Abstract
The generation of superoxide anion radicals (O2•−) and hydrogen peroxide (H2O2) during mitochondrial respiration has been widely postulated to be causally linked to the aging process. The hypothesis that a specific enhancement of mitochondrial O2•−/H2O2 catabolism would delay age-associated physiological changes and extend the lifespan was tested by simultaneous overexpression of MnSOD (manganese superoxide dismutase) and catalase, ectopically targeted to the mitochondrial matrix of transgenic Drosophila melanogaster. The increased activities of these antioxidative enzymes resulted in a decrease of mitochondrial H2O2 release and enhancement of free methionine content. The MnSOD/mitochondrial catalase transgenic flies displayed an enhanced resistance to experimental oxidative stress, induced by dietary H2O2 administration or by exposure to 100% ambient oxygen. However, the lifespan of the flies was decreased, by up to 43%, and this effect coincided with (i) an overall decrease in physical fitness, as measured by the speed of walking, and (ii) an agerelated decrease in mitochondrial state 3 (ADP-stimulated) respiration. These findings support the notion that mitochondrial O2•−/H2O2 production at physiological levels is essential for normal biological processes leading to the attainment of a normal lifespan.
Keywords: aging, catalase, Drosophila, manganese superoxide dismutase, mitochondria, oxidative stress
Abbreviations: MnSOD, manganese superoxide dismutase; OAT, ornithine aminotransferase; Dm2SOD/OCAT, flies containing one copy of the MnSOD transgene and one copy of the OAT-catalase transgene; Dm4SOD/OCAT, flies containing two copies of the MnSOD transgene and two copies of the OAT-catalase transgene; RCR, respiratory control ratio; ROS, reactive oxygen species
INTRODUCTION
The mechanisms that cause the deterioration of cellular functions during the aging process are at present poorly understood. The hypothesis that senescent changes are caused primarily by ROS (reactive oxygen species) and the accumulation of macromolecular oxidative damage generated by ROS, is supported by a considerable body of correlative evidence. For instance, the rates of production of superoxide anion radicals (O2•−) and hydrogen peroxide (H2O2) by mitochondria have been found to increase with age and are negatively correlated with the maximum lifespan of mammalian and insect species [1,2]. Steady-state amounts of oxidatively modified molecules increase as a function of age in virtually all the species that have been examined [2,3]. Furthermore, experimental regimens that prolong lifespan, such as caloric restriction in rodents and prevention of flying activity in insects, are associated with decreased rates of mitochondrial O2•−/H2O2 generation and lower amounts of molecular oxidative damage [4].
It is estimated that, under normal physiological conditions, between 0.1 and 3% of the oxygen utilized by mitochondria is diverted to the generation of O2•− [5,6]. MnSOD (manganese superoxide dismutase), located in the mitochondrial matrix, converts O2•− into H2O2, which in turn is degraded by mitochondrial peroxidases or diffuses into the cytosol [7]. The mitochondrial generation of O2•−/H2O2 has important deleterious consequences. Indeed, increases in the rate of ROS production or decreases in the catalytic degradation of ROS, resulting from ablation of antioxidative defences, cause structural modifications of DNA, proteins and lipids, which are associated with detrimental effects on cellular functions [3,8]. Mitochondrial ROS generation is thus perceived to be an undesirable phenomenon, which is linked inextricably to oxygen consumption.
The main purpose of the present study was to determine whether or not an increase in the mitochondrial capacity to catabolize O2•−/H2O2 would affect the progression of the aging process and the lifespan of Drosophila melanogaster. Specifically, it was hypothesized that an increase of ROS catabolism in mitochondria would result in an enhanced resistance to exogenous ROS, a postponement of age-related loss of physical fitness and extension of the lifespan. This hypothesis was tested by the simultaneous overexpression of MnSOD and ectopic catalase in the mitochondrial matrix of transgenic Drosophila. Flies containing one or two copies of both an MnSOD transgene and a mitochondrially targeted catalase transgene were generated, and the effects on lifespan, motor ability, mitochondrial respiration and resistance to experimental oxidative stress were determined. Contrary to predictions based on the hypothesis, the enhancement of mitochondrial antioxidative activities was associated with a decrease in lifespan.
MATERIALS AND METHODS
Transgenic flies
The generation and characterization of transgenic fly lines expressing catalase ectopically in the mitochondrial matrix have been described previously [9]. The transgene contains a 7 kb genomic fragment of Drosophila DNA, including the entire catalase coding sequence, with the putative mitochondrial presequence of the D. melanogaster OAT (ornithine aminotransferase) gene inserted upstream of the coding region. The generation and characterization of MnSOD transgenic lines, with inserts containing the genomic MnSOD gene of Drosophila, together with an approx. 7 kb upstream and 1.3 kb downstream sequence, have also been described previously [10].
For the present study, transgenic flies overexpressing both MnSOD and catalase in the mitochondrial matrix, designated Dm2SOD/OCAT (flies containing one copy of the MnSOD transgene and one copy of the OAT-catalase transgene), were generated by crosses combining autosomal homologues containing the respective transgenes, permitting recombination between them. Progeny with the most darkly pigmented eyes were selected and outcrossed to strains possessing dominantly marked balancer chromosomes. Control lines (Dm2Control) were constructed using a similar approach involving unmodified pCaSpeR vector transgenes. The individual transgenes recombined in each line are indicated in Table 1. In each case, the presence of both transgenes was confirmed by Southern blot analysis using digoxigenin-labelled probes complementary to either catalase or MnSOD gene sequences, or to the white+ marker gene sequence in the case of pCaSpeR vector control lines. Loss of either transgene in subsequent recombination events was prevented by maintaining the recombinant homologue over either the chromosome 2 balancer, CyO, or the chromosome 3 balancer, TM3, Sb Ser. In experiments involving Dm2SOD/OCAT and Dm2Control lines, adult male flies, heterozygous with respect to the appropriate transgenes, were generated by outcrossing homozygous or balanced stocks with the parental y w strain.
Table 1. Enzyme activities and lifespans of Dm2SOD/OCAT and Dm2Control flies.
