Abstract
Two of the major proteins secreted by the prostate epithelium secretory cells are PSA (prostate-specific antigen) and PSAP (prostatic-specific acid phosphatase). The molecules involved in the secretory machinery of PSA and PSAP, and the regulation of this machinery, remain unknown. In the present paper, we provide evidence that JFC1 [synaptotagmin-like protein (slp1)], a Rab27a- and PtdIns(3,4,5)P3-binding protein, regulates the androgen-dependent secretion of PSAP and PSA in human LNCaP prostate carcinoma cells. Androgen-dependent PSAP secretion was significantly inhibited in cells that expressed the C2A domain of JFC1 [PtdIns(3,4,5)P3-binding-domain], but was unaffected by JFC1 overexpression. Conversely, PSA secretion was not inhibited by the C2A domain of JFC1. We show, using immunofluorescence analysis, that JFC1 co-localizes with PSAP, but rarely with PSA, in prostate granules, suggesting that JFC1 is part of the PSAP secretory machinery. However, PSA secretion was significantly increased in LNCaP cells that overexpressed JFC1, indicating that the secretion of PSA is susceptible to variations in the intracellular concentration of JFC1. Both PSAP and PSA secretion was increased by overexpression of wild-type Rab27a or the constitutively active Rab27aQ78L. The secretion of PSA was partially inhibited in the presence of LY294002, while the secretion of PSAP was completely abolished by the PI3K (phosphoinositide 3-kinase) inhibitor. This supports the view that PI3K plays a differential role in the secretion of prostate secretory markers. In conclusion, we present evidence that JFC1 differentially regulates the secretion of PSAP and PSA, and that Rab27a and PI3K play a central role in the exocytosis of prostate-specific markers.
Keywords: exocytosis, phosphoinositide 3-kinase (PI3K), prostate-specific antigen (PSA), prostatic-specific acid phosphatase (PSAP), synaptotagmin-like protein, vesicular trafficking
Abbreviations: DsRED, red fluorescent protein from Discosoma sp.; EEA1, early endosome antigen 1; EGFP, enhanced green fluorescent protein; NF-κB, nuclear factor κB; PI3K, phosphoinositide 3-kinase; PIP3, PtdIns(3,4,5)P3; PSA, prostate-specific antigen; PSAP, prostate-specific acid phosphatase; slp, synaptotagmin-like protein; Syt, synaptotagmin; t-SNARE, target-associated soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor; VAMP-2, vesicle-associated membrane protein-2
INTRODUCTION
The main cell types distinguished within the normal prostatic epithelium are the secretory luminal cells, the basal cells and the less abundant neuroendocrine cells [1]. The major cell type is the secretory luminal cell, characterized by the production of prostate secretory proteins, and the expression of the androgen receptor that confers the attribute of androgen-dependent cells. Two of the major proteins produced and secreted by the prostate epithelium secretory cells are PSA (prostate-specific antigen) and PSAP (prostatic-specific acid phosphatase). Both are thought to play a central role in human prostate physiology and pathology. PSA, a human kallikrein with serine protease activity [2], has been shown to contribute to seminal clot liquefaction after ejaculation through hydrolysis of the high-molecular-mass seminal vesicle protein [3], which is essential for sperm motility. PSAP, a major component of prostatic fluid of unclear physiological function, has also been shown to cleave synthetic peptidyl substrates derived from the sequence of human semenogelins [4]. The prostate secretory cells are considered to be the cells of origin of most human prostate adenocarcinomas ([5], but see [5a]). Moreover, although the mechanism of secretion by prostate secretory cells remains unclear, several studies have pointed out a connection between the secretory pathways of prostate epithelium and prostate cancer. For example, up-regulation of Rab25 has been related to prostate tumorigenicity [6]; a recurrent missense mutant of DOCK4, a GTPase activator, has been found in prostate carcinoma cell lines [7]; PRC17, an oncogene encoding a Rab GTPase-activating protein is amplified in prostate cancer [8]; furthermore, impaired trafficking has been found to be responsible for a communication deficiency in prostate carcinoma cell lines [9]. Finally, there is an obvious connection between the secretory products of prostate cells and cancer. First, PSA, which is mainly expressed in the prostate, is often elevated in prostate cancer and is broadly used as a blood-borne diagnostic marker of the disease. Moreover, once secreted, PSA degrades the extracellular proteins fibronectin and laminin [10], and this property has been associated with increased invasion by prostate cancer cells. The expression and secretion of PSA are regulated by androgens in normal prostate secretory epithelial cells, and by ErbB-2, MAPK (mitogen-activated protein kinase) [11] and Akt [12] signalling in cells that have become independent of androgen. However, since the secretory machinery that operates during PSA secretion remains elusive, the involvement of these pathways in the regulation of the PSA secretory machinery has not been established. Secondly, human PSAP produced and secreted specifically by prostate epithelial cells has been largely used as a marker of androgen action in prostate epithelium, and as an indication of hormonal therapy effectiveness [13,14]. PSAP is a tyrosine phosphatase [15], and its activity has been linked to dephosphorylation of ErbB2 and the subsequent down-regulation of prostate cancer cell growth [16]. On the other hand, the pathological consequences of elevated PSAP secretion in prostate cancer remain unclear. As for PSA, although androgen-dependent regulatory mechanisms have been described in the signalling that is involved in the secretion of PSAP [17], the secretory machinery implicated in the secretion of this phosphatase remains obscure. The prostate carcinoma cell line LNCaP, widely used as a model for androgen-dependent [18] or androgen-independent (cells become independent of androgens after long passage [11]) prostate cancer, expresses and secretes both PSA [11,19] and PSAP [17].
Previously, we identified and isolated JFC1, a member of the slp [Syt (synaptotagmin)-like protein] family of Rab effectors that are characterized by the presence of a Rab-binding motif in its N-terminus and tandem C2 domains in its C-terminus [20]. We found significant expression of JFC1 in lymphoid and non-lymphoid tissues that have a secretory function, with the highest level of expression observed in the prostate [20]. The analysis of the cellular distribution of JFC1 showed that it is partially and peripherally associated with the cellular membranes [20]. The presence of tandem C2 domains in the JFC1 C-terminus is a characteristic that is generally found in proteins associated with vesicular trafficking [21]. Moreover, the C2A domain of JFC1 is highly similar to the C2B domain present in Syt I, and possesses a close match with the polyphosphate-inositol-binding site K(K/R)KTXXK(K/R) that is found in several members of the Syt family [20]. Syts are a family of transmembrane proteins that contain tandem C2 domains, and are involved in the regulated exocytosis that mediates neurotransmitter release [22]. This function is dependent on their ability to bind to polyphosphorylated inositol lipids (phosphoinositides) [22], in particular phosphoinositides that are phosphorylated in position 3′ of the inositol ring, products of the lipid kinases known as PI3Ks (phosphoinositide 3-kinases) [23]. In previous studies, we demonstrated that JFC1 binds to phosphoinositides in vitro and in vivo, showing preference for the PI3K product PIP3 [PtdIns(3,4,5)P3] [20,24]. Moreover, we established that the C2A domain of JFC1 is responsible for the binding of JFC1 to the plasma membrane via 3′-phosphoinositides in living cells [24]. Owing to similarities between JFC1 and Syt I, it has been proposed that the former could play a similar role in the regulation of exocytosis in non-neural tissues [20,24].
