Abstract
Protein translocation occurs across the energy-conserving bacterial membrane at the SecYEG channel. The crystal structure of the channel has revealed a possible mechanism for gating and opening. This study evaluates the plug hypothesis using cysteine crosslink experiments in combination with various allelic forms of the Sec complex. The results demonstrate that the SecY plug domain moves away from the center of the channel toward SecE during polypeptide translocation, and further show that the translocation-enhancing prlA3 mutation and SecG subunit change the properties of channel gating. Locking the plug in the open state preactivates the Sec complex, and a super-active translocase can be created when combined with the prlA4 mutation located in the pore of the channel. Dimerization of the Sec complex, which is essential for translocase activity, relocates the plug toward the open position. We propose that oligomerization may result in SecYEG cooperative interactions important to prime the translocon function.
Keywords: channel, gating, oligomer, Sec complex, translocon
Introduction
Proteins destined for secretion, membrane integration or organelle import contain signal sequences that direct them to the membrane. Once there, transport machines receive and translocate the substrate protein appropriately into or across the membrane. The Sec system is a universally conserved protein translocation system (Cao and Saier, 2003). In bacteria, the Sec complex is composed of three membrane proteins (SecYEG) which conduct polypeptides through or into the cytosolic membrane (for recent reviews, see Rapoport et al, 2004; Veenendaal et al, 2004). Depending on the type of substrate, the Sec complex cooperates with either the ribosome or the ATPase SecA to push the protein by co- or post-translational mechanisms, respectively (Dalbey and Chen, 2004; Luirink and Sinning, 2004; Maillard et al, 2005).
There is a wealth of available genetic, biochemical and recent structural data such that our understanding of the transport process is at an advanced stage. The medium resolution structure of Escherichia coli SecYEG was determined by electron microscopy in its membrane dimeric form and a detergent-solubilized monomer of SecYEβ from Methanococcus jannaschii was resolved to higher resolution by X-ray crystallography (Breyton et al, 2002; Van den Berg et al, 2004). Both structures identify a putative protein conduction channel, which is held between the two pseudo-symmetric domains of the largest subunit SecY and embraced by the peripheral subunit SecE. The center of the channel is constrained by a ring of hydrophobic residues and capped by a short helical domain, termed the plug (Figure 1). The ring and the plug might, respectively, seal and close the channel. An attractive model proposed following the determination of the X-ray structure of the Sec complex includes the widening of the pore and the displacement of the plug away from the center of the channel by the incoming signal peptide of the polypeptide substrate (Clemons et al, 2004; Van den Berg et al, 2004).
Figure 1.
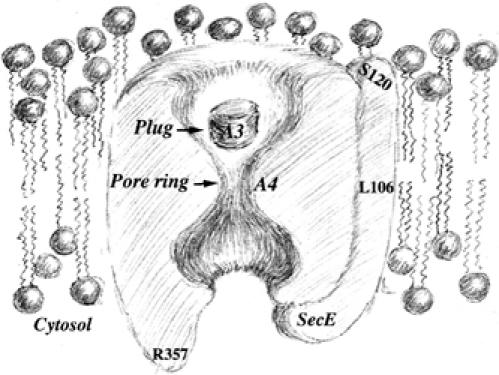
Schematic cross-sectional view of the closed Sec channel depicting the plug, the pore and the amino-acyl positions engineered in this study. The prlA4 (I408N) and prlA3 (F67C) mutations are labeled A4 and A3, respectively. Drawing kindly provided by Ms Kailun Jiang.
The proposed mechanism of channel gating seems to be supported by an earlier in vivo crosslinking experiment. It was shown that cysteines introduced into the plug domain of SecY and at the C-terminal end of SecE can form a disulfide bridge (Harris and Silhavy, 1999). These two cysteines are 20 Å apart in the closed channel structure, so that the observed crosslink is now explained by the movement of the plug out of the center of the channel and toward the periplasmic side of the membrane (Van den Berg et al, 2004). The notion of channel gating is further supported by the location of signal sequence suppressor (prl) mutations (Osborne and Silhavy, 1993). Most of them are located in the center of the channel (e.g. prlA4) or in the plug (e.g. prlA3). These mutations upregulate the translocase activity and allow the transport of secretory proteins with defective or even deleted signal sequences (Derman et al, 1993; Nouwen et al, 1996; Prinz et al, 1996). Thus, the prl mutations may preactivate the translocon by mimicking the effect of signal sequence binding on channel gating. It was therefore proposed that the prl mutations destabilize the closed state of the channel or facilitate its opening (Duong and Wickner, 1999; Van den Berg et al, 2004). These compelling and attractive hypotheses are awaiting further experimental analysis, which should link the structural and functional data together in order to clarify and expand these concepts.
Another unresolved question derived from the structural data is the oligomeric state of the Sec complex and the organization of the protomers. Numerous studies have shown that the translocon is an oligomer composed of 2–4 SecYEG complexes (Meyer et al, 1999; Manting et al, 2000; Beckmann et al, 2001; Bessonneau et al, 2002; Mori et al, 2003), while the crystal structure and functional analysis indicate that the channel itself is comprised within the SecY protomer (Yahr and Wickner, 2000; Duong, 2003; Van den Berg et al, 2004; Cannon et al, 2005). Thus, if a single Sec protomer within the oligomer proves sufficient for formation of the protein-conducting channel, one can ask what function the oligomer would serve. Understanding the functional significance of Sec complex oligomerization has implications for the working mechanism of the translocase, and also applies to other membrane transport systems where a similar paradox exists (Veenhoff et al, 2002; Park et al, 2004).
The present study also provides new insights into the translocation mechanism by building on the recent breakthroughs in the SecYEG structure determination. We investigate the plug hypothesis and address the paradox of the oligomeric state of the translocase. The results establish an experimental link between the translocase activity and the movement of the plug, and extend this structure–function relation to the effect of SecG and prl mutations. The results also provide one of the possible reasons explaining why the translocation reaction would beneficiate from the Sec complex oligomerization.
Results
Cysteine crosslinks between the SecY plug and SecE
The original SecY and SecE cysteine mutations described by Harris and Silhavy (1999) were taken as starting point for this study (Figure 1). The two cysteine mutations, SecY-F67C (also termed prlA3) and SecE-S120C, were placed into HA-tagged SecE and/or HA-tagged SecY and the Sec complex overproduced in E. coli strain BL21. Inner membrane vesicles (IMVs) were prepared from the cells and analyzed by SDS–PAGE and Western blotting. Under nonreducing gel electrophoresis conditions, cysteine-dependent covalent association between SecY and SecE was readily observed (Figure 2A, lanes 5 and 6). Preincubation of the IMVs with a relatively high concentration of reducing agent was required to disrupt the disulfide bond between SecY and SecE (Figure 2B, left panel), suggesting that a stable bond exists between the two cysteines. Native gel electrophoresis analysis showed that the dimeric SecYEG complex can be dissociated into monomers during detergent extraction (Bessonneau et al, 2002), even though the SecY and SecE subunits are crosslinked via the disulfide bridge (Figure 2C). This last result shows that the crosslink between SecY–F67C and SecE–S120C occurs within the same Sec protomer.
