Abstract
To study host response to CagA, human gastric cancer cell line AGS was infected with a Helicobacter pylori type I wild-type or isogenic cagA-negative mutant. Differentially expressed genes were identified using cDNA array technology. By Northern blotting, downregulation of focal adhesion kinase and upregulation of LIM kinase mRNA in the presence of CagA were clearly verified. Furthermore, upregulation of LIM kinase, macrophage inflammatory protein-2, c-myc, and bone morphogenetic protein-1 and downregulation of transcription factor Y-box binding protein-1 and focal adhesion kinase mRNA in response to H. pylori type I infection compared to the uninfected control could be shown by Northern blotting. Hence, these findings identified new targets for further functional studies on H. pylori-associated pathogenesis.
Helicobacter pylori type I strains exclusively harbor the multigene 40-kb cag pathogenicity island (PAI) associated with increased bacterial virulence contributing to a more severe inflammatory response in the host mucosa (5). Based on sequence homology, a putative type IV secretion apparatus encoded by multiple genes of the cag PAI has been described (5). This locus is required for the induction of interleukin (IL)-8 through an NF-κB-dependent pathway in gastric epithelial cells (5, 24). Furthermore, cytoskeletal reorganization, tyrosine phosphorylation of host proteins (23), and differential activation of MAP kinases (12, 14) occur in gastric epithelial cells after attachment of type I strains.
The cagA gene serves as a marker for the cag PAI, encoding an immunodominant size-variant protein of unknown physiologic function (7). Recently, it was shown that CagA is translocated into gastric epithelial cells via the putative type IV secretion system and subsequently becomes tyrosine phosphorylated (3, 16, 22, 25). However, the mode(s) of CagA action in the epithelial cell still needs to be elucidated.
In this study, cDNA array technology was used to investigate the role of CagA in host gene expression. cDNA expression arrays were screened in parallel to detect alterations in gene expression of AGS cells stimulated with an isogenic pair of H. pylori type I strains, a wild-type and a cagA-negative isogenic mutant. Subsequently, differentially expressed genes identified were subjected to further analysis by Northern blotting.
H. pylori growth and infection of AGS cells.
H. pylori strains were routinely cultured for 48 h on H. pylori selective agar (Biotest Laboratories) in a microaerobic atmosphere at 37°C. The human gastric epithelial adenocarcinoma cell line AGS (ATCC CRL 1739) was grown in RPMI 1640 supplemented with 4 mM l-glutamine and 10% fetal calf serum (Life Technologies Inc., Rockville, Md.) at 37°C in a humidified atmosphere of 5% CO2. Prior to infection, viability of H. pylori cultures was routinely assessed by phase contrast microscopy. A monolayer of AGS cells (107) grown to 80% confluency was cocultured with H. pylori at a multiplicity of infection of 100 in culture medium for 4.5 h. As CagA translocation has been shown to occur 30 min after infection but is at its maximum in a time range of about 4 to 5 h (3, 15) the time point of 4.5 h chosen in our study seemed to be appropriate to investigate alterations in gene expression which may primarily reflect events early in host response to CagA.
cDNA array analysis.
H. pylori wild-type P12 (cag PAI+) and the corresponding cagA-negative isogenic mutant P17 derived by insertional mutagenesis (21) were used for stimulation. The absence of CagA protein in P17 was confirmed by Western blotting (results not shown). Total cellular RNA was extracted by the guanidinium thiocyanate single-step method (6) at 4.5 h postinfection and treated with RQ1 DNase (0.5 U/μg of RNA) (Promega, Madison, Wis.). Efficient H. pylori infection was controlled by detecting relative differences in IL-8 expression using semiquantitative reverse transcription (RT)-PCR. Briefly, cDNA was prepared from 5 μg of each preparation of total RNA dedicated to array analysis using Moloney murine leukemia virus reverse transcriptase (1 U/μg) (Promega, Madison, Wis.). Subsequent PCR was carried out under standard conditions using oligonucleotides specific for IL-8 (Table 1). For normalization, β-actin RT-PCR was performed. P12 and P17 were shown to induce IL-8 in contrast to uninfected control and no significant differences in IL-8 mRNA levels could be found between the two samples (data not shown).