Importantly, in a previous study, JFC1 was found to bind specifically to Rab27a [25], a small GTPase that is involved in vesicle-regulated secretion in several tissues [26]. Immunoprecipitation experiments confirmed that JFC1 binds preferentially to the GTP-bound form of Rab27a and its isoform Rab27b (72% identity at the protein level) [27], suggesting that it may be a Rab27 effector. Moreover, the Rab-binding domain of JFC1 co-localized with Rab27a when overexpressed in melanocytes [25], disturbing the association of Rab27a with endogenous effectors. Based on that study, it was suggested that the interaction between JFC1 and Rab27a is physiologically relevant [25]. However, the role that JFC1 plays in Rab27a-dependent regulated secretion has remained obscure for some time. In the present paper, we give evidence that JFC1 differentially regulates the androgen-dependent secretion of PSAP and PSA in human LNCaP prostate carcinoma cells, we show that Rab27a plays an important role in the secretory pathway of LNCaP cells and we describe a differential involvement of the PI3K pathway in the secretion of PSAP and PSA.
EXPERIMENTAL
Cell culture and transfections
The human prostate carcinoma cell line LNCaP-FGC (A.T.C.C.), which is referred to in the present paper as LNCaP, was cultured in RPMI 1640 medium supplemented with 10% of either foetal bovine serum or charcoal-stripped foetal bovine serum (Gemini Bioproducts), 50 units/ml penicillin and 50 μg/ml streptomycin at 37 °C in 5% CO2. LNCaP cells were transfected by nucleoporation using the nucleoporator apparatus (Amaxa, Germany). For these experiments, 5×106 cells were resuspended in 100 μl of Solution V (Amaxa) in the presence of 10 μg of the indicated expression vector. Nucleofection was performed using the electrical setting TO1, and cells were then re-plated and used for analysis 24–48 h post-transfection.
Cloning
The various steps in the cloning of the constructs were performed by standard techniques, and all constructs were verified by sequencing using an automated fluorescent dye-terminator sequencer. The cloning of JFC1 in the expression vectors pCMV-myc (Stratagene) downstream of the sequence encoding the c-myc epitope, and in the expression vector pDsRED1-N1 (Clontech) upstream of the red fluorescent protein from Discosoma sp. (DsRED), was performed by excising the nucleotide sequence encoding JFC1 from the vector pEGFP-JFC1 (described previously [24]) using the restriction enzymes EcoRI and SalI. The fragment was purified and re-ligated into the EcoRI/SalI pre-digested pCMV-myc-3B and pDsRED1-N1 vectors. The cloning of the C2A domain of JFC1 in pCMV-myc was performed exactly as described above for the full-length JFC1, except that the vector pEGFP-C2A, described previously [24], was used as the source of the insert of interest using the restriction enzymes EcoRI and SalI. The wild-type human Rab27a was obtained from UMR cDNA Resource Center (http://www.cdna.org). The constitutively active mutant Rab27aQ78L was generated using the primer 5′-TGGGACACAGCAGGGCTGGAGAGGTTTCGTAGCT-3′ and the QuikChange® Multi Site-Directed Mutagenesis kit (Stratagene).
Immunofluorescence
LNCaP cells were seeded at 70% confluence in an eight-well chambered coverglass, fixed with 3.7% (w/v) paraformaldehyde, washed with PBS (Gibco BRL), permeabilized with 0.01% saponin and blocked with 1.5% BSA in PBS. Appropriate dilutions of the indicated primary antibodies, pre-bleeds or non-specific IgG from the same species as negative controls, were added overnight at 4 °C. The antibody raised against JFC1 (rabbit) has been described previously [20], except that it was purified further using Montage antibody purification kit with PROSEP-A medium (Millipore). The mouse monoclonal antibody against JFC1 was raised using a KLH (keyhole-limpet haemocyanin)-conjugated peptide containing residues 454–466 of JFC1 to immunize 129GIX+ mice. The mouse monoclonal antibody raised against Rab27a was provided by M.C.S. and has been described previously [28]. The polyclonal antibody against Rab27a was raised using recombinant GST (glutathione S-transferase)–Rab27a, the RiBi Adjuvant System (RiBi Immunochem Research, Hamilton, MT, U.S.A.) and Imject® alum (Pierce, Rockford, IL, U.S.A.) to immunize two New Zealand White rabbits at The Scripps Research Institute antibody production core facility. The antibodies raised against PSAP and PSA of mouse and rabbit origin were from NeoMarkers (Union City, CA, U.S.A.) and the goat anti-PSA antibody was from R & D Systems (Minneapolis, MN, U.S.A.). Other antibodies used were: anti-VAMP-2 (vesicle-associated membrane protein-2) and anti-LAMP-2 (lysosome-associated membrane protein-2) (Santa Cruz Biotechnology); anti-EEA1 (early endosome antigen 1) (Transduction Laboratories).
After incubation with the appropriate dilutions of the indicated primary antibodies, the samples were washed three times with PBS for 15 min, and the appropriate combinations of the secondary antibodies (488) and/or (594) Alexa-Fluor-conjugated donkey anti-rabbit, anti-mouse and/or anti-goat (Molecular Probes) were added, and samples were incubated further for 2 h at room temperature (21 °C). Cells were washed twice with PBS, and transferred to and stored in Fluoromount-G (Southern Biotechnology, Birmingham, AL, U.S.A.) until analysed by laser-scanning confocal microscopy on a Bio-Rad MRC1024 attached to a Zeiss Axiovert S100TV microscope. For visualization, fluorescence associated with Alexa-Fluor-594-labelled secondary antibodies was excited using the 568-nm laser line, and collected using a standard Texas Red filter. Fluorescence associated with Alexa-Fluor-488-labelled secondary antibodies was visualized using the 488-nm laser line, and collected using a standard FITC filter set. Images were collected using the Bio-Rad LaserSharp (version 3.2) software.
PSA and PSAP secretion assays
LNCaP cells were cultured as described above, transfected with 10 μg of the indicated expression vectors by nucleofection (Amaxa), and seeded in six-well plates containing RPMI 1640 medium. After 12 h, the medium was replaced with fresh RPMI 1640 medium containing 100 nM 6α-fluorotestosterone (Biomol) or carrier (DMSO). Cells were incubated in the absence or presence of androgen for 24, 48 or 72 h. In some experiments, cells were incubated in the presence of the PI3K inhibitor LY294002 (16 μM) or DMSO for 45 min at 37 °C, before the addition of 6α-fluorotestosterone. The supernatants were collected and kept at −20 °C until analysed. The concentration of PSAP and PSA in the supernatants was determined using ELISA kits specific for the indicated prostatic secretory proteins as described by the manufacturer (Alpha Diagnostics International, San Antonio, TX, U.S.A.). The cells were trypsinized, harvested, counted and lysed in 2% Nonidet P40 in PBS for 30 min on ice. The cell debris was eliminated by centrifugation at 16000 g for 10 min at 4 °C, and the cell lysates were kept at −20 °C for further analysis, including protein determination, protein expression and, in some cases, for the determination of the concentration of unsecreted PSA and PSAP in the lysates. For the analysis of protein expression, 10 μg of the cell lysates was resolved by electrophoresis using 10% NuPAGE gels, and Mops SDS running buffer (Invitrogen), and proteins were then transferred on to nitrocellulose membranes. The antibodies used to determine the level of expression of endogenous and overexpressed JFC1 and Rab27a have been described above. The monoclonal antibody myc-Tag (9B11) raised against the peptide EQKLISEEDL was obtained from Cell Signaling Technology. The secondary antibodies anti-rabbit IgG or anti-mouse IgG conjugated to HRP (horseradish peroxidase) were from Caltag Laboratories. For visualization, we used the chemiluminescence substrate system LumiGlo (Upstate Biotechnology) and Kodak BioMax Light Film (Kodak).
Statistical analysis
Continuous variables were expressed as means±S.E.M. The statistical significance of the difference of the means was analysed by Student's t test. P<0.05 was considered statistically different.