Figure 2.
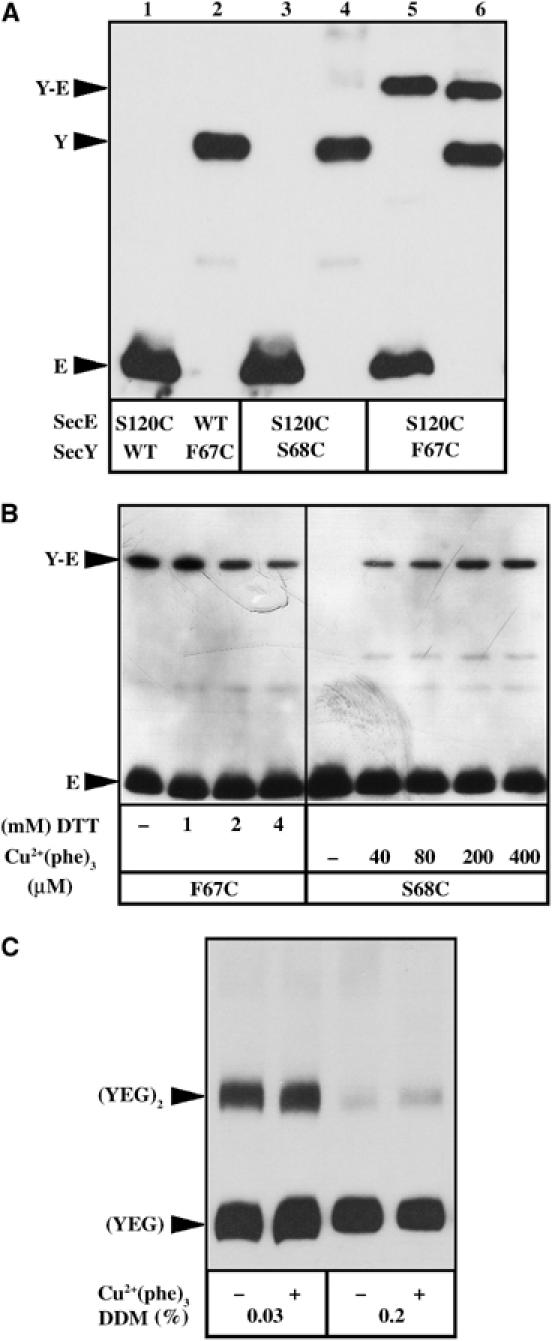
Crosslinking between the SecY plug and SecE. (A) About 1 μg of IMVs enriched for the cysteine-mutagenized SecYEHAG (odd lanes) or SecYHAEG (even lanes) complexes were solubilized with the Laemmli-sample buffer (without reducing agent), then analyzed by 13% SDS–PAGE and transferred onto polyvinyldene difluoride membrane for immunostaining with anti-HA antibodies. (B) IMVs enriched for the SecYEHAG complex carrying the mutations SecY–F67C or SecY–S68C in combination with SecE–S120C (labeled F67C or S68C, respectively) were incubated with reducing (DTT) or oxidizing (Cu2+(phe)3) agent for 5 min at RT at the indicated final concentration. Unreacted cysteines were blocked with NEM (8 mM final, 10 min, RT) prior to IMV solubilization and analysis by SDS–PAGE and Western blotting. (C) IMVs enriched for the SecY–F67C/SecE–S120C SecYEHAG complex were first oxidized with 400 μM Cu2+(phe)3 or reduced with 4 mM DTT, then the cysteines modified by NEM (8 mM final, 5 min, RT). IMVs were solubilized with 0.2% dodecyl-maltoside (DDM) and the oligomeric state of the Sec complex revealed by blue native gel electrophoresis (4–13%) and Western blotting.
That the cysteines SecY–F67C and SecE–S120C spontaneously bridge together in the IMVs is in apparent contradiction with the observation that the two equivalent positions are ∼20 Å apart in the structure of the monomeric M. jannaschii Sec complex (Van den Berg et al, 2004). One of the possible explanation is that the SecY–F67C mutation had destabilized or relocated the plug domain toward SecE, and thus toward the open state of the channel. In support to this hypothesis, the mutation F67C (i.e. prlA3) is known to upregulate the translocation activity of the Sec complex (Figure 3A; Emr et al, 1981; Duong and Wickner, 1999). To test the hypothesis, the cysteine mutation was displaced to residue 68 of SecY. The results show that the mutation SecY–S68C not only abolishes the spontaneous SecY–SecE crosslinks (Figure 2A, lanes 3 and 4) but also restores the translocation activity of the Sec complex to wild-type (WT) level (Figure 3A). Thus, the translocase activity of the Sec complex seems to be correlated to the location of the SecY-plug domain (see below also). Finally, when the SecY–S68C/SecE–S120C IMVs were incubated in oxidative conditions, SecY–SecE cysteine crosslinks were detected (Figure 2B, right panel). This last observation suggests that the plug possess intrinsic flexibility or mobility: the two cysteines SecY–S68C and SecE–S120C may not be in close vicinity, but the molecular dynamics of the SecY plug combined with an oxidative environment which favors the disulfide bridge formation will eventually trap the SecY plug and SecE together.
Figure 3.
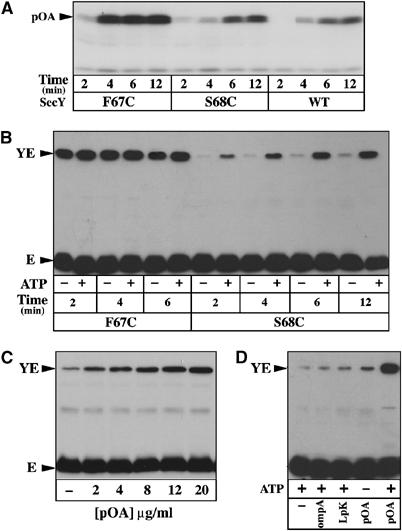
SecY-plug movement during preprotein translocation. (A) IMVs enriched for the WT or cysteine-mutagenized SecYEHAG complexes were tested for their translocase activity using the preprotein substrate [125I]proOmpA, as described in Materials and methods. (B) IMVs were incubated in the same conditions as in (A), but using unlabeled proOmpA. Unreacted cysteines were blocked with NEM (8 mM, 5 min, RT) prior to IMV solubilization and analysis by SDS–PAGE and Western blotting with anti-HA antibodies. (C, D) IMVs were incubated in the same conditions as in (B), but using the indicated concentration of proOmpA, or using a preprotein substrate with a deleted (OmpA) or altered (LpK) leader peptide.