Enzyme activities were determined at 10 days of age in whole body homogenates. Results are expressed as units/mg of protein and are means±S.D.
| Mean lifespan (days) (n)* | ||||
|---|---|---|---|---|
| Drosophila line | MnSOD activity | Catalase activity | Experiment 1 | Experiment 2 |
| Dm2Control lines | ||||
| c23, c6 | 123±8 | 300±15 | 66.6 (99) | 62.3 (98) |
| c8, c9 | 104±13 | 302±19 | 73.3 (93) | 72.9 (99) |
| c3, c10 | 126±5 | 264±2 | 75.2 (95) | 73.1 (102) |
| c2, c5 | 118±4 | 268±32 | 76.0 (101) | 76.9 (99) |
| c22, c24 | 75±6 | 287±39 | 78.5 (100) | 76.4 (101) |
| c24, c23 | 121±8 | 272±1 | 80.5 (99) | 77.1 (96) |
| c21, c7 | 125±11 | 313±12 | 83.2 (100) | 78.0 (98) |
| c5, c8 | 96±7 | 253±18 | 83.4 (98) | 76.0 (100) |
| c7, c24 | 92±1 | 286±10 | 83.7 (100) | 79.7 (102) |
| Mean±S.D. | 109±18 | 283±20 | 77.8±5.7 (885) | 74.7±5.1 (895) |
| Dm2SOD/OCAT lines | ||||
| MnSOD1, OAT-Cat1 | 168±27 | 377±12 | 62.4 (97) | 62.8 (100) |
| MnSOD2, OAT-Cat2 | 168±3 | 422±8 | 64.7 (99) | 64.2 (99) |
| MnSOD3, OAT-Cat1 | 213 | 458±7 | 66.4 (97) | 67.1 (100) |
| MnSOD4, OAT-Cat3 | 161±18 | 287±7 | 66.6 (97) | 64.4 (98) |
| MnSOD5, OAT-Cat4 | 190±20 | 387±8 | 66.8 (97) | 69.2 (99) |
| MnSOD1, OAT-Cat3 | 160±10 | 338±8 | 68.1 (93) | 66.3 (100) |
| MnSOD6, OAT-Cat1 | 168±8 | 435±23 | 68.5 (99) | 67.5 (99) |
| MnSOD7, OAT-Cat5 | 233±39 | 322±28 | 70.1 (97) | 64.7 (97) |
| MnSOD8, OAT-Cat6 | 217±16 | 420±19 | 71.5 (99) | 71.5 (97) |
| MnSOD7, OAT-Cat7 | 101±19 | 561±38 | 73.1 (96) | 71.1 (93) |
| MnSOD6, OAT-Cat8 | 180±6 | 341±8 | 75.2 (95) | 71.8 (100) |
| MnSOD9, OAT-Cat5 | 243±86 | 312±14 | 75.7 (96) | 73.2 (102) |
| MnSOD8, OAT-Cat9 | 132±15 | 359±7 | 78.4 (99) | 76.9 (91) |
| MnSOD8, OAT-Cat7 | 66±2 | 505±13 | 78.8 (97) | 77.8 (96) |
| Mean±S.D. | 171±49 | 395±77 | 70.4±5.1 (1358) | 69.2±4.7 (1371) |
* n is the number of flies in each lifespan experiment.
Transgenic flies bearing two copies of the MnSOD transgene and two copies of the OAT-catalase transgene (Dm4SOD/OCAT flies) were obtained by direct crosses between male and virgin female flies from selected Dm2SOD/OCAT lines. For lifespan studies, a total of six Dm4SOD/OCAT transgene combinations and six controls (Dm4Control) were generated. The genotypes of these flies are indicated in Table 2. The chromosome 2/chromosome 2 control and experimental groups were used for the measurements of H2O2 release, negative geotaxis, mitochondrial respiration, glutathione and methionine contents and resistance to experimental stress.
Table 2. Transgene combinations, enzyme activities and lifespans of Dm4SOD/OCAT and Dm4Control flies.
Enzyme activities were determined at 10 days of age in whole body homogenates except for mitochondrial catalase activity, which was determined at 2 days of age. Results are expressed as units/mg of protein and are means±S.D.; n.d., not determined.
| Catalase activity | |||||
|---|---|---|---|---|---|
| Chromosomes | Transgene combinations | MnSOD activity | Total | Mitochondrial | Mean lifespan (days) (n)* |
| Control | Dm4Control | ||||
| Chr. 2/Chr. 2 | c23, c6/c7, c24 (C1) | 39±2 | 283±3 | 7±23 | 77.2 (100) |
| c22, c24/c6, c21 (C2) | 56±2 | 219±11 | 11±9 | 83.8 (100) | |
| c21, c7/c24, c23 (C3) | 45±1 | 257±7 | 2±9 | 86.2 (100) | |
| Chr. 2; Chr. 3 | c23, c6; c3, c10 (C4) | 48±3 | 259±6 | n.d. | 70.7 (98) |
| c22, c24; c2, c5 (C5) | 47±4 | 178±4 | n.d. | 90.6 (99) | |
| c21, c7; c8, c9 (C6) | 54±11 | 226±16 | n.d. | 89.3 (100) | |
| Mean±S.D. | 48±6 | 237±37 | 83±8 (597) | ||
| Experimental | Dm4SOD/OCAT | ||||
| Chr. 2/Chr. 2 | M8, O6/M2, O2 (E1) | 51±3 | 659±28 | 243±19 | 50.9 (99) |
| M9, O5/M7, O7 (E2) | 95±6 | 517±25 | 194±23 | 47.3 (99) | |
| M5, O4/M7, O5 (E3) | 98±4 | 449±20 | 214±5 | 59.8 (100) | |
| Chr. 2; Chr. 3 | M8, O6; M3, O1 (E4) | 100±7 | 588±27 | n.d. | 65.6 (99) |
| M9, O5; M6, O1 (E5) | 78±1 | 568±7 | n.d. | 69.3 (100) | |
| M5, O4; M1, O3 (E6) | 89±6 | 428±37 | n.d. | 66.3 (99) | |
| Mean±S.D. | 85±19 | 538±88 | 60±9 (596) | ||
* n is the number of flies in the lifespan experiment.
For all experiments, adult male flies were isolated using mild carbon dioxide anaesthesia and housed under constant light, 25 per vial, at 25 °C. The flies were transferred into fresh vials containing standard media every second day, and every single day beyond 25–35 days of age. Flies used in biochemical assays and physiology experiments were provided with fresh vials approx. 2 h before beginning the experiments.
Biochemistry
Whole body homogenates of flies and mitochondrial fractions were obtained as described in earlier reports [9,11]. Catalase activity was measured spectrophotometrically at 240 nm by recording the decomposition of H2O2, as described previously [12,13]. One unit of catalase activity was defined as 1 μmol of H2O2 decomposed per min. This activity was fully (100%) inhibitable by preincubation of fly homogenates or mitochondria with 0.33 M 3-amino-1,2,4-triazole for 5 min at 30 °C.
