Abstract
Shiga toxin-producing Escherchia coli (STEC) comprises a group of attaching and effacing (A/E) enteric pathogens of animals and humans. Natural and experimental infection of calves with STEC may result in acute enteritis or subclinical infection, depending on serotype- and host-specific factors. To quantify intestinal secretory and inflammatory responses to STEC in the bovine intestine, serotypes that are associated with human disease (O103:H2 and O157:H7) were introduced into ligated mid-ileal loops in gnotobiotic and conventional calves, and fluid accumulation and recruitment of radiolabeled neutrophils were measured after 12 h. STEC serotype O103:H2, but not serotype O157:H7, elicited strong enteropathogenic responses. To determine if the inflammatory response to STEC O103:H2 in calves requires Shiga toxin 1 or intimate bacterial attachment to the intestinal epithelium, defined mutations were made in the stx1, eae, and tir genes. Our data indicate that some STEC induce intestinal inflammatory responses in calves by a mechanism that is independent of A/E-lesion formation, intimin, or Shiga toxin 1. This may have implications for strategies to reduce STEC carriage in cattle.
Shiga toxin-producing Escherichia coli (STEC) comprises an emerging group of zoonotic enteric pathogens (41). In humans, STEC infections result in bloody or nonbloody diarrhea, which may be complicated by hemorrhagic colitis and severe renal and neurological sequelae, including hemolytic uremic syndrome (HUS) (45, 55). The pathogenesis of HUS is believed to involve the translocation of Shiga toxin (Stx1 and/or Stx2; also called verocytotoxins), produced by STEC in the intestinal lumen, to systemic sites, where they cause microvascular endothelial cell damage, fibrin-platelet thrombus formation, and thrombocytopenia (45).
Cattle are an important reservoir of STEC (25), and human infections are frequently associated with the consumption of contaminated beef and dairy products (26). Severe cases of infection of susceptible calves with bovine virulent STEC strains may result in atrophy of the villi, epithelial cell damage, diffuse infiltration of neutrophils into the lamina propria and intestinal lumen, and the formation of a pseudomembrane containing blood, fibrin, cellular debris, and neutrophils (29, 46). A similar histopathology occurs in some cases of STEC-associated hemorrhagic colitis in humans (27, 55). An understanding of the induction of intestinal inflammatory responses to STEC is important in the development of strategies to reduce the prevalence of STEC in cattle. By implication, such strategies may lower the incidence of human STEC infections.
Infection of the gastrointestinal tracts of conventional cattle and 5-day-old gnotobiotic calves by STEC serotype O157:H7 is asymptomatic (5, 63). However, E. coli O157:H7 is frequently associated with human disease, and the molecular basis of STEC serotype host specificity is unknown. Infection of neonatal colostrum-deprived calves with E. coli O157:H7 may result in enterocolitis similar to that seen in humans (8); however, such animals are highly susceptible to infection and are not ideal models for studying the enteropathogenesis of STEC in cattle. Unlike E. coli O157:H7, STEC serotype O103:H2 is frequently isolated from diarrheic calves (36, 62), as well as being associated with human disease (38).
Colonization of the gastrointestinal tracts of colostrum-deprived neonatal calves and piglets by E. coli O157:H7 and the induction of colonic edema and diarrhea requires the bacterial outer membrane protein intimin (Eae) (7, 14, 39, 59). The eae gene also plays a role in the pathogenesis of infections by enteropathogenic E. coli (EPEC) in humans (13), rabbit EPEC (REPEC) in infant rabbits (37), and Citrobacter rodentium in mice (30, 53). In all these cases, intimin is required for the formation of intestinal attaching and effacing (A/E) lesions, which are characterized by intimate bacterial attachment to the apical surfaces of enterocytes and the localized destruction of microvilli (22). This histopathology is determined by the chromosomal locus for enterocyte effacement (LEE) and also requires the LEE-encoded Tir protein, which is translocated via a type III protein secretion system into host cells, where it acts as a receptor for intimin (11). Intimin can also bind to β1 integrins (23), though the consequence of this is unknown.