SecY-plug movement during preprotein translocation
To directly assess the plug hypothesis (Van den Berg et al, 2004), the IMVs enriched for the cysteine-mutagenized Sec complex were incubated in the presence of translocation ligands and ATP. The results show that the progress of the preprotein translocation into the IMVs enriched for the SecY–S68C/SecE–S120C complex is concomitant with the appearance of the SecY–SecE cysteine crosslinks (Figure 3B, right panel), and with kinetics comparable to that of the translocation reaction itself (Figure 3A). Moreover, the efficiency of the SecY–SecE crosslink reaction reflects the concentration of preprotein substrate available (Figure 3C), while the amount of the SecY–SecE disulfide bridge depends on both ATP and a functional leader peptide. Neither a protein substrate with a deleted (OmpA) nor altered (LpK) signal sequence can significantly promote the SecY–SecE crosslink reaction (Figure 3D). Altogether, the observations directly support the hypothesis that the SecY plug relocates toward the position of SecE–S120 during preprotein movement across the membrane. Interestingly, under the same experimental conditions, the IMVs enriched for the SecY–F67C/SecE–S120C complex did not show a translocation-dependent increase of the SecY–SecE crosslinks (Figure 3B, left panel). As proposed above, the SecY–F67C mutation may have already displaced the plug domain towards the open state of the channel, so that no further increase of SecY–SecE crosslinking can occur.
SecG and prlA mutations favor the open state of the channel in resting IMVs
To further establish the correlation between the translocase activity of the Sec complex and the location of SecY plug, the effect of the translocation-enhancing factors SecG and prlA4 mutation was investigated. SecG, a nonessential subunit, strongly stimulates the rate of preprotein translocation (Nishiyama et al, 1994). As expected, deletion of this subunit resulted in diminished proOmpA translocation efficiency into both SecY–S68C and SecY–F67C IMVs (Figure 4A, lanes 1–4). Conversely, introduction of the mutation prlA4 into SecY–S68C IMVs resulted in enhanced translocase activity (Figure 4A, compare lane 3 to lane 5). The mutation prlA4 (I408N) is not located in the plug, but in the pore at the center of the channel (Osborne and Silhavy, 1993; Van den Berg et al, 2004).
Figure 4.
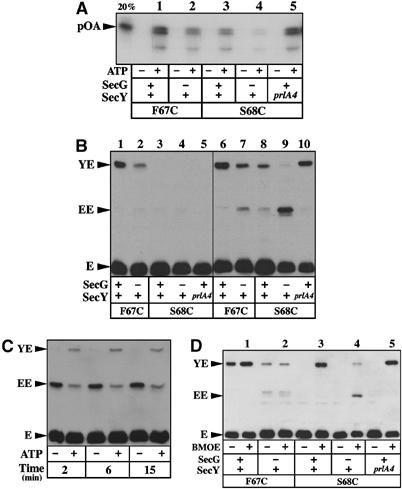
Relation between translocase activity and dynamic of the plug. (A) IMVs enriched for the indicated Sec complexes were tested for their translocase activity using [125I]proOmpA, as descibed in Materials and methods. In all, 20% of [125I]proOmpA added to the reaction was loaded on the gel as standard. (B) The same set of IMVs was analyzed for the SecY–SecE crosslinking efficiency either without (left panel) or with oxidation with 80 μM Cu2+(phe)3 (5 min, RT; right panel), followed by SDS–PAGE and Western blotting with anti-HA antibodies. (C) The translocation-dependent movement of the SecY plug was tested using the IMVs enriched for the SecY–S68C/SecE–S120C complex depleted for SecG. (D) The IMVs were incubated in the presence of the cystein-reactive crosslinker BMOE (0.1 mM final, 5 min, RT), before analysis by SDS–PAGE and Western blotting.
The IMVs were then analyzed for the proximity between the SecY plug and SecE by monitoring the formation of SecY–SecE cysteine crosslinks with or without oxidizing agent (Figure 4B, right and left panels, respectively). With the SecY–F67C IMVs, the amount of SecY–SecE crosslinks was strongly diminished in the absence of SecG (Figure 4B, compare lane 1 to lane 2 or lane 6 to lane 7). A similar decrease also occurred with the SecY–S68C IMVs preparation lacking SecG (compare lane 8 and lane 9). In these last IMVs, the absence of SecG instead favored a cysteine crosslink corresponding to the covalent association of two SecE molecules (Figure 4B, lane 9), while preprotein translocation induced only a limited relocation of the SecY plug toward the position SecE–S120C (Figure 4C). Thus, the absence of SecG diminishes the protein translocation efficiency, relocates the SecY plug away from the position SecE–S120 and seems to restrain its displacement during the preprotein translocation reaction. In contrast, the mutation prlA4 introduced into SecY–S68C resulted in an increased amount of SecY–SecE crosslinks (Figure 4B, compare lane 8 with lane 10), although detection of the SecY plug/SecE interaction required incubation in the presence of oxidizing agent. Altogether, the results confirm that the proximity of the SecY plug to the position SecE–S120 is correlated to the translocase potential of the Sec complex. The translocation-enhancing SecG subunit and prlA4 mutation favor the open state of the channel in resting IMVs.
Finally, the vicinity between the SecY plug and the position SecE–S120 was further tested using a homobifunctional thiol-reactive bridging agent (1,2-bis-maleimidoethane (BMOE), 9.9 Å spacer arm). The amount of BMOE-bridged SecY–SecE molecules was equivalent for the Sec complexes carrying the mutation prlA3, S68C or S68C–prlA4 (Figure 4D, lanes 1, 3 and 5, respectively), showing that the SecY plug and SecE are within the reach of this crosslinker spacer arm. In contrast, only a small amount of BMOE-mediated SecY–SecE crosslinking was obtained when SecG was missing from the SecY–F67C or SecY–S68C complexes (Figure 4D, lanes 2 and 4). Thus, the absence of SecG seems to perturb the dynamic of the plug movement and/or increase the molecular distance between the SecY plug and the position of SecE–S120. In contrast, the prlA mutations and especially prlA3 promote the molecular dynamics of the SecY plug, which in turn reduces the average distance between the two cysteines and thus facilitates disulfide bridge formation (Figure 4B).
The open state of the channel increases the translocase potential of the Sec complex
The above experiments and conclusions lead us to predict that stabilization of the open state of the channel should improve the translocase activity of the Sec complex. The SecY–S68C IMVs were incubated with an oxidizing agent, then re-isolated and analyzed for their potential for polypeptide translocation. The results show that the chemically induced SecY plug/SecE crosslink leads to a spectacular increase in the translocase activity (Figure 5A). Indeed, the translocase activity of the oxidized IMVs reached levels comparable to those obtained with the SecY–F67C (i.e. prlA3) mutation (Figure 5C, compare lane 2 to lane 6). Thus, it is possible to enhance the preprotein translocation reaction via artificial stabilization of the open state of the channel.