MnSOD activity was measured spectrophotometrically at 560 nm, by recording the cyanide-inhibitable reduction of Nitro Blue Tetrazolium [14,15]. One unit of SOD activity was defined as the amount that inhibited Nitro Blue Tetrazolium reduction half-maximally in a 1 ml reaction volume.
The amount of H2O2 released from purified flight muscle mitochondria of 2-day-old flies was quantified by a modification of the fluorimetric procedure of Hyslop and Sklar [16], based on the oxidation of p-hydroxyphenylacetate to a stable fluorescent compound (λex=320 nm and λem=400 nm), as described previously [9].
The amounts of GSH, GSSG and methionine were measured by HPLC coupled with coulometric electrochemical detection, as described by Rebrin et al. [17].
Aconitase activity was measured at 30 °C by a coupled assay in which the formation of β-NADPH was followed spectrophotometrically at 340 nm, using citrate as a substrate, essentially as described in [18].
Physiology
Mitochondrial respiration was measured polarographically at 28 °C, using a Clark-type oxygen electrode connected to a computer-operated Oxygraph control unit (Hansatech Instruments, Norfolk, U.K.). Fly mitochondrial protein (30–60 μg) obtained from isolated thoraces was added to the respiration buffer (120 mM KCl, 5 mM potassium phosphate, 3 mM Hepes, 1 mM EGTA, 1 mM MgCl2 and 0.2% BSA, pH 7.2) in a 1.0 ml reaction chamber, followed by the addition of NAD+-linked substrate (10 mM pyruvate+10 mM proline) and subsequently 100 μM ADP. After recovery of state 4 respiration (ADP-depleted), a second addition of ADP (400 μM) was made and was used to calculate the rate of state 3 respiration. All measurements were performed in quadruplicate, within 1 h after the isolation of mitochondria.
In negative geotaxis experiments, the speed of walking was measured after placing individual flies in disposable plastic pipettes at 25 °C, as described previously [19].
Resistance to exogenous H2O2 and hyperoxia
Exposure to dietary H2O2 or 100% oxygen was initiated 15 days post-eclosion. Exposure to H2O2 was performed in standard polystyrene vials in which the regular food medium was replaced by a strip of filter paper saturated with a 1% sucrose solution containing 0.25, 0.5 or 1.0% H2O2. Flies exposed to the sucrose solution alone served as controls. For the hyperoxia experiment, a steady flow of 100% oxygen was bubbled through water and passed through a sealed Plexiglass® chamber, containing flies in standard polystyrene vials, under a low positive pressure.
Statistical analysis
The significance of differences in enzymatic activities or H2O2 release from mitochondria was assessed by unpaired, two-sided Student's t tests, using SYSTAT 10 software (Systat Software, Richmond, CA, U.S.A.). The mean lifespans of entire cohorts of flies from each line or transgene combination were also compared using unpaired, two-sided t tests. The use of group means in the analysis of survivorship data was deemed necessary because treating individual flies with identical genotypes as independent samples may overestimate the extent of replication in the experiment [20]. This possibility arises to the extent that the effects on survivorship are influenced by the sites of insertion of the transgenes in the genome.
The significance of differences in mean survival times during exposure to exogenous H2O2 was assessed by two-way ANOVA. The factors in the analysis were group (experimental versus control) and H2O2 dosage. Similarly, differences in resistance to 100% oxygen exposure were compared with fly group and replicate experiment as factors. For the analysis of glutathione and methionine content and differences in walking speed, a nested ANOVA was performed with age and group as factors, and individual transgene combinations nested within group type. Nesting was used because only three transgene combinations were examined per group, with multiple replicate measurements per combination. Mitochondrial respiration rates were compared without nesting, because only a single transgene combination from each group was examined.
RESULTS
Simultaneous overexpression of MnSOD and ectopic expression of catalase in mitochondria
Mitochondrial overexpression of MnSOD with simultaneous expression of ectopic, mitochondrially targeted catalase was achieved in D. melanogaster by recombination of autosomal homologues containing MnSOD and OAT-catalase transgenes, as described in the Materials and methods section. A total of 27 Dm2SOD/OCAT lines were generated, containing a single MnSOD transgene and a single OAT-catalase transgene, each at one of ten unique insertion sites. Similarly, 24 Dm2Control lines were obtained, containing two pCaSpeR vector insertions, each at one of 13 unique insertion sites. For subsequent lifespan studies and measurement of resistance to experimentally elevated ROS concentrations, nine Dm2Control lines and 14 Dm2SOD/OCAT lines were selected on the basis of maximum overexpression of one or both enzymes at the whole body level and minimum transgene redundancy. As compared with Dm2Control lines, the MnSOD and catalase activities of these Dm2SOD/OCAT lines were increased by an average of 57 and 40% respectively (Table 1).
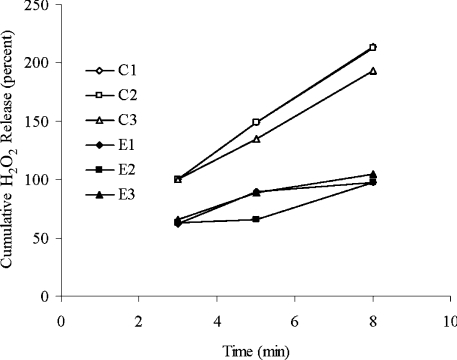
Further overexpression of MnSOD and mitochondrial catalase was achieved by generating transgenic flies with two MnSOD and two OAT-catalase inserts in the genome. A total of six Dm4SOD/OCAT combinations and six Dm4Control combinations were generated by crosses among Dm2SOD/OCAT and Dm2Control lines (Table 2). Enzyme activities of MnSOD and catalase in whole body homogenates of Dm4SOD/OCAT flies were 77 and 126% higher, on average, than the respective activities in Dm4Control flies (P<0.001, Table 2). Mitochondrial catalase activity ranged from 194 to 243 units/mg of protein in Dm4SOD/OCAT flies, but no activity was detected in Dm4Control mitochondria (Table 2). This manipulation of enzymatic antioxidant activities was associated with a 66% slower rate of release of H2O2 from the mitochondria of Dm4SOD/OCAT versus Dm4Control flies (P<0.0005, Figure 1).
Figure 1. Cumulative H2O2 release.