TABLE 1.
Target-specific primer sequences for probe generation by PCR
| GenBank accession no. | Name | Primer 1 (forward) | Primer 2 (reverse) | Positions | Transcript size (bp) |
|---|---|---|---|---|---|
| M22488 | BMP-1 | TCCTGGATACCATTGTCC | AGATGGCTTCGTAGACTG | 874–1333 | 2,487 |
| V00568 | c-myc | TGCAGCCGTATTTCTACTG | CCTCATCTTCTTGTTCCTC | 614–1337 | 2,121 |
| L13616 | FAK | GGTCATCTGGGAAGCCTTG | GAGGGTAGCAAGACGTGCTC | 2879–3422 | 3,791 |
| D26309 | LIMK | CATCTCATGGCAAGCGTG | GGCTGAGTCTTCTCGTCCAC | 607–1570 | 3,262 |
| X53799 | MIP2-α | GAATTCACCTCAAGAACATC | CCTAAGTGATGCTCAAAC | 194–501 | 1,081 |
| M83234 | 4B-1 | CATCAACAGGAATGACAC | GCGTCTATAATGGTTACG | 447–667 | 1,468 |
| M28130 | IL-8a | ATGACTTCCAAGCTGGCCGTGGCT | TCTCAGCCCTCTTCAAAAACTTCTC | 1584–1873 | 5,191 |
| NM001101 | β-Actinb | CATGTACGTTGCTATCCAGGCTGTG | GAAGGTAGTTTCGTGGATGCCACAG | 466–916 | 1,793 |
| M33197 | GAPDHc | CCACCCATGGCAAATTCCATGGCA | TCTAGACGGCAGGTCAGGTCCACC | 212–812 | 1,268 |
Primer pair purchased from Clontech.
Primer pair generously provided by R. Mader (Vienna, Austria).
Primer pair purchased from Stratagene (La Jolla, Calif.).
For array analysis, poly(A)+ RNA was prepared from total RNA by oligo(dT)-latex bead chromatography (Qiagen, Hilden, Germany). Atlas human cDNA expression arrays (Clontech, Palo Alto, Calif.) were used representing a broad range of 588 arrayed human cDNAs and housekeeping controls spotted in duplicate. The array is arranged into six sections of 98 genes each, representing the following functional groups: cell cycle regulator genes and oncogenes (region A), stress response and intracellular signaling-related genes (B), apoptosis-related genes (C), DNA repair and recombination genes (D), transcription factors (E), and cell adhesion molecules and genes involved in cell-cell communication (F). Poly(A)+ RNA (1 μg) was reverse transcribed into 32P-labeled first-strand cDNA according to the manufacturer’s instructions. Equal amounts of cDNA (106 cpm/μl) were hybridized overnight at 65°C to the arrays. Following the high-stringency washes, analysis of hybridization patterns was performed by autoradiography (Fig. 1) and scanning densitometry using the ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). Several film exposures were scanned to ensure that signal intensities were within the linear range of the film. Control spots on arrays were negative for genomic DNA contamination.
FIG. 1.
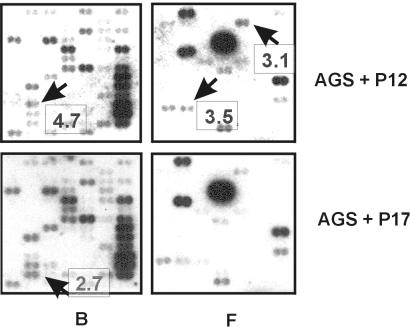
Parallel fingerprints of AGS cells infected for 4.5 h with the P12 wild-type or its isogenic cagA-negative mutant, P17. Region B (stress response) and region F (cell-cell communication) of the human Atlas array are shown. Blots were exposed to X-ray film for 24 h. Arrows mark representative cDNA spots that showed significant differences in gene expression, and boxes indicate fold upregulation of the respective gene in AGS cells stimulated with the indicated H. pylori strain relative to the parallel approach.