RESULTS
The role of JFC1 in the androgen-regulated secretion of PSAP and PSA
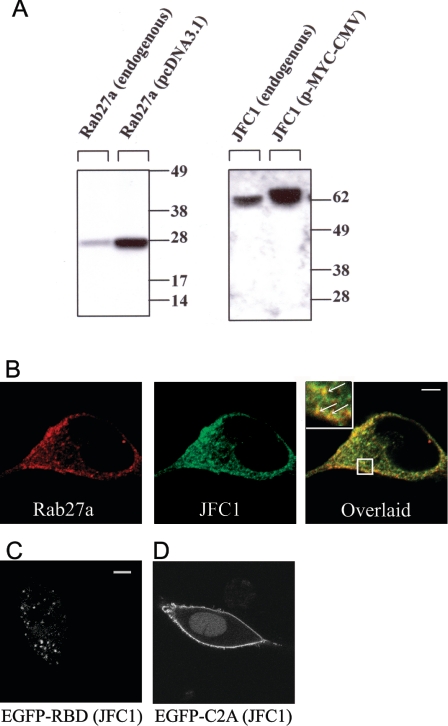
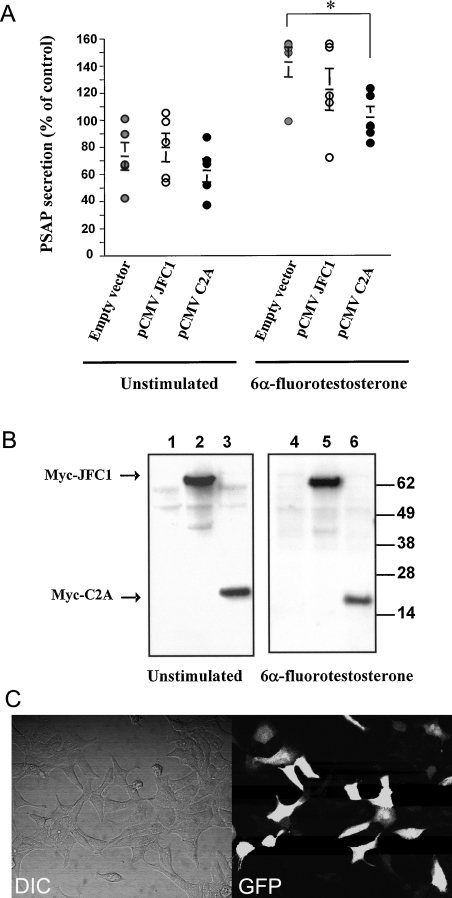
Rab effectors are heterogeneous molecules that are considered to be specific for particular transport systems [29] and can regulate vesicular trafficking in many different ways. A possible role for a Rab effector is to contribute to the docking of the Rab-containing vesicle to the acceptor membrane [30]. Generally, this type of effector contains a Rab-binding domain and an adaptor domain that recognizes membrane lipids or proteins at the docking site [30]. An example of a member of this group is Rabphilin3a, originally described as a Rab3a effector, that associates with target membranes through its tandem C2 domain. In the present paper, we first analysed the expression and distribution of endogenous JFC1 and Rab27a in LNCaP prostate cells. First, the expression of these secretory proteins in LNCaP cells was demonstrated by immunoblot analysis (Figure 1A). Then, using immunofluorescence analysis, we showed that JFC1 and Rab27a co-localize in cellular structures showing a punctate pattern (Figure 1B) [31]. Importantly, some of these structures could be clearly identified in association with the plasma membrane, suggesting that JFC1 and Rab27a may localize to a readily releasable pool of vesicles (Figure 1B). In the present study, we also show that the Rab27a-binding domain of JFC1 lacking the tandem C2 domains localizes on punctate structures, presumably secretory vesicles, when expressed as a chimaera with the enhanced green fluorescent protein (EGFP) in LNCaP cells (Figure 1C). However, those structures were not distributed in the proximity of the plasma membrane, suggesting that the C2 domains of JFC1 play a central role in vesicle docking. This is also supported by the observation that the C2A domain of JFC1 localizes at the plasma membrane when expressed as an EGFP chimaera (Figure 1D and [24]). Then we hypothesized that if JFC1 plays a role in the docking of Rab27a vesicles to the plasma membrane through its C2A domain, the overexpression of this domain would compete with endogenous JFC1 for a docking point at the plasma membrane, and should impair secretion. To test our hypothesis, we transiently transfected LNCaP cells by nucleofection and then evaluated the basal and androgen-regulated secretion of PSAP in transfected cells. Cells expressing the C2A domain of JFC1 showed a significant inhibition in the secretion of PSAP in response to androgens (Figure 2A). This inhibition by the C2A domain, although significant, is rather weak, but this may be a consequence of the transfection efficiency achieved in these experiments (∼33%; Figure 2C). This finding supports the hypothesis that the binding of JFC1 to the plasma membrane via its C2A domain is a necessary step in the secretion of PSAP and therefore suggests that JFC1 plays a central role in androgen-regulated secretion of PSAP. On the other hand, overexpression of the full-length JFC1 did not significantly affect basal or androgen-dependent PSAP secretion (Figure 2A), possibly indicating that the secretory machinery in PSAP-containing granules is fully mature (i.e. PSAP-containing vesicles are saturated with JFC1 molecules). Importantly, androgen treatment did not significantly affect the level of exogenously expressed JFC1 or C2A (Figure 2B) or that of endogenous JFC1 (results not shown).
Figure 1. Expression and subcellular localization of JFC1 and Rab27a in LNCaP cells.
(A) LNCaP cells were transfected with the vectors pcDNA3.1-Rab27a, pCMV-myc-JFC1 or the corresponding empty vectors (endogenous). The cells were lysed and proteins (10 μg) were resolved by gel electrophoresis and transferred on to nitrocellulose membranes. JFC1 and Rab27a were detected by Western blotting using a rabbit polyclonal antibody raised against an N-terminal peptide of JFC1 and a mouse monoclonal antibody against Rab27a. The antibodies are described in the Experimental section. Molecular-mass sizes are given in kDa. (B) Immunofluorescence was performed in 3.7% (w/v) paraformaldehyde-fixed cells as described in the Experimental section. LNCaP cells were labelled for immunofluorescence detection of endogenous JFC1 (green) and endogenous Rab27a (red) using a mouse monoclonal antibody and a rabbit polyclonal antibody respectively. The cells were incubated with anti-JFC1 and anti-Rab27a antibodies or negative control (not shown) overnight at 4 °C. For detection, secondary antibodies Alexa-Fluor-conjugated donkey anti-mouse (488) and donkey anti-rabbit (594) were used. Cells were stored in Fluoromount-G (Southern Biotechnology) until analysed by laser-scanning confocal microscopy. (C and D) The chimaeras consisting of the EGFP–Rab-binding domain (RBD) of JFC1 (C) or the EGFP–C2A domain of JFC1 (D) were expressed in LNCaP cells. The cells were fixed 24–48 h after transfections and were analysed by confocal microscopy. Scale bars, 5 μm.
Figure 2. JFC1 modulates the secretion of PSAP in LNCaP cells.
(A) LNCaP cells were transfected with 10 μg of the pCMV-myc-JFC1 expression vector (pCMV JFC1), the pCMV-myc-C2A (pCMV C2A) or the empty vector pCMV-myc (Empty vector) by nucleofection and were seeded in six-well plates containing RPMI 1640 medium 16 h before treatment. Cells were left untreated (DMSO) or stimulated with 100 nM 6α-fluorotestosterone for 48 h. For each condition, the medium was collected, and the concentration of PSAP was evaluated using a specific ELISA kit. Results are means±S.E.M. for five different experiments. The unstimulated empty vector control was arbitrarily designated as 100% secretion. The significance of the difference between means was calculated using a one-tail paired Student's t test (InStat 3.0); *P<0.02. (B) Cells were lysed in 2% (v/v) Nonidet P40 in PBS, and the level of expression of the overexpressed proteins was analysed by immunoblotting using an antibody raised against the myc-tagged protein. Lanes 1 and 4, pCMV-myc; lanes 2 and 5, pCMV-myc-JFC1; lanes 3 and 6, pCMV-myc-C2A. Molecular-mass sizes are given in kDa. (C) Transfection efficiency of the LNCaP cells by nucleofection was evaluated using the pGFP expression vector. The transfection efficiency after 24 h was ∼33%.