Results are presented as percentage of Dm4Control H2O2 accumulation at 3 min for thoracic mitochondria obtained from 2-day-old Dm4Control flies (C1–C3, open symbols) and Dm4SOD/OCAT flies (E1–E3, closed symbols). P<0.0005 for cumulative H2O2 release from 3 to 8 min.
Curtailment of lifespan
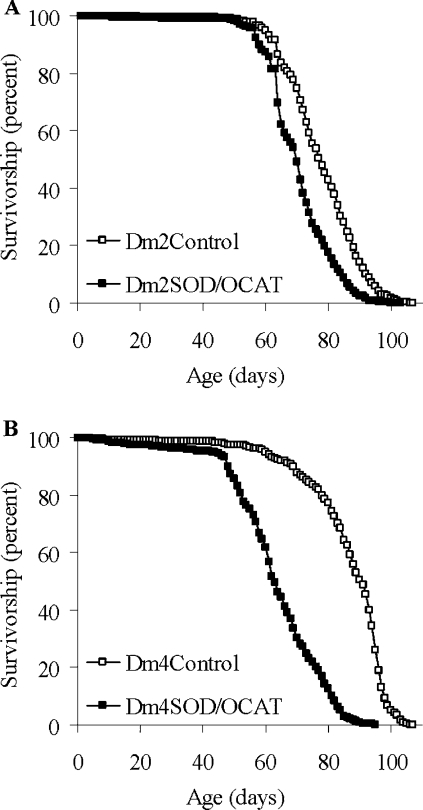
To determine whether or not the enhanced capacity for catalytic removal of mitochondrial ROS influenced longevity under standard, unstressed conditions, lifespan studies were performed at 25 °C for both the Dm2SOD/OCAT and the Dm4SOD/OCAT flies. In two independent experiments, using 14 Dm2SOD/OCAT lines, the average lifespan was not increased as predicted, but was decreased by 9.5% (P=0.004) and 7.4% (P=0.007) in comparison with nine Dm2Control lines (Table 1 and Figure 2A). The lifespans of the flies with two MnSOD and two OAT-catalase inserts in the genome were shortened even more dramatically. In two independent experiments, the lifespans of Dm4SOD/OCAT flies were decreased by 27–28% on average (to 60–63 days, versus 83–86 days for Dm4Control flies, P<0.002), and by as much as 43% for combination E2 (Table 2 and Figure 2B). Similarly, at 30 °C, at which temperature the metabolic rate of Drosophila is increased, the average lifespan of Dm4SOD/OCAT flies was 20–22% shorter than the lifespan of Dm4Control flies (24–25 days versus 31 days, P<0.03).
Figure 2. Lifespans at 25 °C.
(A) Pooled survivorship curves of nine Dm2Control and 14 Dm2SOD/OCAT transgenic lines. Approximately 100 flies were used for each line. Results are shown for experiment 1 (P=0.004) and are representative of two independent experiments. Mean lifespans of the individual lines are presented in Table 1. (B) Pooled survivorship curves of six Dm4Control and six Dm4SOD/OCAT combinations. Approximately 100 flies were used for each combination. Results are shown for experiment 1 (P=0.0007) and are representative of two independent experiments. Mean lifespans of the individual combinations are indicated in Table 2.
Decreased motor performance
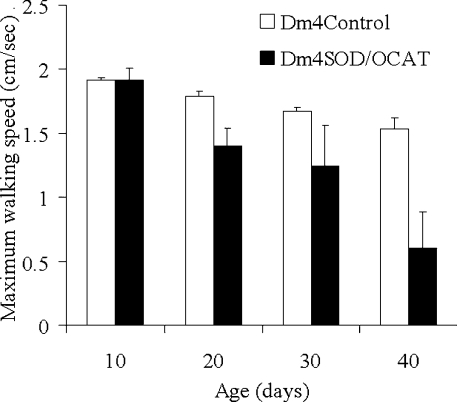
The speed of walking, a marker of physical fitness, was measured in Dm4SOD/OCAT flies. The speed of walking decreased significantly as a function of age (P<0.0005, Figure 3) and the Dm4SOD/OCAT flies had a slower walking speed than the Dm4Control flies (P<0.0005). At 40 days of age, the speed of walking of the Dm4SOD/OCAT flies, averaged among three groups with different transgene combinations, was 60% lower than that of the Dm4Control flies.
Figure 3. Maximum walking speed.
Individual flies were confined in 5 ml graduated pipettes and gently tapped to the base, and the maximum height reached by each fly after 10 s was recorded. The maximum walking speed (cm/s) was determined on the basis of the greatest height attained in three trials, using 15 different flies for each of three Dm4Control groups (C1–C3) and three Dm4SOD/OCAT groups (E1–E3), at each age. Results are pooled data and are expressed as means±S.E.M. for the three groups of Dm4Control flies (white bars) and Dm4SOD/OCAT flies (black bars). The Dm4SOD/OCAT flies were significantly slower than the control flies (P<0.0005) and the walking speed decreased with advancing age (P<0.0005).
Age-related decrease in mitochondrial respiration
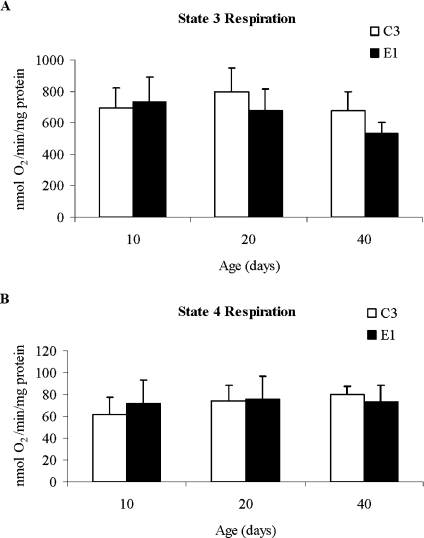
The mitochondrial respiration rates of Dm4SOD/OCAT and Dm4Control flies were measured at different ages, in order to test the hypothesis that impaired mitochondrial respiratory function could be responsible for the decrease in lifespan. Among young adult flies 10 days of age, there were no differences in the state 3 (ADP-stimulated) or state 4 (ADP-depleted) respiration rates, RCR (respiratory control ratio; ratio of state 3 rate/state 4 rate) or in the ADP:O ratio (the number of ADP molecules consumed per oxygen atom during state 3 respiration in the presence of 400 μM ADP) (Table 3). Additionally, there was no difference in the activity of aconitase, a tricarboxylic acid cycle enzyme that is particularly susceptible to oxidative inactivation [21]. However, the state 3 respiration rates of Dm4SOD/OCAT flies were, respectively, 15 and 21% lower than the Dm4Control rates at 20 and 40 days of age (Figure 4A). Overall, there were significant differences between fly types (P=0.01) and age groups (P=0.001), and a significant interaction between fly type and age (P=0.02). State 4 respiration did not differ significantly between fly types or among age groups (Figure 4B).