Ratios of signal intensities between potential differentially expressed genes were calculated. The constitutively expressed genes produced comparable signal intensities on both membranes which did not change by more than 0.25-fold between the arrays. After background reduction, an average of these values was used for normalization of the arrays to correct probe variation between signals to be validated. Subsequently, ratios of expression levels were calculated for each gene producing sufficient signal intensities to be analyzed (176 genes, 29.9%). Overall, 75 of these genes (42.6%) exhibited a more than 0.25-fold difference in abundance between the arrays. As no replicate was performed, a very stringent cutoff for significant up- or downregulation of genes was set to a 2.5-fold difference in transcript abundance to avoid false-positives. However, we are aware that by doing so some genes differentially regulated by small amounts may have been excluded from further analysis.
Figure 1 shows two representative regions of the array, including results from quantitative evaluation of the dots. Hence, three genes, LIM kinase (LIMK), bone morphogenetic protein 1 (BMP-1), and macrophage inflammatory protein 2α (MIP-2α), were identified to be upregulated. Three genes, focal adhesion kinaw (FAK), Y-box binding protein 1 (YB-1), and c-myc, appeared to be downregulated in AGS cells coincubated with P12 compared to those treated with the cagA-negative mutant strain (Table 2).
TABLE 2.
Names and GenBank accession numbers of upregulated transcripts after stimulation with P12 or P17 compared to the respective parallel approach
| Stimulation | Upregulated transcripts (GenBank accession no.) | Fold stimulation | Functional role of gene |
|---|---|---|---|
| AGS + P12 (wild type) | LIMK (D26309) | 4.7 | Intracellular signal transduction, serine/threonine kinase |
| BMP-1 (M22488) | 3.5 | Cell-cell communication, metalloproteinase | |
| MIP-2α (GRO beta) (X53799) | 3.1 | Cell-cell communication, cytokine (C-X-C subgroup) | |
| AGS + P17 (cagA negative) | FAK (L13616) | 2.7 | Intracellular signal transduction, tyrosine kinase |
| YB-1 (M83234) | 2.6 | Transcription factor | |
| myc proto-oncogene (V00568) | 2.5 | Oncogene |
Confirmation of differential gene expression.
Stimulation experiments were subsequently repeated at least three times as described, including other sets of isogenic strains: P12ΔcagA, a cagA knockout of P12 (16), as well as wild-type G27 (cag PAI+) and cagA knockout strain G27ΔcagA (5, 22). As cag PAI-encoded factor CagE is critical for IL-8 induction and tyrosine phosphorylation, being a putative core protein of the predicted type IV system, a cagE-negative strain (10-B4) isogenic to G27 was also included (5). As expected, IL-8 expression was not induced in AGS cells stimulated with 10-B4 compared to stimulation with the G27 wild-type. In contrast, all other strains mentioned above induced IL-8 to a similar extent (results not shown).
For Northern analysis, DNase I-treated total RNA (35 μg) was separated on denaturing 1.2% agarose–formaldehyde gels and capillary transferred onto nylon membranes (Amersham Pharmacia Biotech). To generate the labeled probes, specific cloned cDNA fragments, amplified from the respective mRNAs (Table 1), were biotinylated by PCR using Pwo polymerase (Hoffmann-La Roche, Basel, Switzerland), and 3.5 nmol of dATP was replaced by biotin-14-dATP (0.4 mM; Gibco Life Technologies, Rockville, Md.) in the reaction mixture (0.2 mM deoxynucleoside triphosphates, 2 mM MgCl2). The membranes were prehybridized for 1 h in North2South hybridization buffer (Pierce, Rockford, Ill.) at 65°C and hybridized overnight to the cDNA probe (30 ng/ml of hybridization buffer). Four posthybridization washes were done at 20 min each at 65°C with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate–0.1% sodium dodecyl sulfate (SDS). Detection of cDNA/RNA hybrids was carried out using the North2South chemiluminescent detection system.