Figure 5.
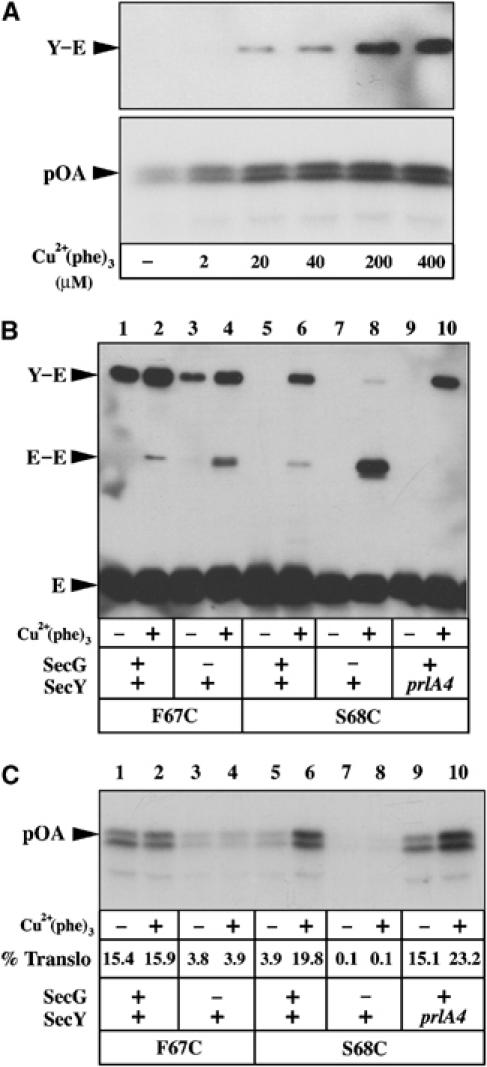
Stabilization of the plug in the open state increases the translocase activity. (A) IMVs enriched for the SecY–S68C/SecE–S120C complex were oxidized with Cu2+(phe)3 at the indicated final concentration (5 min, RT), then re-isolated by ultra-centrifugation. The left lane (−) corresponding to IMVs treated with 1 mM DTT. After IMV resuspension, the amount of SecY–SecE crosslinks obtained was monitored by SDS–PAGE and Western blotting (top panel), while the translocase activity was measured using [125I]proOmpA as substrate (8 min, 37°C; bottom panel). (B, C) IMVs enriched for the indicated Sec complexes were treated and analysed as in (A). The percentage of translocated proOmpA (% translo) is indicated.
The finding was analyzed further using other allelic forms of the Sec complex. As shown above, incubation of the SecY–F67C IMVs with an oxidant results in limited increase of the SecY–SecE crosslinks (Figure 5B, lanes 1 and 2). As expected, if the plug is already in the open conformation, no significant change of translocation activity was detected when these IMVs were treated with the oxidizing agent (Figure 5C, lanes 1 and 2). In contrast, incubation of the SecY–S68C–prlA4 IMVs with the oxidant resulted in a strong increase of the SecY–SecE crosslinks and the concomitant augmentation of the translocase activity (Figure 5B and C, lanes 9 and 10). In fact, analysis of the translocation efficiency showed that the oxidized SecY–S68C–prlA4 IMVs were almost 45% more active than the prlA3 IMVs (Figure 5C, compare lane 2 to lane 10). Thus, at the difference of the prlA3 mutation, the translocation-enhancing effect of the prlA4 mutation can be further improved via chemical stabilization of the open state of the channel. It suggests that the prlA4 and prlA3 mutations facilitate translocation using a distinct molecular mechanism. With the SecY–F67C and SecY–S68C IMVs lacking SecG, incubation with the oxidant did not result in detectable modification of the translocase activity (Figure 5C, lanes 4 and 8). Thus, stabilization of the open state of the channel does not seem sufficient to lower the need for the translocation-stimulatory SecG subunit.
Sec mutations at R357 relocate the plug toward its closed state
Previous targeted random mutagenesis studies identified the conserved arginine residue R357 as functionally important for cell viability (Mori and Ito, 2001). Indeed, mutagenesis of R357 to glutamate leads to a severe reduction of both in vivo and in vitro preprotein translocation efficiency (Mori and Ito, 2001; this study). To test whether this sec mutation also prevents the translocation-dependent movement of the SecY plug, the mutation R357E was placed into the SecY–S68C/SecE–S120C Sec complex. Indeed, compared to the SecY–S68C/SecE–S120C IMVs (labeled WT; Figure 6), the amount of SecY–SecE crosslinks normally induced by polypeptide translocation was severely reduced by the R357E mutation (Figure 6A). However, even in the resting state (i.e. in the absence of the translocation partners and ATP), a diminished amount of SecY–SecE crosslinks was obtained when these IMVs were incubated in oxidizing conditions (Figure 6B, compare the left panel to the middle panel). To confirm and amplify the observation, the mutagenesis was extended toward residues P358 and G359, also shown to be important for the translocase activity when substituted by certain amino acids (Mori and Ito, 2001). The sequence Arg–Pro–Gly (RPG) was thus replaced by the sequence Glu–Asp–Pro (EDP). The results show that the crosslink between SecY and SecE was largely abolished, even upon incubation of the IMVs in fully oxidizing conditions (Figure 6B, right panel). The distance between the SecY plug and SecE–S120 position was then evaluated using the cysteine-reactive crosslinkers BMOE and 1,6-bismaleimodohexane (BMH) (9.9 and 16.1 Å spacer arms, respectively). Only a small amount of the SecY–SecE crosslink was obtained with the R357E-mutagenized Sec complex, and this amount was further lowered by the mutations RPG357EDP (Figure 6C). Thus, compared to WT SecY, the mutations R357E and especially RPG357EDP seem to increased distance between the SecY plug and the position SecE–S120, although the primary effect of these mutations could be a reduced dynamic of the SecY plug.
Figure 6.
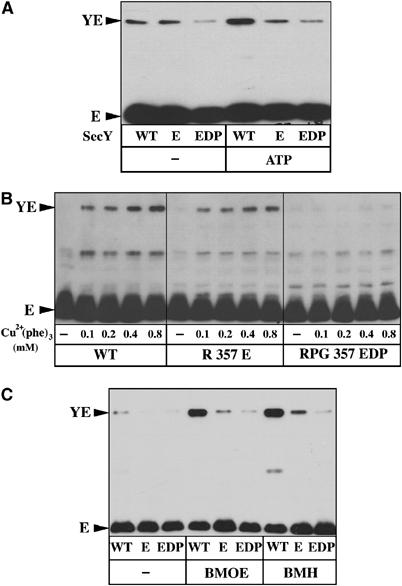
Sec mutations at R357 relocate the plug toward its closed state. (A) IMVs enriched for the SecY–S68C/SecE–S120C complex (labeled ‘WT'), or carrying the mutations R357E or RPG357EDP, were analyzed for the translocation-dependent plug movement (10 min, 37°C). To increase the detection of the SecY–SecE crosslinks, IMVs were incubated with 80 μM Cu2+(phe)3 (5 min, RT) at the end of the translocation reaction. (B) The same set of IMVs was analyzed for the amount of SecY–SecE crosslinks occurring in the absence of preprotein translocation and using the indicated final concentration of oxidizing agent. (C) The same set of IMVs was incubated with the cysteine-reactive crosslinkers BMOE and BMH (0.1 mM final; 5 min, RT), before analysis by SDS–PAGE and Western blotting.