It remains unclear if intimin per se, or intimate bacterial adherence and A/E-lesion formation, is required to elicit intestinal inflammatory responses. In mice, C. rodentium induces a pronounced T helper cell type 1 (Th1) mucosal immune response characterized by mucosal thickening and infiltration of CD4+ T cells (30, 53). A C. rodentium eae null mutant does not induce colonic hyperplasia, whereas killed wild-type C. rodentium and E. coli K-12 strains engineered to express intimin can induce inflammatory responses similar to those seen in wild-type C. rodentium infection (30), suggesting that intimin is required to drive the mucosal inflammatory response. Indeed, a purified C-terminal intimin polypeptide can augment mitogen-stimulated proliferation of T cells (30). In contrast, intimin is not essential for inflammatory responses to REPEC in an infant rabbit model (37), indicating that host- and/or strain-specific differences between the model systems may exist.
The role of Shiga toxins in the intestinal inflammatory response to STEC has also been extensively studied. Shiga toxin is capable of inducing the transmigration of neutrophils across intestinal epithelial cell monolayers in vitro (1) and of stimulating the secretion of the proinflammatory cytokines interleukin-8 (IL-8) (58) and tumor necrosis factor alpha (49), and therefore it may be a strong inducer of mucosal inflammatory responses. In support of this notion, a REPEC strain engineered to produce Shiga toxin produced more severe disease with increased inflammatory changes and elevated mucosal IL-1 activity in infant rabbits compared to the non-Stx-producing parent strain (3). Purified Stx1 induced similar inflammatory responses following intragastric inoculation of rabbits (44). However, differences in the susceptibilities of animals to the intestinal effects of Stx clearly exist, since STEC culture supernatants or purified Stx induces fluid accumulation in rabbit ligated ileal loops (21, 60) but not in the jejunum in gnotobiotic piglets (60) or calf ligated ileal loops (48). Stx is not required for the ability of E. coli O157:H7 strains to cause diarrhea in gnotobiotic piglets (60) and neonatal calves (6). However, a role for Stx in STEC carriage and pathogenesis in cattle cannot be precluded, since Stx1 can inhibit the activation and proliferation of subpopulations of bovine lymphocytes and so may modulate the mucosal immune response (20, 40).
We have used a bovine ligated intestinal loop assay to quantify secretory and inflammatory responses to STEC. The roles of intimin, Tir, and Stx1 in the induction of enteropathogenic responses in the bovine intestine were addressed by the construction of defined STEC mutants.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
E. coli strain PMK5 (O103:H2; stx1) was isolated from a child with HUS in France (38). E. coli 84-289 (O157:H7; stx1 stx2) was isolated from a food handler in Canada (60). Plasmids pKNG101 (strAB [32]) and pCVD442 (blaM [12]) are positive-selection suicide vectors containing the Bacillus subtilis sacBR genes, the mob region from plasmid RP4, and a pir-dependent R6K origin of replication. Plasmid pSB315 (24) was the source of the kanamycin resistance (aphT) gene used to make nonpolar insertions in eae and tir. Plasmids were maintained in E. coli K-12 DH5α, except for suicide plasmids (and derivatives), which were maintained in E. coli CC118 λpir (9) or in E. coli S17-1λpir (54) for conjugation. Bacteria were isolated on Luria-Bertani (LB) agar and cultivated in LB broth with appropriate antibiotics at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 25 μg/ml; nalidixic acid, 25 μg/ml; and streptomycin, 100 μg/ml. For enteropathogenesis assays, bacteria were amplified in brain heart infusion broth for 18 h at 37°C, and the absorbance at 600 nm was adjusted to within 0.1 U.
Cell lines.
HeLa cells (ATCC CCL2) were cultivated in RPMI 1640 buffered with 2 g of sodium bicarbonate/liter and supplemented with 10% (vol/vol) fetal calf serum (PAA Laboratories GmbH, Linz, Austria) and 0.3 g of l-glutamine/liter. For fluorescent actin staining (FAS) tests, cells were seeded at 2 × 105/35-mm-diameter dish on glass coverslips and grown for 18 h at 37°C in a 5% CO2 atmosphere.
Routine DNA manipulation.
Standard procedures were used for DNA extraction, cloning, PCR, and the verification of mutants by Southern hybridization (50).
Construction of mutations in eae, tir, and stx1.
Defined mutations were built into E. coli strain PMK5 (intimin type ɛ; Tir type β; stx1AB [43]). To construct an eae mutant, the PMK5 eae gene was first amplified by PCR using primers cesD-sens (5′ TATGATGATCTATGGCGTCTGT 3′) and escD-asens (5′ TATTTTCAAAAAGAATGATGTC 3′) and cloned into pCR-XL-TOPO (Invitrogen). A 3.3-kb EcoRI fragment containing the cloned eae gene was then subcloned into pUC19, resulting in plasmid pUC-eae. An aphT gene lacking a transcription terminator was excised from pSB315 using HincII and inserted in an EcoRV site in pUC-eae 993 bp downstream of the eae start codon. A ca. 4.5-kb PvuII fragment containing the inactivated eae gene was then subcloned into SmaI-cut pKNG101, generating pKNG-eae::kanR.