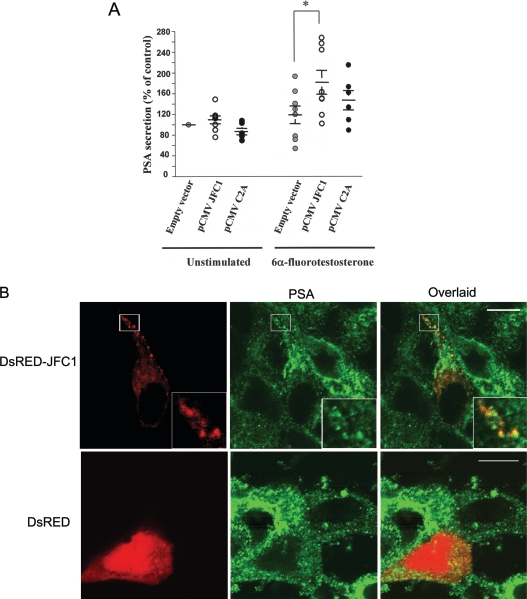
As mentioned above, LNCaP cells produce and secret both PSAP and PSA. Moreover, the secretion of both PSAP and PSA has been demonstrated to be dependent on androgen stimulation in LNCaP cells. However, it is not clear whether these proteins are stored in intracellular vesicles that share identical secretory machinery or, conversely, whether they are stored in independent compartments that undergo differential regulation. To answer this question, we first evaluated the secretion of PSA in LNCaP cells that overexpressed full-length JFC1 or its C2A domain. We show that the overexpression of the C2A domain of JFC1, which acts as a dominant negative for PSAP granule exocytosis, did not significantly inhibit PSA secretion (Figure 3A). This is the first evidence that suggests that the secretory machinery that functions in PSAP-containing vesicles differs from that regulating PSA secretion. Interestingly, although the basal secretion of PSA was not significantly affected by JFC1 overexpression, the overexpression of JFC1 significantly increased the secretion of PSA in response to androgens (Figure 3A). These data suggest that, in contrast with that observed for PSAP-containing granules, the secretion of the subpopulation of vesicles containing PSA is responsive to variations in the intracellular level of JFC1. Therefore the secretion of this prostate marker may increase under circumstances in which JFC1 is up-regulated (see the Discussion). This is supported by the observation that, although endogenous JFC1 co-localizes poorly with PSA-containing vesicles (Figure 4A), overexpressed JFC1 clearly distributes to PSA-containing granules (Figure 3B).
Figure 3. JFC1 regulates the secretory pathway of PSA in LNCaP cells.
(A) LNCaP cells were transfected with 10 μg of the pCMV-myc-JFC1 expression vector (pCMV JFC1), the pCMV-myc-C2A (pCMV C2A) or the empty vector pCMV-myc (Empty vector) by nucleofection and were seeded in six-well plates containing RPMI 1640 medium 16 h before treatment. Cells were left untreated (DMSO) or stimulated with 100 nM 6α-fluorotestosterone for 48 h. For each condition, the medium was collected, and the concentration of PSA was analysed using a specific ELISA kit. Results are means±S.E.M. for eight different experiments. The unstimulated empty vector control was arbitrarily designated 100% secretion. The significance of the difference between means was calculated using a one-tail paired Student's t test (InStat 3.0); *P<0.01. (B) PSA co-localizes with overexpressed JFC1. LNCaP cells were transfected with the expression vector pDsRED-JFC1 (upper panel) or with the control vector pDsRED (lower panel), and, 24 h after transfection, the cells were fixed, and the subcellular distribution of endogenous PSA was evaluated by immunofluorescence.
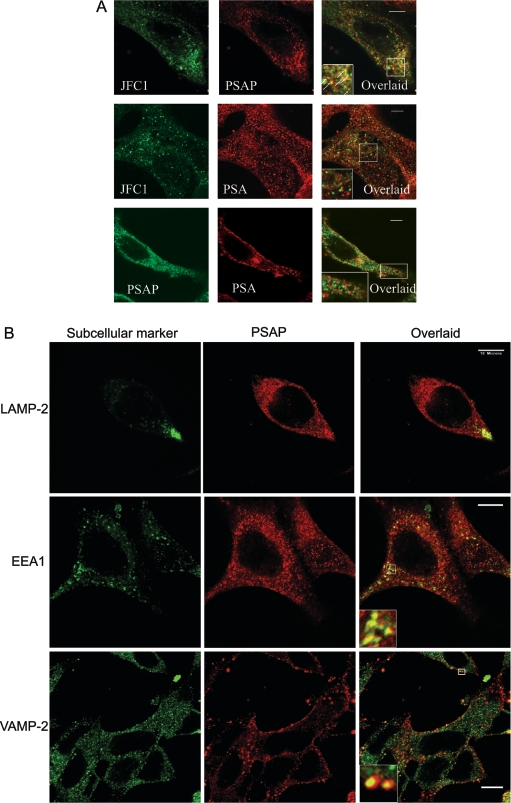
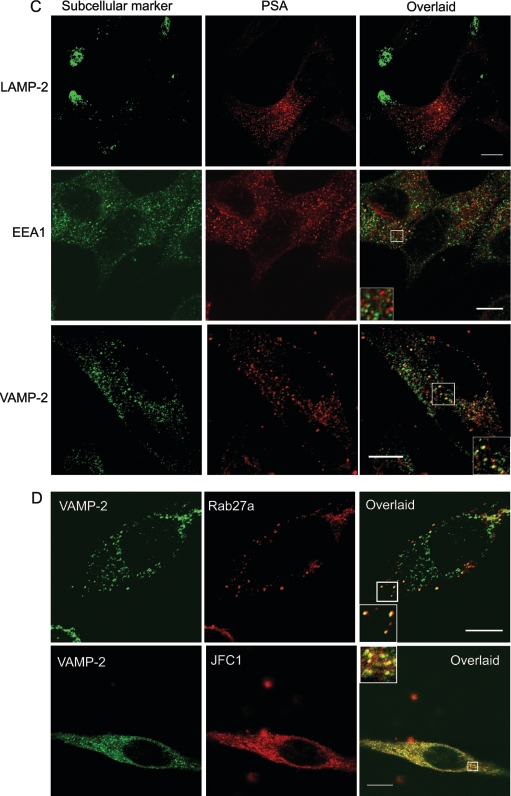
Figure 4. Subcellular localization of JFC1 in association with PSAP and PSA in LNCaP cells.
Immunofluorescence analysis was performed as described for Figure 1 and in the Experimental section. (A) Upper panels: subcellular localization of JFC1 and PSAP was analysed using the anti-JFC1 antibody described in Figure 1 and a mouse monoclonal antibody raised against PSAP (NeoMarkers). The inset shows the magnified image of the area selected in the overlaid panel. The arrows indicate co-localization of JFC1 and PSAP on punctate structures. Middle panels: the experiments were performed as described above, except that an anti-PSA antibody was used in the place of the anti-PSAP antibody. The inset shows a magnified image of representative punctate structures that were positive for PSA staining, but negative for JFC1 and vice versa. Bottom panels: immunofluorescence analysis of the distribution of PSA and PSAP in LNCaP cells is shown. Experiments were performed as described above. The magnified image shows an area of the cells where co-localization is undetectable. Co-localization between PSA and PSAP was only detectable in the perinuclear area. Scale bar, 5 μm. (B, C and D) Subcellular distribution of PSAP, PSA, JFC1 and Rab27a in relation to distinct subcellular organelle markers was evaluated by immunofluorescence. LAMP-2, lysosome-associated membrane protein-2. Insets show magnified images of the indicated areas. Scale bar, 10 μm.