Sec mutations at R357 monomerize the Sec complex
To further characterize the structural impact of these mutations, the oligomeric state of the Sec complex was analyzed by BN–PAGE. Both SecYEG monomers and dimers were detected from the detergent extract prepared from the SecY–S68C/SecE–S120C membranes (labeled WT; Figure 7A). In contrast, the ratio between monomer and dimer was significantly decreased by the mutations R357E and especially RPG357EDP (Figure 7A). The same observations were made from the BN–PAGE analysis of the purified Sec complexes (Figure 7B). Our previous work showed that the SecYEG complex is a labile structure that reversibly associates into dimers upon dilution of the detergent (Figure 7C; Bessonneau et al, 2002). In contrast, the mutations RPG357EDP induced a dramatic reduction in the ability of the purified Sec complex to re-form dimers. Further dilution of the detergent beyond the critical micellar concentration led to aggregation of the Sec complex (Figure 7C, labeled Top), but no dimeric intermediate was observed. Finally, to probe the oligomeric state of the complex when it is membrane-embedded, the mutations R357E and RPG357EDP were combined with the mutation SecE–L106C. The structure shows that this position is located at the interface of the SecYEG dimer (Breyton et al, 2002) and, indeed, some disulfide-bridged SecE–L106C dimers can be observed (Kaufmann et al, 1999; Figure 7D, left panel). In contrast, the SecE–SecE crosslink obtained upon oxidation of the L106C IMVs was significantly impaired by the R357E and RPG357EDP mutations (Figure 7D, left panel). Thus, although a significant fraction of the overproduced and mutagenized Sec complex can still form disulfide-linked SecE dimers, the result suggests that the mutations also tend to monomerize the membrane-embedded Sec complex. This conclusion was confirmed using the cysteine-reactive crosslinker BMOE, since a diminished amount of SecE dimers was observed with the IMVs enriched for the mutagenized Sec complexes (Figure 7D, right panel). The crosslinking agent BMH was inefficient in attaching together the SecE–L106C molecules (Figure 7D, right panel). The rigid spacer arm of BMH may be too long to efficiently bridge the L106C cysteines if they are too close to each other.
Figure 7.
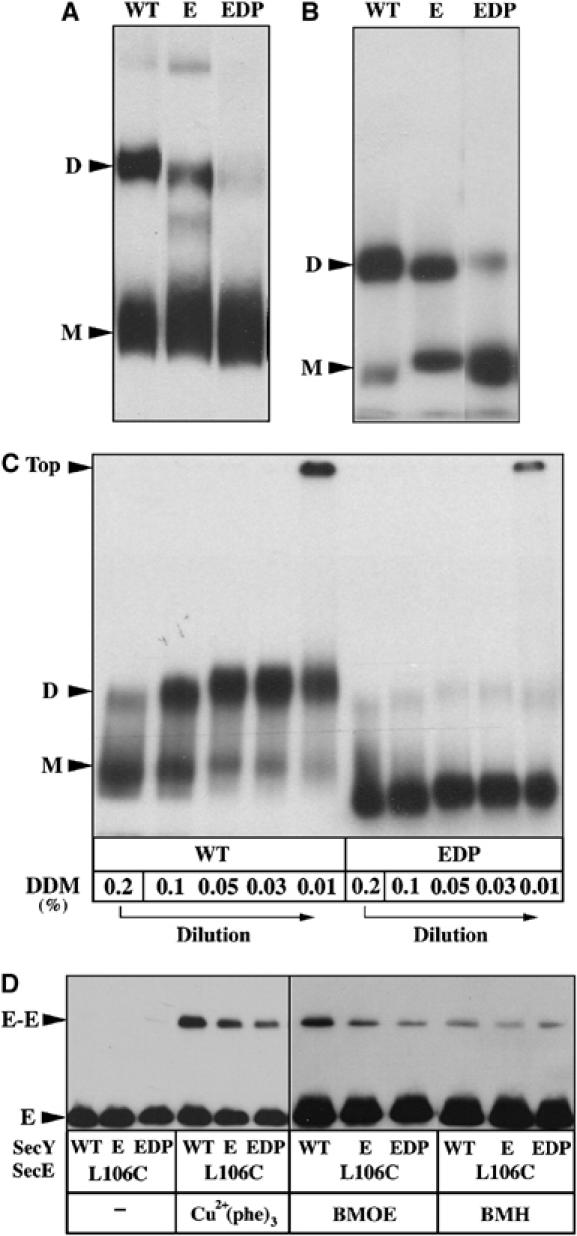
Sec mutations at R357 monomerize the Sec complex. (A) IMVs enriched for the SecY–S68C/SecE–S120C complex and carrying the mutations R357E or RPG357EDP were solubilized with DDM (0.06%). The membrane extracts were analyzed by BN–PAGE and Western blotting using anti-HA antibodies. The monomeric (M) and dimeric (D) forms of the Sec complex are indicated. (B) The R357-mutagenized Sec complexes were purified and radiolabeled, as described in Materials and methods, then analyzed by BN–PAGE and autoradiography. (C) The reversible dissociation of the 125I-labeled and purified Sec complex was analyzed by BN–PAGE. A stock solution of [125I]YEG (in 0.2% DDM) was diluted on ice to the indicated detergent concentration, as previously described (Bessonneau et al, 2002). (D) IMVs enriched for the SecYEHAG complex carrying the mutation SecE–L106C and R357E or RPG357EDP were incubated with Cu2+(phe)3 (80 μM final; 5 min, RT) or with the cysteine-reactive crosslinker BMOE and BMH (0.1 mM final; 5 min, RT). Unreacted cysteines were blocked with NEM (8 mM; 5 min, RT) before analysis of the crosslinked products by SDS–PAGE and Western blotting using anti-HA antibodies.
The results thus show that mutagenesis around residue R357 of SecY leads to decreased translocase activity, diminished Sec complex oligomerization and finally relocation of the SecY plug such as it cannot be readily crosslinked to SecE–S120. Altogether, these results suggest that the monomeric state of the Sec complex corresponds to a further closed state of the channel. Alternatively, monomerization of the channel may reduce the dynamic of the SecY plug.