RNA loadings were normalized by blot hybridization to a glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific probe. Sizes of the transcripts were determined by relative migration versus eukaryotic 28S and 18S rRNAs. Blots were exposed to Kodak X-Omat films (Eastman Kodak, Rochester, N.Y.), and autoradiograms were quantified as described. Statistical analysis of quantitative evaluation was performed by comparing means using the one-way analysis of variance procedure and Bonferroni post hoc range test for multiple comparisons. Probability (P) values less than 0.05 were considered significant.
BMP-1, YB-1, MIP-2, and c-myc are differentially expressed in H. pylori type I infection.
For these genes, results obtained by Northern blotting were not consistent with the differential data from array analysis, as no significant difference in expression between AGS cells stimulated with either the wild-types or the corresponding cagA-negative mutants was found. Importantly, all of these genes were found to be differentially regulated in response to H. pylori type I infection. In our opinion, the highly reproducible results (P < 0.01) obtained in triplicate series of Northern blot experiments (Fig. 2) are assumed to be more relevant than the data obtained from array analysis.
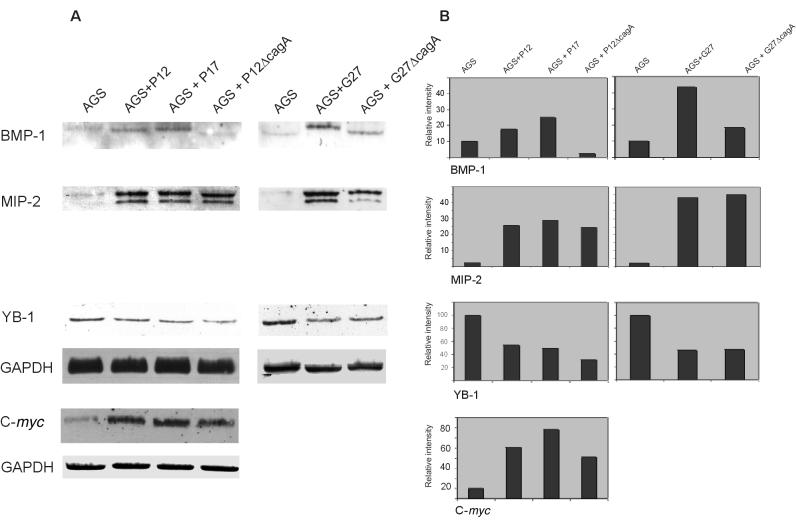
FIG. 2.
Representative series of Northern blots hybridized to the indicated gene-specific probes. Three independent experiments were performed with similar results. After 4.5 h of coincubation using either wild-type P12 and cagA-negative isogenic strain P17 or P12ΔcagA or the isogenic pair G27 and G27ΔcagA, total RNA was isolated and subjected to Northern blotting as described (A). Total RNA from uninfected AGS cells was included as a control. The blots were hybridized to one probe, stripped, and rehybridized sequentially with the other probes indicated. Quantitation is indicated as fold intensity versus the control (B).
Transcription of BMP-1, a metalloproteinase which is putatively involved in morphogenesis and wound repair by altering cell-matrix interactions (10), was found to be upregulated following stimulation with the tested H. pylori wild-type G27 by up to 4.4-fold compared to the unstimulated control. Infection with P17 induced BMP-1 expression to similar levels as the wild-type P12. However, using P12ΔcagA or G27ΔcagA as the stimulus, a marked downregulation of the gene comparable to the AGS control could be found.
Furthermore, the transcript specific for YB-1 reproducibly appeared to be downregulated in all H. pylori-treated AGS populations, by up to the 3.2-fold (P12ΔcagA). Transcription factor YB-1 has been suggested to play a role in promoting cell proliferation, since Y-boxes are present in the promoters of several genes associated with cell division (26). It could therefore be speculated that a downregulation of YB-1 may be due to growth retardation of gastric epithelial cells occurring during H. pylori infection, as described (1). Our results provide first evidence that YB-1 and BMP-1 might be involved in host response to infection with H. pylori.