Endogenous JFC1 localizes at PSAP-containing granules in LNCaP cells
The most obvious explanation for the results of our secretory experiments is that PSAP and PSA are stored in different intracellular compartments in LNCaP cells, and that these vesicles have different secretory machineries. At this point, it is worth mentioning that the subcellular distribution of PSA and PSAP in the prostate luminal secretory cells is controversial. A recent study has shown that PSA and PSAP localize exclusively in prostate-specific granules [32]. However, the presence of a minor cytosolic form of PSAP has been demonstrated [11]. Moreover, in a previous study, the ultrastuctural localization of PSA was detected in vesicles and endoplasmic reticulum, while PSAP immunoreactivity was observed in lysosomal granules in hyperplastic and neoplastic human prostates [33]. The controversy over the subcellular localization of these prostate markers may be due to the particularities of the samples analysed, the fixation techniques utilized to prepare the samples, the quality of antibodies used, or a combination of all three. In the present study, we carried out immunofluorescence analysis to determine the subcellular localization of endogenous JFC1 in relation to that of PSAP and PSA. We observed that JFC1 co-localizes with PSAP at punctate structures in LNCaP cells (Figure 4A, upper panels). Conversely, co-localization of PSA and JFC1 was barely detectable (Figure 4A, middle panels) unless JFC1 was overexpressed (Figure 3B), suggesting that PSA and PSAP may localize in different granule/vesicle subpopulations. This was confirmed by our immunofluorescence analysis that shows that PSA and PSAP localize in different vesicular structures in LNCaP cells (Figure 4A, lower panels), although some level of co-localization was observed in the perinuclear area. Then, we evaluated the localization of PSAP and PSA in relation to the distribution of various organelle markers by immunofluorescence in LNCaP cells. We observed that, while PSAP was identified in structures that were positive for the secretory vesicle marker VAMP-2, and in others that were positive for the early endosome marker EEA1, PSA only co-localized with secretory vesicles that expressed VAMP-2 (Figures 4B and 4C respectively). Finally, in Figure 4(D), we show that both JFC1 and Rab27a co-localize with VAMP-2. However, while the association of JFC1 with VAMP-2 can be observed on vesicles distributed homogeneously throughout the cell, the co-localization of Rab27a and VAMP-2 is only evident at the periphery of the cell where the GTPase is mainly located (Figure 4D).
Rab27a up-regulates the secretion of PSAP and PSA
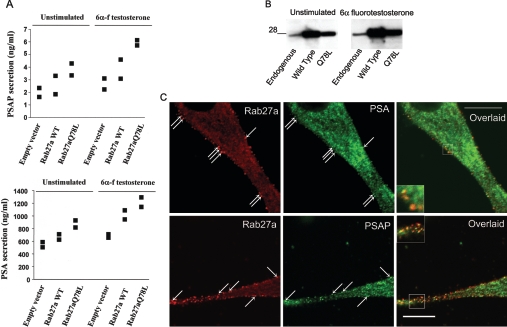
So far, JFC1 is considered to bind specifically to Rab27, but not to other Rab proteins [34]. Therefore the data presented above suggest a role for Rab27a in the secretory machinery in LNCaP cells. To determine whether this was actually the case, we evaluated the secretion of PSAP and PSA in LNCaP cells that overexpressed wild-type Rab27a or the constitutively active mutant Rab27aQ78L, which is thought to remain in the GTP-bound form due to the loss of its GTPase activity [25]. Unfortunately, the level of expression of the dominant-negative mutant Rab27aT23N was too low because of protein instability under our experimental conditions, a phenomenon described previously [35]. In Figures 5(A) and 5(B), we show that the secretion of both PSAP and PSA is increased by the overexpression of Rab27a. Moreover, the effect was manifested further when the constitutively active mutant was expressed. Importantly, we observed that endogenous PSA and PSAP co-localize with endogenous Rab27a in vesicles distributed in the proximity of the plasma membrane in LNCaP cells (Figure 5C), suggesting that this small GTPase is part of the secretory machinery of these prostatic secretory markers, and that Rab27a-containing vesicles possibly belong to the readily releasable pool of secretory vesicles. Altogether, these data suggest that Rab27a, but not JFC1, is a limiting factor in the PSAP secretory machinery. On the other hand, although it is clear that Rab27a can up-regulate the secretion of PSA, it is less clear how this GTPase mediates this effect. One possibility is that Rab27a may bind to a Rab27a effector that is different from JFC1 in vesicles containing PSA. Another possibility is that the cytosolic pool of JFC1 [36] mediates the regulation of PSA secretion by interacting with PSA-containing vesicle-associated Rab27a, after activation. Supporting this, we recently demonstrated that, while the subcellular localization of Rab27a is restricted to vesicle membranes in LNCaP cells, JFC1 is co-distributed between the vesicles and the cytosol, and its subcellular localization and its ability to cycle between the two pools is tightly regulated by phosphorylation in these cells [36].
Figure 5. Secretion of PSAP and PSA is increased by overexpression of Rab27a.
(A) Secretion experiments were performed as in Figures 2 and 3, except that LNCaP cells were transfected with wild-type Rab27a (Rab27a WT), the constitutively active mutant Rab27aQ78L or the empty vector pcDNA3.1 (Invitrogen) by nucleofection. Results from two independent experiments are shown as individual symbols, expressed as ng/ml of secreted protein. (B) The level of expression of Rab27a or the mutant Rab27aQ78L was evaluated by Western blotting. A 28 kDa size is indicated. C, The co-localization of endogenous Rab27a with endogenous PSAP or PSA was determined by indirect immunofluorescence and subsequent confocal microscopy analysis. The arrows indicate the distribution of Rab27a in vesicles located in the proximity of the plasma membrane that co-localize with PSA or PSAP.
Secretion of PSAP and PSA is regulated by the PI3K pathway
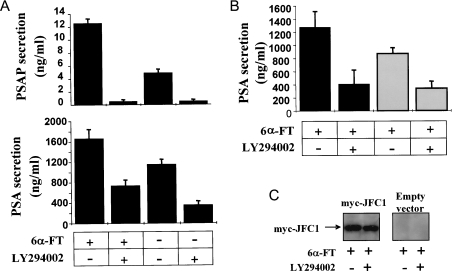
In a previous study, we demonstrated that JFC1 binds with relatively high specificity to the PI3K product PIP3, which is present only at the plasma membrane [20]. Moreover, we showed that the C2A domain of JFC1 mediates the binding to PIP3 in vivo and in vitro [24]. These data suggest that PI3K may play a central role in JFC1-mediated secretion. Our observation that the PI3K inhibitor LY294002 dramatically decreases androgen-stimulated secretion of PSAP and PSA (Figure 6A) supports further this hypothesis. However, the differences observed in the susceptibility of the secretion of these prostate markers to the PI3K inhibitor suggest again that the secretory mechanisms of PSA and PSAP are dissimilar. While PSAP secretion was completely abolished by LY294002, PSA secretion was still detectable after LY294002 treatment, suggesting that PI3K-independent mechanisms are partially responsible for the secretion of PSA in LNCaP cells in response to androgens. Importantly, the increase in the secretion of PSA observed in response to JFC1 overexpression in androgen treated-LNCaP cells was abolished by treating these cells with the PI3K inhibitor (Figure 6B), suggesting a role for PI3K products in the secretory mechanism that involves JFC1.
Figure 6. Androgen-dependent secretion of PSAP and PSA is down-regulated by PI3K inhibition.