To inactivate the tir gene, the PMK5 tir region was amplified using the primers orf19-sens (5′ GAAAGTTGATGAGCAGTGTGG 3′) and cesD-asens (5′ GACGACTTCTATTTCATTCT 3′) and cloned into pCR-TOPO-2.1 (Invitrogen). A 2.5-kb HindIII fragment containing the cloned tir gene was then subcloned into pUC19. The resulting plasmid, pUC-tir, was then opened with EcoRV and ligated to the HincII fragment of pSB315 containing the aphT cassette gene (the insertion is 515 bp downstream of the tir start codon). The inactivated tir gene was excised on a 2.5-kb EcoRI-AflIII fragment, the ends were filled in using T4 polymerase, and the gene was cloned into SmaI-cut pKNG101, generating pKNG-tir::kanR.
Approximately 3 μg of pKNG-eae::kanR and pKNG-tir::kanR was separately used for electroporation of PMK5 in 0.2-cm-electrode gap cuvettes at 2.5 or 12.5 kV/cm and 25 mF, using a 5-ms pulse at 4°C and a Bio-Rad GenePulser. After incubation in SOC medium for 1 h, merodiploids were selected on LB agar containing streptomycin and kanamycin. Double recombinants were selected by growing the merodiploids to late logarithmic phase in LB broth lacking streptomycin and plating them onto LB agar (minus NaCl) containing 5% (wt/vol) sucrose and kanamycin at 30°C. Strains resistant to sucrose and kanamycin but sensitive to streptomycin were shown to carry the mutations by PCR and Southern hybridization.
The PMK5 Δstx1 mutant contains a large internal deletion of the stx1A gene that also removes the start codon for the B subunit. Sequences flanking the PMK5 stx1A gene were separately amplified by PCR with Vent proofreading DNA polymerase (New England Biolabs) using the primer pairs stxI3 (5′ ATATATGAGCTCCTTGACCAGATATGTTAAGG 3′) plus stxI4 (5′ ATTAATAATGTTTTTTTCAAGAGCGAATGACATTCAGC 3′) and stxI5 (5′ TCATTCGCTCTTGAAAAAAACATTATTAATAGCTGC 3′) plus stxI6 (5′ ATATATGAGCTCTCTAACACATCTATTATCAG 3′) (based on the sequence of the bacteriophage J93 slt1 gene [accession no. M19473]). The primary PCR products were gel purified and combined in an overlapping PCR (31) using the flanking primers stxI3 and stxI6. The secondary PCR product was then cloned into pCVD442 using SacI sites incorporated into the primers. The resulting plasmid, pCVDΔstx1, was introduced into a nalidixic acid-resistant derivative of PMK5 by conjugation from S17-1λpir, and a merodiploid was isolated on LB agar containing ampicillin. Double recombinants were selected on sucrose-containing medium, screened for the deletion by colony PCR, and verified by Southern hybridization. The deletion results in fusion of the region encoding the Stx1A N-terminal 44 amino acids to a stop codon within the stx1B sequence, ensuring that a fusion protein with the B subunit cannot be made.
Quantification of enteropathogenesis.