Our probe specific for MIP-2α (GRO beta), which is 95% homologous to the MIP-2β (GRO gamma) gene (8), detected both of the MIP-2-related mRNAs. Thus, the Northern blots revealed a profound upregulation of up to 45-fold of MIP-2, which has similar activities on neutrophils as IL-8, upon infection of AGS cells with all H. pylori type I strains. These results are in agreement with Yamaoka et al., who described an increase in GRO expression due to H. pylori infection in gastric biopsy specimens (27). To our knowledge, our results obtained with the characterized isogenic H. pylori strains are the first to indicate that CagA does not influence induction of MIP-2.
Finally, a significant upregulation of the c-myc proto-oncogene by up to 6.8-fold (P17) was demonstrated upon infection with all H. pylori strains. These findings are consistent with those of Nardone et al., who showed enhanced prevalence of c-myc expression in patients with gastric atrophy who were chronically infected with type I strains (13).
Expression of FAK and LIMK is regulated in a CagA-dependent manner.
Data from array analysis were confirmed by quantitative evaluation of Northern blots. FAK and LIMK are both involved in signaling cascades regulating organization of the actin cytoskeleton (28, 29). As a consequence, FAK and LIMK are both involved in regulation of cell motility and growth control. LIMK overexpression appears to retard cell growth indirectly by affecting processes related to cell proliferation, such as cytoskeletal organization (9), whereas overexpression of FAK leads to increased cell migration and survival (11).
Northern blots revealed a very low basal transcription of LIMK in AGS cells which was increased by up to 3.5-fold (G27) upon incubation with H. pylori wild-type strains. Expression of LIMK mRNA was 2.5-fold lower in P17-treated AGS cells compared to cells infected with the wild-type P12. Similar results were also achieved with the other pairs of isogenic strains differing in cagA presence or absence (Table 3). In contrast, transcription of FAK was decreased to 4.8-fold after exposure to H. pylori wild-type strains P12 and G27. Furthermore, incubation with a cagA-negative strain led to upregulation of FAK mRNA by up to 3.5-fold in AGS cells compared to infection with the wild type. With respect to both kinases, results similar to those shown with cagA-negative mutants were obtained by infection with strain 10-B4 (cagE-negative) (Table 3). Hence, it can be assumed that translocation of CagA through the putative type IV secretion apparatus might be necessary for transcription modulation of both genes.
TABLE 3.
Decrease or increase in LIMK and FAK expression due to incubation of AGS cells with pairs of isogenic H. pylori strains for 4.5 ha
| Cells + strain | FAK expression (fold)
|
LIMK expression (fold)
|
||
|---|---|---|---|---|
| Decrease versus uninfected control | Increase versus wild type | Increase versus uninfected control | Decrease versus wild type | |
| AGS + P12 | 4.78 ± 0.07* | 2.98 ± 0.13* | ||
| AGS + P17 | 1.28 ± 0.17 | 3.01 ± 0.17* | 1.17 ± 0.19 | 2.54 ± 0.19* |
| AGS + P12ΔcagA | 1.27 ± 0.18 | 3.48 ± 0.18* | 1.46 ± 0.16 | 2.04 ± 0.16* |
| AGS + G27 | 4.54 ± 0.10* | 3.49 ± 0.52* | ||
| AGS + G27ΔcagA | 1.43 ± 0.08 | 3.19 ± 0.08* | 1.38 ± 0.17 | 2.52 ± 0.17* |
| AGS + 10-B4 (cagE) | 1.31 ± 0.11 | 3.47 ± 0.11* | 1.25 ± 0.15 | 2.79 ± 0.15* |
Data from three independent Northern blot experiments were quantitatively evaluated, and means were compared statistically. *, P < 0.01 versus indicated control.