(A) LNCaP cells were seeded in six-well plates containing RPMI 1640 medium and cultured as described in the Experimental section. After 12 h, the medium was replaced with fresh RPMI 1640 medium, and, where indicated, cells were incubated in the presence of the PI3K inhibitor LY294002 (16 μM) (+) or DMSO (−) for 45 min at 37 °C before the addition of 100 nM 6α-fluorotestosterone [6α-FT (+)] or DMSO (−). The cells were incubated for a further 48 h, and the medium was collected and kept at −20 °C until analysed. The concentration of PSAP and PSA in the media was determined using respective ELISA kits as described in the Experimental section. Results are means±S.E.M. for three independent experiments. (B) Experiments were performed exactly as described above, except that LNCaP cells were transfected with 10 μg of the pCMV-myc-JFC1 expression vector (black bars) or the empty vector pCMV-myc (grey bars) by nucleofection and were seeded in 12-well plates containing RPMI 1640 medium 12 h before treatment. (C) For each condition, the cells were lysed in 2% (v/v) Nonidet P40 in PBS, and the expression of the indicated proteins was analysed by immunoblotting using an antibody directed against the myc-tagged protein.
DISCUSSION
In the present study, we provide strong evidence that JFC1, a member of the slp family of Rab27a binding proteins, is a central component of the secretory machinery of prostate carcinoma secretory cells. Moreover, our data identify a definitive role for JFC1 in secretion, suggest the participation of Rab27a in the secretion of prostate specific markers and help to elucidate the mechanism of vesicle-regulated secretion in prostate cells by identifying PI3K as a mediator of this process.
The androgen-regulated secretion of PSAP and PSA plays a central role in prostate physiology and pathophysiology. Thus elevated serum levels of PSA are usually observed in patients with prostate cancer and benign prostatic hyperplasia. Moreover, PSA is considered to be a marker for disease progression in patients with androgen-independent prostate cancer [37,38]. Similarly, serum levels of PSAP have been used in the past as a marker for disease progression and treatment response [13,14,39]. However, lack of correlation between PSA or PSAP serum levels and their expression in biopsy specimens have been described in [39], highlighting the importance of their secretory mechanism. Based on the observation that pharmacological intervention may regulate differentially and independently prostate marker secretion and cell proliferation, Figg and collaborators suggest that, for the interpretation of clinical data based on serial PSA measurements to be valid, it is important to know the effects of a particular pharmacological agent on the regulation of PSA secretion [40]. In this context, the identification of the molecular machinery involved in the secretion of PSA and PSAP, and the characterization of its regulation, are essential steps for the understanding of the pathophysiology of prostate cancer and for the analysis of its progression based on the detection of secreted prostatic markers. Therefore, in order to understand the mechanism that takes place during secretion of PSAP and PSA, we analysed the role that JFC1, which is highly expressed in prostate [20,41], plays in this process. JFC1 specifically binds to Rab27a [25], and so far is not known to bind to other small GTPases from the Rab family [34]. We have shown that Rab27a, which has been found to exert a general role in regulated exocytosis [26], is also highly expressed in LNCaP cells, supporting the concept that the secretory role of Rab27a is not restricted to cytotoxic T-lymphocytes and melanocytes, cells in which a defect in Rab27a is considered to lead to the appearance of the phenotype observed in Griscelli syndrome. In the present study, we provide evidence that JFC1 and Rab27a are involved in the secretion of PSAP and PSA in LNCaP cells. The overexpression of the C2A domain of JFC1 acts as a dominant negative that, presumably by competing with endogenous JFC1, prevents the secretion of PSAP-containing vesicles. Importantly, similar approaches involving overexpression of C2 domains have been largely utilized to characterize the secretory capacity of Syts (for a review, see [42]), thus supporting our experimental design. An interesting observation from the secretory data of the present study is that the secretion of PSAP and PSA are regulated differently by JFC1. Thus, contrary to that observed for PSAP, the overexpression of the C2A domain of JFC1 did not affect the secretion of PSA. This correlates with our immunofluorescence analysis that shows that endogenous JFC1 is present in PSAP-containing vesicles, but is almost undetectable in PSA vesicles. Strikingly, overexpression of wild-type full-length JFC1 significantly increased PSA secretion, but not that of PSAP. The increase of PSA secretion in response to JFC1 overexpression possibly indicates that the Rab effector could be forced to localize to PSA vesicles when its intracellular concentration is increased; this is confirmed by our observation that the fluorescent chimaera DsRED–JFC1 co-localizes with PSA-containing granules when overexpressed. An important observation in connection with this finding is that JFC1 is transcriptionally activated by the NF-κB (nuclear factor κB) transcription factor in LNCaP cells [41]. Since NF-κB is part of a major survival pathway that is frequently up-regulated in prostate cancer [18], those results, together with the observation of the present study that the overexpression of JFC1 increases PSA secretion, suggest that the transcriptional activation of jfc1 by NF-κB may have physiological significance, and establish a link between the pathophysiological role of NF-κB and the PSA secretory machinery.
Importantly, the secretion of both PSAP and PSA was upregulated by overexpression of wild-type Rab27a or the constitutively active mutant Rab27aQ78L, thus strongly supporting a role for this small GTPase in the secretory pathways of LNCaP cells. Since the secretion of PSA was not affected by overexpression of JFC1-C2A, we propose that Rab27a may interact with a Rab27a effector different from JFC1 on PSA vesicles. However, the Rab27a effector MUNC13-4 was undetectable in prostate by Northern blots [43]. Moreover, neither granuphilin, whose expression is restricted to pancreatic β-cells and the pituitary gland [44], nor melanophilin, which is only expressed in melanocytes, seem to be a good candidate for playing a role in the secretion of PSA. Importantly, JFC1 exists in LNCaP cells as two independent pools, a vesicle-associated and a soluble cytosolic pool [36]. Therefore another possibility is that the localization of JFC1 on PSA-containing vesicles is an androgen-dependent mechanism, and that, after activation, the cytosolic pool of JFC1 mobilizes towards PSA-containing vesicles, thus regulating PSA secretion. Further experiments are necessary to test this hypothesis.
An important observation from our secretory data is that JFC1 does not seem to be involved in the basal secretion of PSAP or PSA. Differential regulatory mechanisms for basal and stimulated secretion have also been observed for another Rab27a effector, granuphilin, during insulin secretion in pancreatic β-cells [45]. In that case, while overexpression of granuphilin caused an increase in basal insulin secretion, it significantly inhibited high K+-induced insulin secretion. Granuphilin has been proposed to regulate insulin secretion by docking insulin-containing granules to the plasma membrane. Although this and the data presented here support a role for slp family members in vesicle tethering during exocytosis, the structures recognized by these two Rab27a effectors at the plasma membrane seem to be different. Thus granuphilin has been proposed to promote the plasma membrane targeting of insulin granules via interaction with the t-SNARE (target-associated soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor) syntaxin1a [45]. From this, it seems likely that members of the slp family of Rab effectors play a similar role by binding to different docking molecules in diverse cellular systems, although it is still unclear whether JFC1 has the ability to bind to t-SNAREs.