Discussion
The plug hypothesis is one of the new concepts derived from the structural data which required further experimental investigation. For instance, the previously identified disulfide bridge between the SecY plug and SecE resulted in a dominant-negative phenotype (Harris and Silhavy, 1999). Since most intermolecular crosslinks also inactivate the translocon (Kaufmann et al, 1999; van der Sluis et al, 2002), thus showing that the Sec complex requires an intrinsic flexibility, the functional significance of the plug movement did not necessarily follow from this earlier crosslinking observation. A recent study already provided strong evidences that translocating polypeptides indeed pass through the pore of the SecY complex and also contact residues forming the plug domain (Cannon et al, 2005). The present study provides further evidences linking together the functional and structural aspects of the plug hypothesis, while providing new insight into the dynamic behavior of channel gating.
Our first experimental observation confirms the structural prediction that the SecY-plug domain moves out of the channel toward the C-terminal end of SecE. This displacement occurs within a Sec protomer and during polypeptide translocation. According to the structure of the monomeric Sec complex of M. jannaschii, the movement corresponds to a ∼22 Å translation of the plug towards SecE, as well as a shift of about 12 Å towards the external side of the membrane, assuming that the C-terminal of SecE itself remains stationary (Van den Berg et al, 2004). Such a large-amplitude movement is predicted to fully open the translocation channel and, indeed, our results show that it occurs during polypeptide translocation. Incubation with SecA only was not sufficient to induce this large-amplitude movement, whether nucleotides were present or not (data not shown). Interestingly, prolonged incubation with the preprotein substrate in the absence of ATP increased slightly the amount the SecY–SecE crosslinks (Figure 3B). Thus, although binding of the preprotein itself may result in limited channel opening, ATP binding and hydrolysis by SecA seem essential to fully promote the SecY-plug displacement. We notice that prolonged incubation in translocation conditions did not allow the total population of SecY and SecE molecules to become crosslinked together. A fraction of the overproduced Sec complex may be incompetent for translocation. Alternatively, opening of the translocation channel may occur only within a fraction of the Sec complexes forming the translocon.
The second observation, predicted from the first observation, is that stabilization of the open state of the channel (or destabilization of the closed state) results in enhanced translocase activity. Stabilization of the open state could be either chemically induced (i.e. crosslinking the plug to SecE-S120C) or genetically favored (i.e using the prlA3 mutation). Interestingly, the prlA3 mutation upregulates the translocase, but also allows translocation of preprotein with defective leader peptide (Flower et al, 1994). We assume that stabilization of the open state by disulfide crosslinking should also result in suppression of signal sequence defect. The observations also suggest that the plug displacement is a rate-limiting step of the translocation reaction, since a bypass of this step via chemical crosslinking leads to increased translocation efficiency. How the prlA3 induces the displacement of the plug will require further investigation. The plug, defined by the helical domain TM2a, is a flexible structure not particularly hydrophobic and buried in the vicinity of the ring of hydrophobic residues forming the center of the pore (Van den Berg et al, 2004). The mutation prlA3 (i.e F67C), but not the mutation S68C, increases further the dynamics of the plug such that the SecY–SecE crosslinks are readily observed without oxidizing agent. This observation may recall that of Harris and Silhavy (1999), showing that cysteine mutagenesis at some positions in SecY is lethal when combined to SecE–S120C, while neighboring positions are not. Thus, only certain mutations, such as prlA3, may increase the flexibility of the plug and may do so by lowering its interaction with residues forming the channel. Such hypothesis can be paralleled with the previous observation that the prlA3 mutation loosens the SecYEG intersubunit interactions (Duong and Wickner, 1999).
Although the mutation prlA4 (i.e I408N) also increases the translocase activity and results in relaxation of the interaction between SecY and SecE (van der Wolk et al, 1998; Duong and Wickner, 1999), the present study suggests that prlA4 acts distinctly from prlA3. This conclusion arises from the observation that the translocase-enhancing effect of prlA4 can be further increased by locking the plug in the open position (Figure 5C). Accordingly, the prlA4 mutation is not located in the plug domain but in the pore, and may destabilize or widen the channel structure (Van den Berg et al, 2004). Thus, the combination of prlA3 and prlA4 mutations may result in a ‘super-prl' activity due to the bypass of two distinct substeps of the translocation reaction: displacement of the plug and widening of the pore, respectively. Although it is unknown whether the prlA3–prlA4 combination is viable when present as a single copy on the chromosome, we were unable to overexpress the SecYEG complex carrying both mutations (data not shown).
The third observation is that SecG favors the open state of the channel, while its absence leads the position SecE–S120 of two neighboring SecE molecules to become closer to each other (Figure 4B). These perturbations are probably indirect since SecG is located at the periphery of the SecYE core complex (Breyton et al, 2002; Satoh et al, 2003a; Van den Berg et al, 2004). It is possible that the translocation-enhancing SecG subunit changes the conformation of the SecYE complex or SecYE oligomer, which would indirectly lead the plug closer to its open position. Accordingly, a previous BN–PAGE analysis has suggested that SecG modulates the quaternary structure of the SecYE assemblies (Bessonneau et al, 2002).
Our last observation suggests that the oligomeric state of the Sec complex influences the location of the plug, and therefore the open state of the channel. Mutation of the conserved residue R357 and adjacent residues results in both monomerization and inactivation of the complex, and also decreased ability of the plug to move to the open position. In support of this finding, a recent model of the structure of the membrane-embedded and dimeric E. coli Sec complex reveals that, compared to the soluble and monomeric Sec complex, the plug has shifted about 5 Å outward the channel and towards the periplasmic space (M Bostina, B Moshin, W Kühlbrandt and I Collinson, submitted). That mutations around R357 tend to monomerize the Sec complex was unexpected, and it cannot be exluded that it is an indirect effect. Previous genetic studies suggest that the mutagenesis of this region rather impairs an aspect of the SecY–SecA interaction, since the mutations are partially suppressed by a super-active form of SecA (Mori and Ito, 2001). Furthermore, the R357 region is not located at the interface of the SecYEG dimer, but in the cytosolic loop connecting the transmembrane segments TM8 and TM9 of SecY. However, crosslinking analysis also showed that this region is physically close to the central cytosolic loop of SecE (Satoh et al, 2003b). Thus, mutations around residue R357 may provoke a general conformational change, which would propagate and destabilize the interface of the SecYEG oligomer. It should be noted that the oligomeric state as well as the organization of the oligomers is still a matter of contention and it cannot be excluded that mutations around R357 affect only a subpopulation of these oligomers. Nevertheless, the results indicate that monomerization of the Sec complex is somehow correlated to the relocation of the plug towards a closed and inactive state of the translocation channel. We thus propose that the oligomerization of the Sec complex may result in conformational changes important to prime the channel towards its open state, and thus to facilitate its subsequent activation by signal sequences at the initiation of the translocation reaction. Such a preactivation step would partly explain why the translocon exists as an oligomeric assembly (Manting et al, 2000; Duong, 2003; Van den Berg et al, 2004).