Table 3. Mitochondrial function of Dm4SOD/OCAT and Dm4Control flies.
Mitochondrial respiration rates (states 3 and 4) were determined using pyruvate+proline as substrates and flies 10–15 days of age. Rates are expressed as nmol O2·min−1·mg of protein−1, and are means±S.D. for four measurements. The RCR is the ratio of state 3 rate/state 4 rate, and the ADP:O ratio is the number of ADP molecules per oxygen atom during state 3 respiration. Results from a representative experiment are shown; the experiment was repeated independently three times (pooled results are presented in Figure 4). Aconitase activity is expressed as mM β-NADPH·min−1·mg of protein−1 and values are means±S.D. for three independent experiments, each involving two different preparations, with four measurements per preparation.
| Control (C3) | Experimental (E1) | |
|---|---|---|
| Respiration rate (nmol of O2·min−1·mg of protein−1) | ||
| State 3 | 633.2±13 | 631.5±44 |
| State 4 | 58.8±3 | 71.5±7 |
| RCR | 10.8±1 | 8.9±1 |
| ADP:O ratio | 3.1 | 3.0 |
| Aconitase activity (mM β-NADPH·min−1·mg of protein−1) | 0.50±0.07 | 0.53±0.04 |
Figure 4. Mitochondrial respiration as a function of age.
Mitochondrial respiration rates (states 3 and 4) of Dm4Control C3 and Dm4SOD/OCAT E1 flies were measured using pyruvate+proline as substrates, and expressed as nmol of O2·min−1·mg of protein−1. Results are means±S.D. for three independent experiments at each age. (A) State 3 respiration (in the presence of excess substrate and ADP) was the same in both groups at 10 days of age, but it was lower in experimental versus control flies at 20 and 40 days of age. (B) State 4 respiration (in the presence of excess substrate after exhaustion of ADP) was well maintained at all ages tested.
Effects on GSH/GSSG ratio and content of free methionine
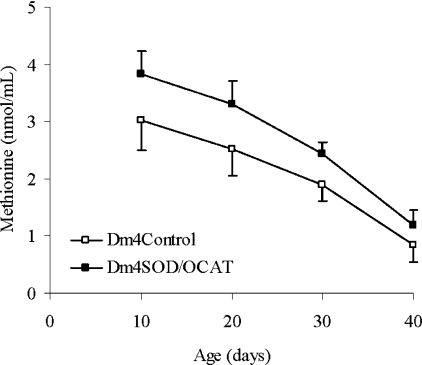
The ratio between GSH and GSSG is considered to be an indicator of the overall redox state [22], which is defined as the ratio of the concentrations of oxidizing and reducing equivalents. Free methionine is a precursor in cysteine biosynthesis, and it may also act as an antioxidant via reversible oxidation to methionine sulphoxide [23]. To determine whether the level of oxidative stress and the redox state of the organism as a whole were affected by the manipulation of mitochondrial O2•−/H2O2 catabolism, GSH, GSSG and methionine were quantified in whole body homogenates of 10- to 40-day-old Dm4SOD/OCAT and Dm4Control flies. The GSH and GSSG contents and the GSH/GSSG ratio were not significantly different between groups (results not shown), but the concentration of free methionine was significantly higher in Dm4SOD/OCAT flies versus control flies (P<0.0005, Figure 5) and decreased as a function of age (P<0.0005). This higher level of free methionine was also observed in enriched mitochondrial fractions obtained from thoracic homogenates (results not shown).
Figure 5. Methionine content.
Results are presented as means±S.D. of the data for three Dm4Control (C1–C3; □) and three Dm4SOD/OCAT combinations (E1–E3, ■) and are expressed as nmol/ml of initial preparation [5% (w/v) homogenates in 5% (w/v) metaphosphoric acid]. Results for each combination, at each age, were obtained from four independent preparations of 50 flies each. The methionine content was significantly higher in Dm4SOD/OCAT flies than in Dm4Control flies (P<0.0005) and decreased as a function of age (P<0.0005).
Increased protection against acute oxidative stress
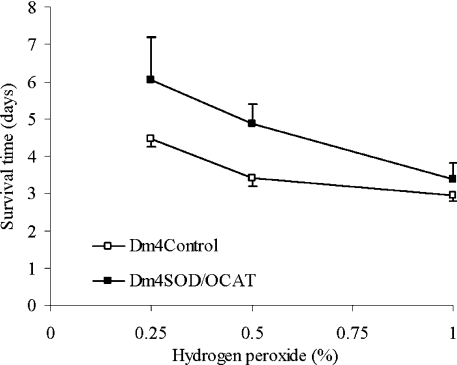
To investigate whether the specific enhancement of mitochondrial catalytic O2•−/H2O2 removal would result in protection against acute oxidative stress, flies were exposed to various concentrations of exogenous H2O2. The survival times of the Dm2SOD/OCAT flies, fed solutions of 0.25, 0.5 and 1.0% exogenous H2O2 in 1% sucrose, were increased by 33, 30 and 22% respectively when compared with Dm2Control flies (P<0.0005). Similarly, Dm4SOD/OCAT flies exposed to 0.25, 0.5 and 1.0% H2O2 exhibited increases in survival times of 35, 43 and 14% respectively when compared with Dm4Control flies (P<0.0005, Figure 6).
Figure 6. Resistance to H2O2 exposure.
H2O2 was administered to control flies (□) and experimental flies (■) in 1% sucrose solutions. Survival times (mean±S.D.) were pooled for three Dm4Control groups (C1–C3) versus three Dm4SOD/OCAT groups (E1–E3) (P<0.001).
The transgenic flies were also exposed to 100% oxygen, which causes an increase in mitochondrial ROS production [24]. In two independent experiments involving 9–10 Dm2Control lines and 13–14 Dm2SOD/OCAT lines, the average survival times of the Dm2SOD/OCAT lines were 12–15% greater than those of the controls (P=0.02, results not shown). However, the Dm4SOD/OCAT flies were not significantly more resistant to hyperoxia than the Dm4Control flies.