There is increasing evidence that PI3K and, in particular, its product PIP3 are directly involved in vesicular trafficking. For example, it has been recently established that a synapsin-I-associated PI3K mediates synaptic vesicle delivery to the readily releasable pool of vesicles [46]. Moreover, the release of intracellular calcium and subsequent degranulation were completely inhibited by PI3K inhibitors in mast cells [47], and the participation of PIP3 in the regulation of Ca2+ entry via Btk (Bruton's tyrosine kinase) has been demonstrated in platelets [48]. It has been proposed that the participation of PIP3 in vesicular trafficking may serve two functions [49]. First, PIP3 might contribute to the recruitment of a secretory effector to the plasma membrane. Secondly, PIP3 may be directly involved in the effector activation. Our previous studies suggest that JFC1 is affected by PIP3 in at least one of these two possible ways. That is, we demonstrated that JFC1 binds to a PIP3 in LNCaP cells [20]. Thus we hypothesize that JFC1 could act as a key molecule to position the Rab-containing vesicle into a specific docking point at the plasma membrane by binding to Rab27a via its N-terminal domain and to PIP3 via its C2A domain. Our experimental observations that the overexpression of the C2A domain resulted in a marked inhibition on the secretory pathway in androgen-stimulated LNCaP cells support further our hypothesis. Moreover, in the present study, the secretion of PSA and PSAP was dramatically attenuated by treatment of LNCaP cells with the PI3K inhibitor LY294002, supporting further the role of this lipid kinase in the secretory pathway in LNCaP cells. A role for the enzyme PI3K in the secretory pathway of prostatic markers is particularly relevant because the lack of function of the tumour suppressor PTEN (phosphatase and tensin homologue deleted on chromosome 10) and consequent elevated levels of PIP3 and Akt activity have been frequently associated with prostate cancer progression to androgen independence [1,50,51]. However, while PSAP secretion was abolished completely in the presence of the PI3K inhibitor, the secretion of PSA was only partially attenuated. This again suggests that the regulation of PSAP and PSA secretion is differentially regulated, and that a PI3K-independent mechanism is responsible for a large proportion of the PSA that is secreted in response to androgens. It also suggests that elevated levels of PSAP, but not PSA, may correlate with the frequently observed up-regulation of PI3K in patients with prostate cancer.
In conclusion, in the present study, we provide strong evidence that JFC1 differentially regulates the androgen-dependent secretion of PSAP and PSA in human LNCaP prostate carcinoma cells, we established that Rab27a plays a central role in the secretory pathway of LNCaP cells, and we describe a differential involvement of the PI3K pathway in the secretion of PSAP and PSA.
Acknowledgments
Supported in part by U.S. Public Health Service Grants AI-044434 and AI-024227, and by the Sam and Rose Endowment Fund. Edited before submission by Dr Peter Atterton.
References
- 1.Catz S. D., Johnson J. L. BCL-2 in prostate cancer: a minireview. Apoptosis. 2003;8:29–37. doi: 10.1023/a:1021692801278. [DOI] [PubMed] [Google Scholar]
- 2.Yousef G. M., Diamandis E. P. Human tissue kallikreins: a new enzymatic cascade pathway? Biol. Chem. 2002;383:1045–1057. doi: 10.1515/BC.2002.113. [DOI] [PubMed] [Google Scholar]
- 3.Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J. Clin. Invest. 1985;76:1899–1903. doi: 10.1172/JCI112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brillard-Bourdet M., Rehault S., Juliano L., Ferrer M., Moreau T., Gauthier F. Amidolytic activity of prostatic acid phosphatase on human semenogelins and semenogelin-derived synthetic substrates. Eur. J. Biochem. 2002;269:390–395. doi: 10.1046/j.0014-2956.2001.02667.x. [DOI] [PubMed] [Google Scholar]
- 5.Denmeade S. R., Lin X. S., Isaacs J. T. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28:251–265. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5a.Erratum. Prostate. 1996;28:414. [Google Scholar]
- 6.Calvo A., Xiao N., Kang J., Best C. J., Leiva I., Emmert-Buck M. R., Jorcyk C., Green J. E. Alterations in gene expression profiles during prostate cancer progression: functional correlations to tumorigenicity and down-regulation of selenoprotein-P in mouse and human tumors. Cancer Res. 2002;62:5325–5335. [PubMed] [Google Scholar]
- 7.Yajnik V., Paulding C., Sordella R., McClatchey A. I., Saito M., Wahrer D. C., Reynolds P., Bell D. W., Lake R., van den Heuvel S., et al. DOCK4, a GTPase activator, is disrupted during tumorigenesis. Cell. 2003;112:673–684. doi: 10.1016/s0092-8674(03)00155-7. [DOI] [PubMed] [Google Scholar]
- 8.Pei L., Peng Y., Yang Y., Ling X. B., Van Eyndhoven W. G., Nguyen K. C., Rubin M., Hoey T., Powers S., Li J. PRC17, a novel oncogene encoding a Rab GTPase-activating protein, is amplified in prostate cancer. Cancer Res. 2002;62:5420–5424. [PubMed] [Google Scholar]
- 9.Govindarajan R., Zhao S., Song X. H., Guo R. J., Wheelock M., Johnson K. R., Mehta P. P. Impaired trafficking of connexins in androgen-independent human prostate cancer cell lines and its mitigation by α-catenin. J. Biol. Chem. 2002;277:50087–50097. doi: 10.1074/jbc.M202652200. [DOI] [PubMed] [Google Scholar]
- 10.Webber M. M., Waghray A., Bello D. Prostate-specific antigen, a serine protease, facilitates human prostate cancer cell invasion. Clin. Cancer Res. 1995;1:1089–1094. [PubMed] [Google Scholar]
- 11.Lee M. S., Igawa T., Yuan T. C., Zhang X. Q., Lin F. F., Lin M. F. ErbB-2 signaling is involved in regulating PSA secretion in androgen-independent human prostate cancer LNCaP C-81 cells. Oncogene. 2003;22:781–796. doi: 10.1038/sj.onc.1206066. [DOI] [PubMed] [Google Scholar]
- 12.Wen Y., Hu M. C., Makino K., Spohn B., Bartholomeusz G., Yan D. H., Hung M. C. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 13.Huggins C., Hodges C. V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Ca Cancer J. Clin. 1972;22:232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 14.Gutman A. B. The development of the acid phosphatase test for prostatic carcinoma: the Sixth Ferdinand C. Valentine Memorial Lecture. Bull. N.Y. Acad. Med. 1968;44:63–76. [PMC free article] [PubMed] [Google Scholar]
- 15.Lin M. F., Clinton G. M. Human prostatic acid phosphatase has phosphotyrosyl protein phosphatase activity. Biochem. J. 1986;235:351–357. doi: 10.1042/bj2350351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin M. F., Lee M. S., Zhou X. W., Andressen J. C., Meng T. C., Johansson S. L., West W. W., Taylor R. J., Anderson J. R., Lin F. F. Decreased expression of cellular prostatic acid phosphatase increases tumorigenicity of human prostate cancer cells. J. Urol. 2001;166:1943–1950. [PubMed] [Google Scholar]
- 17.Lin M. F., Garcia-Arenas R., Chao Y. C., Lai M. M., Patel P. C., Xia X. Z. Regulation of prostatic acid phosphatase expression and secretion by androgen in LNCaP human prostate carcinoma cells. Arch. Biochem. Biophys. 1993;300:384–390. doi: 10.1006/abbi.1993.1052. [DOI] [PubMed] [Google Scholar]
- 18.Catz S. D., Johnson J. L. Transcriptional regulation of bcl-2 by nuclear factor κB and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 19.Kampa M., Papakonstanti E. A., Hatzoglou A., Stathopoulos E. N., Stournaras C., Castanas E. The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. FASEB J. 2002;16:1429–1431. doi: 10.1096/fj.02-0131fje. [DOI] [PubMed] [Google Scholar]