A role for CagA has been proposed for development of a specific cellular phenotype leading to dramatic elongation and spreading of gastric epithelial cells, which superimposes the stress fiber-associated (SFA) morphology induced by H. pylori attachment (22). Signal transduction pathways induced by attachment of H. pylori type I causing the morphological changes are not well characterized. Recently, involvement of small Rac GTPase in the signal transduction cascade leading to actin reorganization induced by H. pylori has been shown (18). Rho and Rac have both been shown to induce activation of LIMK, suggesting that these two G-proteins are mutually integrated in a highly dynamic process to regulate LIMK action and actin reorganization (17, 28).
FAK is a peripheral membrane protein mainly located in focal adhesions, where active stress fibers contact the cell membrane. A role of FAK in integrin-mediated activation of Rac and actin cytoskeleton organization has been described (4). In this context, it should be considered that FAK may play a role in SFA phenotype, which is inevitably caused by all H. pylori isolates. Downregulation of FAK and upregulation of LIMK might be regarded as an indication of the involvement of CagA in downregulating the SFA cellular phenotype in infected cells to favor development of the specific morphological phenotype caused by type I isolates.
Following CagA translocation, dephosphorylation of host cell proteins in the size range of 80 kDa and 120 to 130 kDa is observed (15). Interestingly, FAK has a molecular mass of 125 kDa. The Yersinia YopH protein, which is a highly active tyrosine phosphatase and disrupts focal adhesions by dephosphorylating FAK (19), is also delivered into the eukaryotic cell by a type III secretion system functionally similar to type IV and effectively inhibits bacterial uptake by epithelial cells and macrophages. It may therefore be hypothesized that CagA or other products of the cag PAI may play a similar role by regulating FAK activity. However, only divergent data on H. pylori resistance to phagocytosis are available at present (2, 15, 20).
In summary, differential expression of FAK and LIMK was clearly confirmed to be influenced by the presence of CagA in this study. However, from our recent data, we cannot definitely conclude whether the activities of LIMK and FAK are affected by CagA. Further functional studies to investigate if and how the biological activities of FAK and LIMK are influenced as a consequence of infection with H. pylori type I strains are currently being performed.
Acknowledgments
This study was financially supported in part by the European Helicobacter pylori Study Group (EHPSG).
We kindly thank Antonello Covacci (Siena, Italy), Wolfgang Fischer and Rainer Haas (Munich, Germany), and Nina Salama and Ellyn Segal (Stanford, Calif.) for generously providing the H. pylori isogenic strains used in this study.
Editor: V. J. DiRita
REFERENCES
- 1.Ahmed, A., D. Smoot, G. Littleton, R. Tackey, C. S. Walters, F. Kashanchi, C. R. Allen, and H. Ashtorab. 2000. Helicobacter pylori inhibits gastric cell cycle progression. Microbes Infect. 2:1159–1169. [DOI] [PubMed] [Google Scholar]
- 2.Allen, L. A., L. S. Schlesinger, and B. Kang. 2000. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 191:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asahi, M., T. Azuma, S. Ito, Y. Ito, H. Suto, Y. Nagai, M. Tsubokawa, Y. Tohyama, S. Maeda, M. Omata, T. Suzuki, and C. Sasakawa. 2000. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J. Exp. Med. 191:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cary, L. A., D. C. Han, and J. L. Guan. 1999. Integrin-mediated signal transduction pathways. Histol. Histopathol. 14:1001–1009. [DOI] [PubMed] [Google Scholar]
- 5.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. Cag, a pathogenicity island of Helicobacter pylori encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648–14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chomczynski, P., and N. Sacchi. 1987. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156–159. [DOI] [PubMed] [Google Scholar]