DISCUSSION
Results of the present study indicate that an experimental increase in mitochondrial O2•−/H2O2 removal capacity, induced by simultaneous overexpression of MnSOD and ectopic expression of catalase in the mitochondria of transgenic D. melanogaster, caused a decrease in the rate of mitochondrial H2O2 release and an enhancement of resistance to acute oxidative stress, induced by exposure to exogenous H2O2. However, the lifespan was significantly shortened, both under standard conditions (25 °C) and at an elevated ambient temperature (30 °C), the walking speed was decreased and the active state of mitochondrial respiration (state 3) was impaired at older ages.
Studies on the effects of overexpression of antioxidative enzymes in transgenic Drosophila have given rise to two seemingly contradictory interpretations. One group of studies has shown that the lifespan is extended, particularly when the overexpression of SOD is restricted to the adult phase of the life cycle [25] or to motor neurons [26]. These results are consistent with the traditional free radical hypothesis of aging [27], which postulates that oxidative damage resulting from ROS production is a major causal factor in the aging process. Indeed, the accrual of such damage is correlated with rates of ROS production and aging in animals [28,29]. However, a second set of studies has shown that overexpression of genes encoding antioxidative enzymes, using native cis-regulatory sequences, does not affect the aging process if long-lived fly strains are used as controls [30,31]. In fact, small decreases in lifespans can be observed in the survivorship data of flies overexpressing single copies of either genomic MnSOD or ectopic, mitochondrially targeted catalase transgenes [10,32], or of some individual long-lived lines in which overexpression of Cu–Zn SOD was induced only during adult life [33]. The seeming discrepancies among these findings may be explained either in terms of differences in the intracellular sites of the overexpressed enzymes or of the lifespans of the control flies employed in the various studies. A negative correlation has indeed been noted previously between the control lifespan and the degree of lifespan extension associated with overexpression of several antioxidant enzymes [31,34]. On this basis, the augmentation of antioxidants may be postulated to compensate for the adverse effects of specific genetic backgrounds and environmental conditions, which may compromise the lifespan of flies for reasons unrelated to the normal aging process.
In the present study, flies in long-lived backgrounds were used to test the hypotheses that (i) the length of life is positively correlated with levels of antioxidative defences and negatively correlated with rates of ROS production, and (ii) the simultaneous overexpression of multiple antioxidants in mitochondria, a major site of ROS production in Drosophila, would have a particularly strong impact on lifespan. To test these hypotheses, flies were generated containing one or two copies of both genomic MnSOD and OAT-catalase transgenes, resulting in significantly enhanced MnSOD and mitochondrial catalase activities. In contrast with the effects of either transgene alone [10,32] or the simultaneous overexpression of cytosolic SOD/catalase [36], there was a consistent and highly significant decrease in longevity. In addition, the decrease in lifespan was much more pronounced in flies containing two copies versus one copy of both the MnSOD and the OAT-catalase transgenes. These findings illustrate the importance of the subcellular site of the overexpressed enzymes and demonstrate (i) that some removal of mitochondrial ROS can be tolerated before longevity is affected and (ii) that the increased capacity for removal of both mitochondrial O2•− and H2O2 is more harmful than for either O2•− or H2O2 alone.
Direct measurement of H2O2 production by mitochondria from Dm4SOD/OCAT flies confirmed that the increase in SOD and catalase activities and decrease in lifespan coincided with a substantial curtailment of H2O2 release. However, under the assay conditions used in the present study, H2O2 release was also virtually abolished in OAT-catalase flies [32]. The dramatic differences in lifespan effects and minor differences in rates of H2O2 release between OAT-catalase and Dm4SOD/OCAT flies could be accounted for by differential effects on O2•− levels, resulting from the additional overexpression of MnSOD. Alternatively, differences in the amount of mitochondrial catalase activity could be primarily responsible for the decrease in lifespan. Indeed, the levels of catalase overexpression in OAT-catalase, Dm2SOD/OCAT and Dm4SOD/OCAT flies averaged 35, 40 and 126% respectively, whereas the average decreases in lifespan, in relation to the respective controls, were 2–6, 7–9 and 27–28%. These results suggest that the levels of catalase activity required to cause a large decrease of lifespan are higher than those needed to abolish H2O2 release by isolated mitochondria. This differential effect could arise, notwithstanding the complete abolition of H2O2 release by OAT-catalase mitochondria under in vitro assay conditions, if the additional mitochondrial catalase in Dm4SOD/OCAT flies quenches H2O2 release that might otherwise occur in vivo.
An alternative possibility is that the mere presence of excessive amounts of the overexpressed proteins in the matrix could cause mitochondrial dysfunction, independent of the levels of enzymatic activity. In theory, this idea could be tested by overexpressing an inert, mutant form of the ectopic catalase in Drosophila mitochondria. In practice, however, a protein of this type could be misfolded, and consequently more susceptible either to proteolysis or aggregation, resulting in an artifact of the type it was intended to exclude. Accordingly, a control experiment of this type was not performed in the present study. However, the plausibility of the hypothesis that the lifespan was decreased as an artifactual effect, caused by the physical presence of the ectopic protein, was decreased by evidence obtained from both structural and functional studies. First, mitochondrial respiratory activity was indistinguishable between Dm4SOD/OCAT and Dm4Control flies after 10 days of adult life. Instead, the impairment of state 3 respiration, specifically at older ages, and the decrease in locomotor activity, suggested that the Dm4SOD/OCAT flies were aging more quickly than the controls. Secondly, there was no obvious difference in the profile of protein expression between Dm2Control and Dm2SOD/OCAT flies (results not shown). Collectively, these findings suggest that the ectopic catalase was not sufficiently abundant to displace or compromise the activities of other mitochondrial proteins, or to prevent their import or assembly into electron-transport complexes, as would be predicted if the mitochondrial protein translocation pores were jammed.
Recent studies indicate that ectopic expression of catalase in mammalian mitochondria has positive effects on lifespan and stress resistance. Specifically, overexpression of MnSOD and mitochondrial targeting of catalase had additive, protective effects against glucose-deprivation-induced cytotoxicity and GSSG accumulation in human PC-3 cells [37]. Very recently, Schriner et al. [38] reported that mice expressing ectopic human catalase at high levels in heart and skeletal muscle mitochondria live significantly longer than controls. These findings illustrate that the presence of ectopic catalase within mitochondria does not have adverse effects on lifespan in all species, suggesting either that any artifactual mitochondrial dysfunction caused by the presence of ectopic catalase is minor (not sufficient to offset the life-extending effect in mice) or that it is species-specific. The reasons for the contrasting effects of mitochondrial catalase in mice and flies are unknown, but they might imply that flies are more sensitive to perturbation of physiological levels of H2O2 production. Such a sensitivity could involve either the cellular signalling or bactericidal activities of H2O2, or some additional, unknown role. In either case, it appears that the rapid rate of mitochondrial O2•−/H2O2 production in Drosophila could account for the rapid accrual of oxidative damage and short lifespan of this species, but the physiological roles of ROS interfere with tests of this hypothesis involving a direct decrease in O2•−/H2O2 production.