A Hereford-Aberdeen Angus hysterotomy-derived gnotobiotic calf was delivered into a positive-pressure isolator (10), fed sterile condensed milk (Carnation; Nestlé), and maintained aseptically for 7 days prior to use. Conventional Friesian bull calves (25 to 30 days old) were fed on powdered milk and screened for the excretion of STEC or Salmonella by enrichment on Sorbitol MacConkey agar containing tellurite and cefixime (Oxoid, Basingstoke, United Kingdom) or Brilliant Green agar, respectively. The calves were observed twice daily for 7 days prior to surgery, and animals with diarrhea or excreting STEC were excluded from the analysis. The bovine ligated ileal loop assay has been described previously (61). Briefly, calves were anesthetized for the duration of the experiment (ca. 14 h) with pentobarbitone sodium (Sagatal; 0.44 ml/kg of body weight), and the mid ileum was flushed with intestinal wash solution (5.61 g of NaCl/liter, 0.11g of KCl/liter, 1.09 g of KH2PO4/liter, 0.16 g of Na2HPO4/liter, 7.04 g of trisodium citrate/liter, and 5 g of N-acetyl cysteine/liter). The calves were maintained at 38.5 to 39.5°C by the use of heated mats. Loops 6 cm in length with 1-cm spacers were ligated with surgical silk and inoculated with 5 ml of bacterial culture (ca. 5 × 109 CFU) or sterile medium as a negative control. Each strain was tested in triplicate in any one animal, and the experiment was repeated in a total of three calves. Approximately 70 ml of venous blood was collected in 12 ml of acid citrate, and neutrophils were isolated according to the method of Carlson and Kaneko (4). The neutrophils were resuspended in 2 ml of Ca2+-free Tyrodes buffer, radiolabeled by mixing the suspension with 3.7 MBq of 111In-oxinate (Mallinckrodt, Petten, The Netherlands) for 5 min, washed in Tyrodes buffer, and reinjected into the calf within 2 h of inoculation of the loops with bacteria.
Twelve hours after inoculation, enteropathogenesis was assessed with respect to fluid accumulation, neutrophil infiltration, and histological changes. The animals were killed by an overdose of pentobarbitone, and 5 ml of 10% neutral buffered formaldehyde was injected into the loops to inactivate the bacteria prior to collection of the loop contents and mucosa. Fluid secretion was measured as a ratio of the volume of fluid accumulated to loop length (V/L), and the mean ± standard error of the mean (SEM) was calculated from the replicates. Radioactivity associated with the loop contents and mucosa was detected using a Wallac 1275 gamma counter and was corrected for differences in loop length. Neutrophil infiltration was expressed as the ratio of 111In activity/L in the test loops to that in the negative control loops. Pairwise comparisons were performed at the overall 0.05 level of significance by two-way analysis of variance (Proc mixed [Statistical Analysis System; SAS Institute, Cary, N.C.]).
Tissue sampling and light microscopy.
Samples of intestinal mucosa for histological analysis were fixed in situ by injecting ligated loops antemortem with 5 ml of 4% (wt/vol) paraformaldehyde in phosphate-buffered saline (PBS) and were then excised and immersed in fixative overnight at 4°C. For light microscopy, 5-μm-thick transverse wax-embedded sections were stained with hematoxylin and eosin and viewed at ×400 using a Leica DMLS microscope. Images were captured with a Polaroid digital microscope camera.
Fluorescence microscopy.
FAS for the detection of F-actin under sites of bacteria adhesion to HeLa cells was performed as described previously (33), except that bacteria were incubated on the cells for a total of 8 h. Transverse wax-embedded sections of mucosa from infected ileal loops were dewaxed and permeabilized with 0.5% (vol/vol) Triton X-100 in PBS for 30 min, and nonspecific binding sites were blocked with 0.5% (wt/vol) bovine serum albumin in PBS. Bacteria were detected with 1:100 rabbit anti-O103 typing serum (Veterinary Laboratories Agency, Weybridge, United Kingdom) and 1:100 anti-rabbit immunoglobulin-Alexa568 (Molecular Probes, Leiden, The Netherlands). F-actin was stained with 1:10 Oregon green 514-phalloidin (Molecular Probes). The stained sections were washed extensively with PBS, mounted with Vectashield (Vector Laboratories, Burlingame, Calif.), and analyzed at ×630 to ×1,000 using a Leica TCS NT confocal laser scanning microscope with version 1.6.587 software.
RESULTS
Bovine enteropathogenic responses to different STEC serotypes.
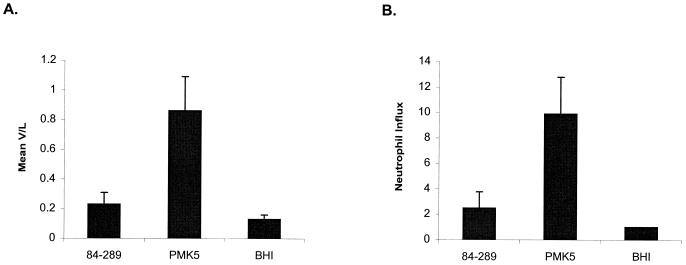
We investigated the abilities of STEC serotypes O103:H2 and O157:H7 to elicit secretory and inflammatory responses in a bovine ligated ileal loop assay. Ligated loops were constructed in the mid-ileum in three ca. 28-day-old conventional calves, and fluid accumulation and infiltration of 111In-labeled neutrophils was quantified 12 h after inoculation of the loops. Fluid accumulation and neutrophil infiltration were significantly greater in the loops inoculated with strain PMK5 (P < 0.05) than in the control loops injected with sterile medium (Fig. 1). The enteropathogenic responses to STEC serotype O157:H7 were not significantly greater than those to the negative control (P values >0.05), and this is consistent with recent observations (48). No differences in the level of adherence of the O157:H7 and O103:H2 strains to bovine ileal mucosa could be detected by immunostaining and confocal microscopy (data not shown).