The first view of the translocon structure has provided dramatic new insights into the mechanism of preprotein translocation across the membrane. Still, it is clear from this and earlier biochemical findings that much experimentation will be needed to fully integrate together functional and structural information. Further crucial issues now include how the channel opens to accommodate its substrate and what are the subreactions and conformational changes, which must occur in a highly dynamic and tightly orchestrated manner. Further biochemical analysis will keep complementing the structural findings to help us get across the present barriers in our understanding of protein translocation.
Materials and methods
Plasmids
Plasmids pBadEHAYG, pBadYHAEG and pBadEHisYG, and cognate versions deleted for SecG, were described previously (Douville et al, 1995; Duong and Wickner, 1997; Collinson et al, 2001). The various point mutations described in this study were introduced by site-directed mutagenesis, using the Clontech Transformer Mutagenesis kit and following the manufacturer's instructions. All mutations were verified by sequencing the relevant coding regions. Note that the prlA4 mutant is actually a double mutation consisting of the I408N substitution in TM10 and F286Y in TM7 (Osborne and Silhavy, 1993). The stability of the I408N-mutagenized SecYEG complex requires the presence of the F286Y mutation (Duong and Wickner, 1999; de Keyzer et al, 2002). Plasmids were maintained in BL21 with 80 μg/ml of ampicillin and overproduction of the Sec complex was achieved with 0.2% arabinose (Duong and Wickner, 1997).
Materials
ATP, lipid-free BSA, proteinase K, BMOE, BMH, N-ethylmaleimide (NEM) and anti-HA antibodies were purchased from Sigma. A stock solution of 40 mM Cu2+ (phenantroline)3 was prepared as described (Kaufmann et al, 1999). SecA and the mature and precursor form of outer membrane protein A (proOmpA) were purified as described (Crooke et al, 1988; Cunningham et al, 1989). The proOmpA mutant termed LpK carries the mutation A11K in the leader peptide. The purification of the SecYEhisG complexes was achieved by Ni2+-chelating chromatography, according to the procedure described by Collinson et al (2001). After radiolabeling (Bessonneau et al, 2002), the specific activities of the [125I]SecYEG complexes and [125I]proOmpA were ∼1 × 106 and ∼1.2 × 106 c.p.m./μg, respectively. IMVs were prepared from E. coli BL21 overproducing the Sec complex as described previously (Douville et al, 1995). Membranes were resuspended at the concentration of 10 mg/ml in TS buffer (50 mM Tris–HCl, pH 7.9; 50 mM NaCl; 1 mM DTT) and stored at −80°C.
ProOmpA translocation assay
ProOmpA translocation assays were performed in 50 μl of TL buffer (50 mM Tris–HCl, pH 7.9; 50 mM NaCl; 50 mM KCl; 5 mM MgCl2) containing SecA (40 μg/ml), BSA (200 μg/ml), IMVs (50 μg/ml), ATP (2 mM) and [125I]proOmpA (∼60 000 c.p.m.; 5 μg/ml). After 8 min incubation at 37°C, translocation reactions were stopped on ice, then treated with proteinase K (1 mg/ml, 15 min), TCA-precipitated and analyzed by 12% SDS–PAGE and autoradiography. Translocation efficiency was compared to [125I]proOmpA standard and evaluated by scanning densitometry using the AlphaImager software.
Crosslinking assays
The SecY–SecE crosslinking assay was performed in 50 μl of TL buffer containing BSA (200 μg/ml), IMVs (100 μg/ml) and the indicated concentration of Cu2+(phe)3 or cysteine-reactive crosslinker. The translocation-dependent plug movement assay was performed in 50 μl of TL buffer containing SecA (40 μg/ml), BSA (200 μg/ml), IMVs (100 μg/ml), ATP (2 mM) and proOmpA (20 μg/ml). Unreacted cysteines were blocked with NEM (8 mM final, 5 min, room temperature (RT)). Reisolation of oxidized IMVs was performed by layering the membranes over one volume of 0.2 M sucrose in TL buffer, followed by ultracentrifugation (10 min, 4°C, 55 000 r.p.m., Beckman TL55 rotor). Sediments were rinsed and resuspended by brief sonication in TL buffer. About 1 μg of IMVs proteins were analysed by SDS–PAGE and Western blotting techniques.
Other methods
Linear gradient blue native gels, electrophoretic conditions and electroblotting were performed as described by Schägger and von Jagow (1991). Immunoblots were visualized using the ECL reagents (Amersham). Protein concentrations were determined using the Bradford reagent (Biorad) using BSA as a standard.
Acknowledgments
We are grateful to Dr Mark Paetzel for critical reading of the manuscript, to Chaslynn Chatwin for great assistance throughout this work and to Dr I Collinson for communicated results prior publication. APM is an INSERM-CIHR visiting-scientist scholar. This work was supported by the Canada Research Chair program, the Canada Foundation for Innovation and the Canadian Institutes of Health Research (CIHR).