- 20.McAdara-Berkowitz J. K., Catz S. D., Johnson J. L., Ruedi J. M., Thon V., Babior B. M. JFC1, a novel tandem C2 domain-containing protein associated with the leukocyte NADPH oxidase. J. Biol. Chem. 2001;276:18855–18862. doi: 10.1074/jbc.M011167200. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda M., Mikoshiba K. Synaptotagmin-like protein 1–3: a novel family of C-terminal-type tandem C2 proteins. Biochem. Biophys. Res. Commun. 2001;281:1226–1233. doi: 10.1006/bbrc.2001.4512. [DOI] [PubMed] [Google Scholar]
- 22.Schiavo G., Gu Q. M., Prestwich G. D., Sollner T. H., Rothman J. E. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin T. F. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu. Rev. Cell Dev. Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- 24.Catz S. D., Johnson J. L., Babior B. M. The C2A domain of JFC1 binds to 3′-phosphorylated phosphoinositides and directs plasma membrane association in living cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11652–11657. doi: 10.1073/pnas.172382799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strom M., Hume A. N., Tarafder A. K., Barkagianni E., Seabra M. C. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 2002;277:25423–25430. doi: 10.1074/jbc.M202574200. [DOI] [PubMed] [Google Scholar]
- 26.Tolmachova T., Anders R., Stinchcombe J., Bossi G., Griffiths G. M., Huxley C., Seabra M. C. A general role for Rab27a in secretory cells. Mol. Biol. Cell. 2004;15:332–344. doi: 10.1091/mbc.E03-07-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramalho J. S., Tolmachova T., Hume A. N., McGuigan A., Gregory-Evans C. Y., Huxley C., Seabra M. C. Chromosomal mapping, gene structure and characterization of the human and murine RAB27B gene. BMC Genet. 2001;2:2. doi: 10.1186/1471-2156-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hume A. N., Collinson L. M., Rapak A., Gomes A. Q., Hopkins C. R., Seabra M. C. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 2001;152:795–808. doi: 10.1083/jcb.152.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segev N. Ypt/rab GTPases: regulators of protein trafficking. Science STKE. 2001;2001:RE11. doi: 10.1126/stke.2001.100.re11. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez L., Jr, Scheller R. H. Regulation of membrane trafficking: structural insights from a Rab/effector complex. Cell. 1999;96:755–758. doi: 10.1016/s0092-8674(00)80585-1. [DOI] [PubMed] [Google Scholar]
- 31.Cohen R. J., Beales M. P., McNeal J. E. Prostate secretory granules in normal and neoplastic prostate glands: a diagnostic aid to needle biopsy. Hum. Pathol. 2000;31:1515–1519. doi: 10.1053/hupa.2000.20885. [DOI] [PubMed] [Google Scholar]
- 32.Cohen R. J., McNeal J. E., Edgar S. G., Robertson T., Dawkins H. J. Characterization of cytoplasmic secretory granules (PSG), in prostatic epithelium and their transformation-induced loss in dysplasia and adenocarcinoma. Hum. Pathol. 1998;29:1488–1494. doi: 10.1016/s0046-8177(98)90020-x. [DOI] [PubMed] [Google Scholar]
- 33.Warhol M. J., Longtine J. A. The ultrastructural localization of prostatic specific antigen and prostatic acid phosphatase in hyperplastic and neoplastic human prostates. J. Urol. 1985;134:607–613. doi: 10.1016/s0022-5347(17)47311-3. [DOI] [PubMed] [Google Scholar]
- 34.Kuroda T. S., Fukuda M., Ariga H., Mikoshiba K. The Slp homology domain of synaptotagmin-like proteins 1–4 and Slac2 functions as a novel Rab27A binding domain. J Biol. Chem. 2002;277:9212–9218. doi: 10.1074/jbc.M112414200. [DOI] [PubMed] [Google Scholar]
- 35.Ramalho J. S., Anders R., Jaissle G. B., Seeliger M. W., Huxley C., Seabra M. C. Rapid degradation of dominant-negative Rab27 proteins in vivo precludes their use in transgenic mouse models. BMC Cell Biol. 2002;3:26. doi: 10.1186/1471-2121-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson J. L., Pacquelet S., Lane W. S., Eam B., Catz S. D. Akt regulates the subcellular localization of the Rab27a-binding protein JFC1 by phosphorylation. Traffic. 2005;6:667–681. doi: 10.1111/j.1600-0854.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 37.Ferro M. A., Gillatt D., Symes M. O., Smith P. J. High-dose intravenous estrogen therapy in advanced prostatic carcinoma: use of serum prostate-specific antigen to monitor response. Urology. 1989;34:134–138. doi: 10.1016/0090-4295(89)90248-3. [DOI] [PubMed] [Google Scholar]
- 38.Kelly W. K., Scher H. I., Mazumdar M., Vlamis V., Schwartz M., Fossa S. D. Prostate-specific antigen as a measure of disease outcome in metastatic hormone-refractory prostate cancer. J. Clin. Oncol. 1993;11:607–615. doi: 10.1200/JCO.1993.11.4.607. [DOI] [PubMed] [Google Scholar]
- 39.Vitali A., Ardoino S., Durante P., Ferro M. A., Li C. F., Parodi C., Sanguineti G., Gaffuri M., Paerachino M., Salvadori R. P. Correlation between immunohistochemical patterns and serum levels of PSA and PSAP in prostatic pathology: evaluation of 198 prostatic fine needle biopsies. Anticancer Res. 1994;14:1503–1507. [PubMed] [Google Scholar]
- 40.Dixon S. C., Knopf K. B., Figg W. D. The control of prostate-specific antigen expression and gene regulation by pharmacological agents. Pharmacol. Rev. 2001;53:73–91. [PubMed] [Google Scholar]
- 41.Catz S. D., Babior B. M., Johnson J. L. JFC1 is transcriptionally activated by nuclear factor-κB and up-regulated by tumour necrosis factor α in prostate carcinoma cells. Biochem. J. 2002;367:791–799. doi: 10.1042/BJ20020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai J., Chapman E. R. The C2 domains of synaptotagmin – partners in exocytosis. Trends Biochem. Sci. 2004;29:143–151. doi: 10.1016/j.tibs.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Feldmann J., Callebaut I., Raposo G., Certain S., Bacq D., Dumont C., Lambert N., Ouachee-Chardin M., Chedeville G., Tamary H., et al. Munc13–4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 44.Wang J., Takeuchi T., Yokota H., Izumi T. Novel rabphilin-3-like protein associates with insulin-containing granules in pancreatic β cells. J. Biol. Chem. 1999;274:28542–28548. doi: 10.1074/jbc.274.40.28542. [DOI] [PubMed] [Google Scholar]
- 45.Torii S., Zhao S., Yi Z., Takeuchi T., Izumi T. Granuphilin modulates the exocytosis of secretory granules through interaction with syntaxin 1a. Mol. Cell. Biol. 2002;22:5518–5526. doi: 10.1128/MCB.22.15.5518-5526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cousin M. A., Malladi C. S., Tan T. C., Raymond C. R., Smillie K. J., Robinson P. J. Synapsin I-associated phosphatidylinositol 3-kinase mediates synaptic vesicle delivery to the readily releasable pool. J. Biol. Chem. 2003;278:29065–29071. doi: 10.1074/jbc.M302386200. [DOI] [PubMed] [Google Scholar]
- 47.Huber M., Helgason C. D., Scheid M. P., Duronio V., Humphries R. K., Krystal G. Targeted disruption of SHIP leads to Steel factor-induced degranulation of mast cells. EMBO J. 1998;17:7311–7319. doi: 10.1093/emboj/17.24.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pasquet J. M., Quek L., Stevens C., Bobe R., Huber M., Duronio V., Krystal G., Watson S. P. Phosphatidylinositol 3,4,5-trisphosphate regulates Ca2+ entry via Btk in platelets and megakaryocytes without increasing phospholipase C activity. EMBO J. 2000;19:2793–2802. doi: 10.1093/emboj/19.12.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cockcroft S. New York: Oxford University Press; 2000. Biology of phosphoinositides. [Google Scholar]
- 50.Murillo H., Huang H., Schmidt L. J., Smith D. I., Tindall D. J. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–4805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 51.Graff J. R., Konicek B. W., McNulty A. M., Wang Z., Houck K., Allen S., Paul J. D., Hbaiu A., Goode R. G., Sandusky G. E., et al. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol. Chem. 2000;275:24500–24505. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]