, Inoue K, Umeda M (1996) Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. PNAS 93: 12867–12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto K, Umeda M (2000) An essential role for a membrane lipid in cytokinesis: regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J Cell Biol 149: 1215–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field C, Li R, Oegema K (1999) Cytokinesis in eukaryotes: a mechanistic comparison. Curr Opin Cell Biol 11: 68–80 [DOI] [PubMed] [Google Scholar]
- Finger FP, White JG (2002) Fusion and fission: membrane trafficking in animal cytokinesis. Cell 108: 727–730 [DOI] [PubMed] [Google Scholar]
- Glotzer M (2001) Animal cell cytokinesis. Ann Rev Cell Dev Biol 17: 351–386 [DOI] [PubMed] [Google Scholar]
- Gromley A, Yeaman C, Jurczyk A, Redick S, Doxsey SJ (2004) Centriolin-anchoring of exocyst and SNARE complexes at the midbody is required for localised secretion and abscission during cytokinesis. Mol Biol Cell 15: 141a. [DOI] [PubMed] [Google Scholar]
- Hales CM, Griner R, Hobdy-Henderson KC, Dorn MC, Hardy D, Kumar R, Navarre J, Chan EK, Lapierre LA, Goldenring JR (2001) Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem 276: 39067–39075 [DOI] [PubMed] [Google Scholar]
- Hickson GRX, Matheson J, Riggs B, Maier VH, Fielding AB, Prekeris R, Sullivan W, Barr FA, Gould GW (2003) Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol Biol Cell 14: 2908–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S-C, Hazuka CD, Foletti DL, Scheller RH (1999) Targeting vesicles to specific sites on the plasma membrane: the role of the sec6/8 complex. Trends Cell Biol 9: 150–153 [DOI] [PubMed] [Google Scholar]
- Junutula JR, Schonteich E, Wilson GM, Peden AA, Scheller RH, Prekeris R (2004) Molecular Characterization of Rab11 Interactions with Members of the Family of Rab11-interacting Proteins. J Biol Chem 279: 33430–33437 [DOI] [PubMed] [Google Scholar]
- Meyers JM, Prekeris R (2002) Formation of mutually exclusive Rab11 complexes with members of the family of Rab11-interacting proteins regulates Rab11 endocytic targeting and function. J Biol Chem 277: 49003–49010 [DOI] [PubMed] [Google Scholar]
- O'Halloran TJ (2000) Membrane traffic and cytokinesis. Traffic 1: 921–926 [PubMed] [Google Scholar]
- Peden AA, Schonteich E, Chun J, Junutula JR, Scheller RH, Prekeris R (2004) The RCP-Rab-11 complex regulates endocytic protein sorting. Mol Biol Cell 15: 3530–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ, Chang F (2002) Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature 419: 82–86 [DOI] [PubMed] [Google Scholar]
- Pelissier A, Chauvin J-P, Lecuit T (2003) Trafficking through Rab11 endosomes is required for cellularisation during Drosophila embryogenesis. Curr Biol 13: 1848–1857 [DOI] [PubMed] [Google Scholar]
- Prekeris R, Davies JM, Scheller RH (2001) Identification of a novel Rab11/25 binding domain present in Eferin and Rip proteins. J Biol Chem 276: 38966–38970 [DOI] [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Scheller RH (2000) A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol Cell 6: 1437–1448 [DOI] [PubMed] [Google Scholar]
- Prigent M, Dubois T, Raposo G, Derrien V, Tenza D, Rosse C, Camonis J, Chavrier P (2003) ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J Cell Biol 163: 1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs B, Rothwell W, Mische S, Debec A, Hickson GRX, Matheson J, Gould GW, Hays TS, Sullivan W (2003) Actin cytoskeleton remodelling during metaphase and cellular furrow formation requires recycling endosomal components Nuclear-fallout and Rab11. J Cell Biol 163: 143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DN, Spudich JA (2004) Mechanics and regulation of cytokinesis. Curr Opin Cell Biol 16: 182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell WF, Fogarty P, Field CM, Sullivan W (1998) Nuclear-fallout, a Drosophila protein that cycles from the cytoplasm to the centrosomes, regulates cortical microfilament organisation. Development 125: 1295–1303 [DOI] [PubMed] [Google Scholar]
- Schafer DA, D'Souza-Schorley C, Cooper JA (2000) Actin assembly at membranes is controlled by Arf6. Traffic 1: 892–903 [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, D'Souza-Schorey C (2002) Localization and activation of the ARF6 GTPase during cleavage furrow ingression and cytokinesis. J Biol chem 277: 27210–27216 [DOI] [PubMed] [Google Scholar]
- Shin OH, Couvillon AD, Exton JH (2001) Arfophilin is a common target of both class II and class III ADP ribosylation factors. Biochemistry 40: 10846–10852 [DOI] [PubMed] [Google Scholar]
- Shin O-H, Ross AH, Mihai I, Exton JH (1999) Identification of arfophilin, a target protein for GTP-bound class II ADP-ribosylation factors. J Biol Chem 274: 26609–26615 [DOI] [PubMed] [Google Scholar]
- Skop AR, Bergmann D, Mohler WA, White JG (2001) Completion of cytokinesis in C. elegans requires brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr Biol 11: 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P (1996) The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J 15: 6483–6494 [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tang X, Liu J, Trautmann S, Balasundaram D, McColloum D, Balasubramanian MK (2002) The multiprotein exocyst complex is essential for cell separation in Schizosaccharaomyces pombe. Mol Biol Cell 13: 515–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Peden AA, Hames R, Fry A, Gould GW, Prekeris R (2005) The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell 16: 849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Wright JR, Nelson WJ (2001) Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J Cell Biol 155: 593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Calafat J, Janssen H, Greenberg S (1999) ARF6 is required for growth factor- and Rac-mediated membrane ruffling in macrophages at a stage distal to Rac membrane targeting. Mol Cell Biol 19: 8158–8168 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie 1
Supplemental Movie 2
Legend to Supplemental Movies 1 and 2
Supplemental Figure 1
Supplemental Figure 2