The decreased lifespans of flies overexpressing MnSOD and mitochondrial catalase suggest that a basal level of mitochondrial ROS production is essential for the attainment of a normal length lifespan. In support of this view, the level of H2O2 affects the expression of at least 80 different genes or proteins, including numerous components of the mitogen-activated protein kinase and nuclear factor κB signalling pathways [39]. ROS may also modulate other signal-transduction pathways [40]. Accordingly, a spatially and quantitatively controlled generation of H2O2 is believed to have beneficial, physiological roles. In contrast, ROS also have adverse effects, including lipid, protein and DNA oxidative damage initiated by •OH, the product of metal-catalysed scission of H2O2 [41,42].
This apparent duality of effects of O2•−/H2O2 provides a plausible explanation for the decrease in lifespan coinciding with increased resistance to experimental oxidative stress in flies overexpressing MnSOD and OAT-catalase. Supraphysiological concentrations of O2•−/H2O2, induced by H2O2 feeding or exposure to normobaric hyperoxia, likely accelerate oxidative damage and possibly interfere with signal transduction mediated by O2•− and H2O2. Overexpression of MnSOD and OAT-catalase would be expected to attenuate these adverse effects. However, when the overexpressed enzymes decrease O2•−/H2O2 below threshold levels, the resulting interference with cellular signalling presumably outweighs any benefit resulting from the slower accrual of oxidative damage. Other reports from this laboratory have shown that either thioredoxin reductase gene overexpression [19] or genetic selection for delayed reproduction [43] also had opposite effects on lifespan and resistance to hyperoxia. These findings are incompatible with the idea that there is a simple relationship between the rate of aging and resistance to experimental oxidative stress. Thus notwithstanding the partial overlap of patterns of gene expression induced by aging and hyperoxia [44], increased resistance to experimental oxidative stress is not a reliable predictor of extended lifespan under normal conditions, and it could not account for the differences in lifespan observed in this study.
Perturbation of the glutathione or thioredoxin redox state and redox-sensitive signalling pathways is one plausible mechanism by which alteration of the O2•−/H2O2 steady state could shorten the lifespan of flies. However, the differences in GSH and GSSG concentrations between MnSOD/OAT-catalase transgenic flies and controls in the present study were not significant. Thus among the various possible factors which could explain the decreased lifespan of the flies, those dependent on changes in the total GSH and GSSG content may be excluded. In contrast, the increased amounts of free methionine at all ages in Dm4SOD/OCAT flies are indicative of a biochemical impact of increased mitochondrial O2•−/H2O2 catabolism at the organismal level. H2O2 has been shown to oxidize methionine to methionine sulphoxide, and this reversible oxidation is believed to regulate protein function [45] and to provide an antioxidative defence mechanism [23]. Similarly, the observed decrease in free methionine content as a function of age is consistent with increased H2O2 production in older flies [2]. A possible role for methionine metabolism in the aging process is supported by a direct relationship between methionine sulphoxide reductase activity and lifespan in flies [47] as well as mice [48], and by the observation that Fischer 344 rats live longer when fed a methionine-restricted diet [49]. However, the shorter life expectancy of flies with either increased or decreased methionine content (Dm4SOD/OCAT transgenics and aged flies respectively) argues against a direct causal relationship between free methionine content and life expectancy. Instead, the results suggest that methionine content may serve as a useful biomarker of the level of oxidative stress in Drosophila.
In conclusion, the results of the present study demonstrate that enhancement of the capacity for mitochondrial O2•−/H2O2 catabolism leads to progressive decreases in the longevity of flies. Although the underlying mechanisms remain to be determined, the present findings do establish that the rate of mitochondrial H2O2 release is not simply and negatively related to the lifespan. Although this finding is seemingly at odds with the oxidative stress hypothesis of aging and with recent results obtained using transgenic mice [38], it remains possible that rates of ROS release are directly proportional to the rate of accumulation of oxidatively damaged macromolecules, which in turn govern the rate of aging under normal conditions. However, decreasing O2•−/H2O2 levels artificially could shorten the lifespan of flies by interfering with various signal-transduction pathways, thereby masking any beneficial effect on the underlying rate of aging. Thus mitochondrial ROS generation, at normal physiological levels, may be necessary for the attainment of a full-length lifespan in Drosophila.
Acknowledgments
Dr I. Rebrin (University of Southern California) assisted with the quantification of aminothiols and M. Orona (University of Southern California) assisted with fly husbandry. This work was supported by grant RO1 AG7657 from the National Institute on Aging – National Institutes of Health.