References
- Beckmann R, Spahn CM, Eswar N, Helmers J, Penczek PA, Sali A, Frank J, Blobel G (2001) Architecture of the protein-conducting channel associated with the translating 80S ribosome. Cell 107: 361–372 [DOI] [PubMed] [Google Scholar]
- Bessonneau P, Besson V, Collinson I, Duong F (2002) The SecYEG preprotein translocation channel is a conformationally dynamic and dimeric structure. EMBO J 21: 995–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyton C, Haase W, Rapoport TA, Kuhlbrandt W, Collinson I (2002) Three-dimensional structure of the bacterial protein-translocation complex SecYEG. Nature 418: 662–665 [DOI] [PubMed] [Google Scholar]
- Cannon KS, Or E, Clemons WM Jr, Shibata Y, Rapoport TA (2005) Disulfide bridge formation between SecY and a translocating polypeptide localizes the translocation pore to the center of SecY. J Cell Biol 169: 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao TB, Saier MH (2003) The general protein secretory pathway: phylogenetic analyses leading to evolutionary conclusions. Biochim Biophys Acta 1609: 115–125 [DOI] [PubMed] [Google Scholar]
- Clemons WM Jr, Menetret JF, Akey CW, Rapoport TA (2004) Structural insight into the protein translocation channel. Curr Opin Struct Biol 14: 390–396 [DOI] [PubMed] [Google Scholar]
- Collinson I, Breyton C, Duong F, Tziatzios C, Schubert D, Or E, Rapoport T, Kuhlbrandt W (2001) Projection structure and oligomeric properties of a bacterial core protein translocase. EMBO J 20: 2462–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke E, Brundage L, Rice M, Wickner W (1988) ProOmpA spontaneously folds in a membrane assembly competent state which trigger factor stabilizes. EMBO J 7: 1831–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K, Lill R, Crooke E, Rice M, Moore K, Wickner W, Oliver D (1989) SecA protein, a peripheral protein of the Escherichia coli plasma membrane, is essential for the functional binding and translocation of proOmpA. EMBO J 8: 955–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey RE, Chen M (2004) Sec-translocase mediated membrane protein biogenesis. Biochim Biophys Acta 1694: 37–53 [DOI] [PubMed] [Google Scholar]
- de Keyzer J, van der Does C, Swaving J, Driessen AJ (2002) The F286Y mutation of PrlA4 tempers the signal sequence suppressor phenotype by reducing the SecA binding affinity. FEBS Lett 510: 17–21 [DOI] [PubMed] [Google Scholar]
- Derman AI, Puziss JW, Bassford PJ Jr, Beckwith J (1993) A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J 12: 879–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douville K, Price A, Eichler J, Economou A, Wickner W (1995) SecYEG and SecA are the stoichiometric components of preprotein translocase. J Biol Chem 270: 20106–20111 [DOI] [PubMed] [Google Scholar]
- Duong F (2003) Binding, activation and dissociation of the dimeric SecA ATPase at the dimeric SecYEG translocase. EMBO J 22: 4375–4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong F, Wickner W (1997) Distinct catalytic roles of the SecYE, SecG and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J 16: 2756–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong F, Wickner W (1999) The PrlA and PrlG phenotypes are caused by a loosened association among the translocase SecYEG subunits. EMBO J 18: 3263–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr SD, Hanley-Way S, Silhavy TJ (1981) Suppressor mutations that restore export of a protein with a defective signal sequence. Cell 23: 79–88 [DOI] [PubMed] [Google Scholar]
- Flower AM, Doebele RC, Silhavy TJ (1994) PrlA and PrlG suppressors reduce the requirement for signal sequence recognition. J Bacteriol 176: 5607–5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CR, Silhavy TJ (1999) Mapping an interface of SecY (PrlA) and SecE (PrlG) by using synthetic phenotypes and in vivo cross-linking. J Bacteriol 181: 3438–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A, Manting EH, Veenendaal AK, Driessen AJ, van der Does C (1999) Cysteine-directed cross-linking demonstrates that helix 3 of SecE is close to helix 2 of SecY and helix 3 of a neighboring SecE. Biochemistry 38: 9115–9125 [DOI] [PubMed] [Google Scholar]
- Luirink J, Sinning I (2004) SRP-mediated protein targeting: structure and function revisited. Biochim Biophys Acta 1694: 17–35 [DOI] [PubMed] [Google Scholar]
- Maillard AP, Chen K, Duong F (2005) Preprotein translocation through the Sec translocon in bacteria. In Protein Movement Across Membranes, Eichler J (ed), pp 1–13. Eurekah Bioscience Collection: Landes [Google Scholar]
- Manting EH, van der Does C, Remigy H, Engel A, Driessen AJ (2000) SecYEG assembles into a tetramer to form the active protein translocation channel. EMBO J 19: 852–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TH, Menetret JF, Breitling R, Miller KR, Akey CW, Rapoport TA (1999) The bacterial SecY/E translocation complex forms channel-like structures similar to those of the eukaryotic Sec61p complex. J Mol Biol 285: 1789–1800 [DOI] [PubMed] [Google Scholar]
- Mori H, Ito K (2001) An essential amino acid residue in the protein translocation channel revealed by targeted random mutagenesis of SecY. Proc Natl Acad Sci USA 98: 5128–5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Tsukazaki T, Masui R, Kuramitsu S, Yokoyama S, Johnson AE, Kimura Y, Akiyama Y, Ito K (2003) Fluorescence resonance energy transfer analysis of protein translocase. SecYE from Thermus thermophilus HB8 forms a constitutive oligomer in membranes. J Biol Chem 278: 14257–14264 [DOI] [PubMed] [Google Scholar]
- Nishiyama K, Hanada M, Tokuda H (1994) Disruption of the gene encoding p12 (SecG) reveals the direct involvement and important function of SecG in the protein translocation of Escherichia coli at low temperature. EMBO J 13: 3272–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwen N, de Kruijff B, Tommassen J (1996) prlA suppressors in Escherichia coli relieve the proton electrochemical gradient dependency of translocation of wild-type precursors. Proc Natl Acad Sci USA 93: 5953–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne RS, Silhavy TJ (1993) PrlA suppressor mutations cluster in regions corresponding to three distinct topological domains. EMBO J 12: 3391–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PS, Filipek S, Wells JW, Palczewski K (2004) Oligomerization of G protein-coupled receptors: past, present, and future. Biochemistry 43: 15643–15656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA, Spiess C, Ehrmann M, Schierle C, Beckwith J (1996) Targeting of signal sequenceless proteins for export in Escherichia coli with altered protein translocase. EMBO J 15: 5209–5217 [PMC free article] [PubMed] [Google Scholar]
- Rapoport TA, Goder V, Heinrich SU, Matlack KE (2004) Membrane-protein integration and the role of the translocation channel. Trends Cell Biol 14: 568–575 [DOI] [PubMed] [Google Scholar]
- Satoh Y, Matsumoto G, Mori H, Ito K (2003a) Nearest neighbor analysis of the SecYEG complex. 1. Identification of a SecY–SecG interface. Biochemistry 42: 7434–7441 [DOI] [PubMed] [Google Scholar]
- Satoh Y, Mori H, Ito K (2003b) Nearest neighbor analysis of the SecYEG complex. 2. Identification of a SecY–SecE cytosolic interface. Biochemistry 42: 7442–7447 [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199: 223–231 [DOI] [PubMed] [Google Scholar]
- Van den Berg B, Clemons WM Jr, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA (2004) X-ray structure of a protein-conducting channel. Nature 427: 36–44 [DOI] [PubMed] [Google Scholar]
- van der Sluis EO, Nouwen N, Driessen AJ (2002) SecY–SecY and SecY–SecG contacts revealed by site-specific crosslinking. FEBS Lett 527: 159–165 [DOI] [PubMed] [Google Scholar]
- van der Wolk JP, Fekkes P, Boorsma A, Huie JL, Silhavy TJ, Driessen AJ (1998) PrlA4 prevents the rejection of signal sequence defective preproteins by stabilizing the SecA–SecY interaction during the initiation of translocation. EMBO J 17: 3631–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenendaal AK, van der Does C, Driessen AJ (2004) The protein-conducting channel SecYEG. Biochim Biophys Acta 1694: 81–95 [DOI] [PubMed] [Google Scholar]
- Veenhoff LM, Heuberger EH, Poolman B (2002) Quaternary structure and function of transport proteins. Trends Biochem Sci 27: 242–249 [DOI] [PubMed] [Google Scholar]
- Yahr TL, Wickner WT (2000) Evaluating the oligomeric state of SecYEG in preprotein translocase. EMBO J 19: 4393–4401 [DOI] [PMC free article] [PubMed] [Google Scholar]