- 7.Covacci, A., S. Censini, M. Bugnoli, R. Petracca, D. Burroni, G. Macchia, A. Massone, E. Papini, Z. Xiang, N. Figura, and R. Rappuoli. 1993. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA 90:5791–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haskill, S., A. Peace, J. Morris, S. A. Sporn, A. Anisowicz, S. W. Lee, T. Smith, G. Martin, P. Ralph, and R. Sager. 1990. Identification of three human related gro genes encoding cytokine functions. Proc. Natl. Acad. Sci. USA 87:7732–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higuchi, O., G.-H. Baeg, T. Akiyama, and K. Mizuno. 1996. Suppression of fibroblast cell growth by overexpression of LIM-kinase 1. FEBS Lett. 396:81–86. [DOI] [PubMed] [Google Scholar]
- 10.Kessler, E., K. Takahara, L. Biniaminov, M. Brusel, and D. S. Greenspan. 1996. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science 271:360–362. [DOI] [PubMed] [Google Scholar]
- 11.Kornberg, L. J. 1998. Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck 20:745–752. [DOI] [PubMed] [Google Scholar]
- 12.Meyer-ter-Vehn, T., A. Covacci, M. Kist, and H. L. Pahl. 2000. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J. Biol. Chem. 275:16064–16072. [DOI] [PubMed] [Google Scholar]
- 13.Nardone, G., S. Staibano, A. Rocco, E. Mezza, F. P. D’Armiento, L. Insabato, A. Coppola, G. Salvatore, A. Lucariello, N. Figura, G. De Rosa, and G. Budillon. 1999. Effect of Helicobacter pylori infection and its eradication on cell proliferation, DNA status, and oncogene expression in patients with chronic gastritis. Gut 44:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naumann, M., S. Wessler, C. Bartsch, B. Wieland, A. Covacci, R. Haas, and T. F. Meyer. 1999. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J. Biol. Chem. 274:31655–31662. [DOI] [PubMed] [Google Scholar]
- 15.Odenbreit, S., B. Gebert, J. Puls, W. Fischer, and R. Haas. 2001. Interaction of Helicobacter pylori with professional phagocytes: role of the cag pathogenicity island and translocation, phosphorylation and processing of CagA. Cell. Microbiol. 3:21–31. [DOI] [PubMed] [Google Scholar]
- 16.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497–1500. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi, K., K. Nagata, M. Maekawa, T. Ishizaki, S. Narumiya, and K. Mizuno. 2000. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J. Biol. Chem. 275:3577–3582. [DOI] [PubMed] [Google Scholar]
- 18.Palovuori, R., A. Perttu, Y. Yan, R. Karttunen, S. Eskelinen, and T. J. Karttunen. 2000. Helicobacter pylori induces formation of stress fibers and membrane ruffles in AGS cells by rac activation. Biochem. Biophys. Res. Commun. 269:247–253. [DOI] [PubMed] [Google Scholar]
- 19.Persson, C., N. Carballeira, H. Wolf-Watz, and M. Fällmann. 1997. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16:2307–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramarao, N., S. D. Gray-Owen, S. Backert, and T. F. Meyer. 2000. Helicobacter pylori inhibits phagocytosis by professional phagocytes involving type IV secretion components. Mol. Microbiol. 37:1389–1404. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt, W., and R. Haas. 1994. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol. Microbiol. 12:307–319. [DOI] [PubMed] [Google Scholar]
- 22.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: Involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559–14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal, E. D., C. Lange, A. Covacci, L. S. Tompkins, and S. Falkow. 1997. Induction of host signal transduction pathways by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 94:7595–7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma, S. A., M. K. R. Tummuru, M. J. Blaser, and L. D. Kerr. 1998. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor κB in gastric epithelial cells. J. Immunol. 160:2401–2407. [PubMed] [Google Scholar]
- 25.Stein, M., R. Rappuoli, and A. Covacci. 2000. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. USA 97:1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolffe, A. P., S. Tafuri, M. Ranjan, and M. Familari. 1992. The Y-box-factors: a family of nucleic acid binding proteins conserved from Escherichia coli to man. New Biol. 4:290–298. [PubMed] [Google Scholar]
- 27.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, T. Tanahashi, K. Kashima, and J. Imanishi. 1998. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut 42:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang, N., O. Higuchi, K. Ohashi, K. Nagata, A. Wada, K. Kangawa, E. Nishida, and K. Mizuno. 1998. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393:809–812. [DOI] [PubMed] [Google Scholar]
- 29.Zachary, I. 1997. Focal adhesion kinase. Int. J. Biochem. Cell Biol. 29:929–934. [DOI] [PubMed] [Google Scholar]