References
- 1.Ku H.-H., Brunk U. T., Sohal R. S. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radical Biol. Med. 1993;15:621–627. doi: 10.1016/0891-5849(93)90165-q. [DOI] [PubMed] [Google Scholar]
- 2.Sohal R. S., Sohal B. H., Orr W. C. Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative damage and longevity in different species of flies. Free Radical Biol. Med. 1995;19:499–504. doi: 10.1016/0891-5849(95)00037-x. [DOI] [PubMed] [Google Scholar]
- 3.Beckman K. B., Ames B. N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 4.Sohal R. S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 6.Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell. 2004;3:13–16. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 7.Fridovich I. Superoxide dismutases. Annu. Rev. Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong L. K., Mockett R. J., Bayne A.-C. V., Orr W. C., Sohal R. S. Decreased mitochondrial hydrogen peroxide release in transgenic Drosophila melanogaster expressing intramitochondrial catalase. Arch. Biochem. Biophys. 2000;383:303–308. doi: 10.1006/abbi.2000.2093. [DOI] [PubMed] [Google Scholar]
- 10.Mockett R. J., Orr W. C., Rahmandar J. J., Benes J. J., Radyuk S. N., Klichko V. I., Sohal R. S. Overexpression of Mn-containing superoxide dismutase in transgenic Drosophila melanogaster. Arch. Biochem. Biophys. 1999;371:260–269. doi: 10.1006/abbi.1999.1460. [DOI] [PubMed] [Google Scholar]
- 11.Orr W. C., Sohal R. S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 12.Lück H. Catalase. In: Bergmeyer H.-U., editor. Methods of Enzymatic Analysis. New York: Academic Press; 1965. pp. 885–894. [Google Scholar]
- 13.Orr W. C., Arnold L. A., Sohal R. S. Relationship between catalase activity, life span and some parameters associated with antioxidant defenses in Drosophila melanogaster. Mech. Ageing Dev. 1992;63:287–296. doi: 10.1016/0047-6374(92)90006-y. [DOI] [PubMed] [Google Scholar]
- 14.Spitz D. R., Oberley L. W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 15.Mockett R. J., Bayne A.-C. V., Sohal B. H., Sohal R. S. Biochemical assay of superoxide dismutase activity in Drosophila. Methods Enzymol. 2002;349:287–292. doi: 10.1016/s0076-6879(02)49343-3. [DOI] [PubMed] [Google Scholar]
- 16.Hyslop P. A., Sklar L. A. A quantitative fluorimetric assay for the determination of oxidant production by polymorphonuclear leukocytes: its use in the simultaneous fluorimetric assay of cellular activation processes. Anal. Biochem. 1984;141:280–286. doi: 10.1016/0003-2697(84)90457-3. [DOI] [PubMed] [Google Scholar]
- 17.Rebrin I., Bayne A. C., Mockett R. J., Orr W. C., Sohal R. S. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem. J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das N., Levine R. L., Orr W. C., Sohal R. S. Selectivity of protein oxidative damage during aging in Drosophila melanogaster. Biochem. J. 2001;360:209–216. doi: 10.1042/0264-6021:3600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mockett R. J., Sohal R. S., Orr W. C. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. FASEB J. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- 20.Tatar M. Transgenes in the analysis of life span and fitness. Am. Nat. 1999;154:S67–S81. doi: 10.1086/303284. [DOI] [PubMed] [Google Scholar]
- 21.Yan L.-J., Levine R. L., Sohal R. S. Oxidative damage during aging targets mitochondrial aconitase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schafer F. Q., Buettner G. R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 23.Levine R. L., Mosoni L., Berlett B. S., Stadtman E. R. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. U.S.A. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turrens J. F., Freeman B. A., Crapo J. D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch. Biochem. Biophys. 1982;217:411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- 25.Sun J., Folk D., Bradley J., Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkes T. L., Elia A. J., Dickinson D., Hilliker A. J., Phillips J. P., Boulianne G. L. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat. Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 27.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 28.Sohal R. S., Mockett R. J., Orr W. C. Current issues concerning the role of oxidative stress in aging: a perspective. In: Hekimi S., editor. The Molecular Genetics of Aging. Berlin: Springer; 2000. pp. 45–66. [DOI] [PubMed] [Google Scholar]
- 29.Barja G. Rate of generation of oxidative stress-related damage and animal longevity. Free Radical Biol. Med. 2002;33:1167–1172. doi: 10.1016/s0891-5849(02)00910-3. [DOI] [PubMed] [Google Scholar]
- 30.Seto N. O., Hayashi S., Tener G. M. Overexpression of Cu-Zn superoxide dismutase in Drosophila does not affect life-span. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4270–4274. doi: 10.1073/pnas.87.11.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orr W. C., Sohal R. S. Does overexpression of Cu,Zn-SOD extend life span in Drosophila melanogaster? Exp. Gerontol. 2003;38:227–230. doi: 10.1016/s0531-5565(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 32.Mockett R. J., Bayne A. C., Kwong L. K., Orr W. C., Sohal R. S. Ectopic expression of catalase in Drosophila mitochondria increases stress resistance but not longevity. Free Radical Biol. Med. 2003;34:207–217. doi: 10.1016/s0891-5849(02)01190-5. [DOI] [PubMed] [Google Scholar]
- 33.Sun J., Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol. Cell. Biol. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohal R. S., Mockett R. J., Orr W. C. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radical Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 35. Reference deleted.
- 36.Orr W. C., Mockett R. J., Benes J. J., Sohal R. S. Effects of overexpression of copper-zinc and manganese superoxide dismutases, catalase, and thioredoxin reductase genes on longevity in Drosophila melanogaster. J. Biol. Chem. 2003;278:26418–26422. doi: 10.1074/jbc.M303095200. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad I. M., Aykin-Burns N., Sim J. E., Walsh S. A., Higashikubo R., Buettner G. R., Venkataraman S., Mackey M. A., Flanagan S. W., Oberley L. W., et al. Mitochondrial O2•− and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J. Biol. Chem. 2005;280:4254–4263. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 38.Schriner S. E., Linford N. J., Martin G. M., Treuting P., Ogburn C. E., Emond M., Coskun P. E., Ladiges W., Wolf N., Van Remmen H., et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 39.Allen R. G., Tresini M. Oxidative stress and gene regulation. Free Radical Biol. Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 40.Finkel T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 41.Stadtman E. R. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 42.Halliwell B., Gutteridge J. M. 3rd edn. New York: Oxford University Press; 1999. Free Radicals in Biology and Medicine. [Google Scholar]
- 43.Mockett R. J., Orr W. C., Rahmandar J. J., Sohal B. H., Sohal R. S. Antioxidant status and stress resistance in long- and short-lived lines of Drosophila melanogaster. Exp. Gerontol. 2001;36:441–463. doi: 10.1016/s0531-5565(00)00258-8. [DOI] [PubMed] [Google Scholar]
- 44.Landis G. N., Abdueva D., Skvortsov D., Yang J., Rabin B. E., Carrick J., Tavare S., Tower J. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7663–7668. doi: 10.1073/pnas.0307605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoshi T., Heinemann S. T. Regulation of cell function by methionine oxidation and reduction. J. Physiol. (Cambridge, U.K.) 2001;531:1–11. doi: 10.1111/j.1469-7793.2001.0001j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reference deleted.
- 47.Ruan H., Tang X. D., Chen M.-L., Joiner M.-L. A., Sun G., Brot N., Weissbach H., Heinemann S. H., Iverson L., Wu C.-F., et al. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moskovitz J., Bar-Noy S., Williams W. M., Requena J., Berlett B. S., Stadtman E. R. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmerman J. A., Malloy V., Krajcik R., Orentreich N. Nutritional control of aging. Exp. Gerontol. 2003;38:47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]