FIG. 1.
Enteropathogenic responses to STEC strains 84-289 (O157:H7) and PMK5 (O103:H2) and sterile brain heart infusion (BHI) broth in the intestines of ca. 28-day-old conventional calves. (A) Fluid accumulation. Each strain was tested in triplicate mid-ileal loops, and the mean V/L ratio (+ SEM) from the three independent experiments is shown. (B) Neutrophil infiltration. The total 111In activity in the loop contents and mucosa was corrected for loop length, and the mean of three measurements in the mid-ileum was derived. Neutrophil influx is the ratio of the mean 111In activity in the test loops to 111In activity in the negative control loops. The mean neutrophil influx (+ SEM) from the three calves is shown.
Enteropathogenic responses to E. coli PMK5 were also tested in midileal ligated loops in a 7-day-old gnotobiotic calf. Fluid accumulation was significantly greater in loops injected with PMK5 (V/L, 0.63 ± 0.04) compared to control loops (V/L, 0.40 ± 0.03; P < 0.05), and the mean neutrophil influx was 12.6-fold greater than in loops injected with sterile medium. The enteropathogenic responses to strain 84-289 were not significantly greater than those to the controls and were not improved by extending the in vivo incubation time to 18 h, by inoculation of loops with mid-logarithmic-phase bacteria grown in tissue culture medium (Dulbecco's modified Eagle's medium) or by inoculating loops constructed in the distal ileum (data not shown).
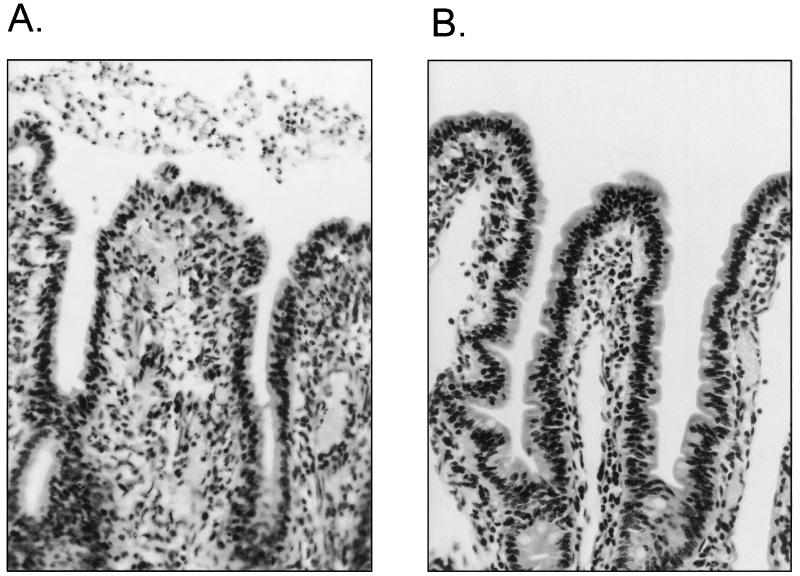
Microscopic analysis of hematoxylin- and eosin-stained sections of intestinal mucosa from ileal loops inoculated with PMK5 revealed lesions similar to those seen in natural and experimental infection of susceptible calves with STEC (Fig. 2) (7, 8, 29, 46). Subjective analysis of PMK5-infected mucosa indicated slight villus atrophy, with foci of exfoliated or rounded epithelial cells, especially on villus tips, and diffuse infiltration of neutrophils into the lamina propria and submucosa compared with control mucosa. A pseudomembrane containing large numbers of neutrophils was present in the lumen overlying the epithelium (Fig. 2A and 3C), which is consistent with the high 111In activity detected in the loop contents.
FIG. 2.
Histological analysis of mid-ileal mucosa from ligated loops inoculated with E. coli PMK5 (A) or sterile medium (B). Hematoxylin and eosin staining was used. Magnification, ×400.
FIG.3.
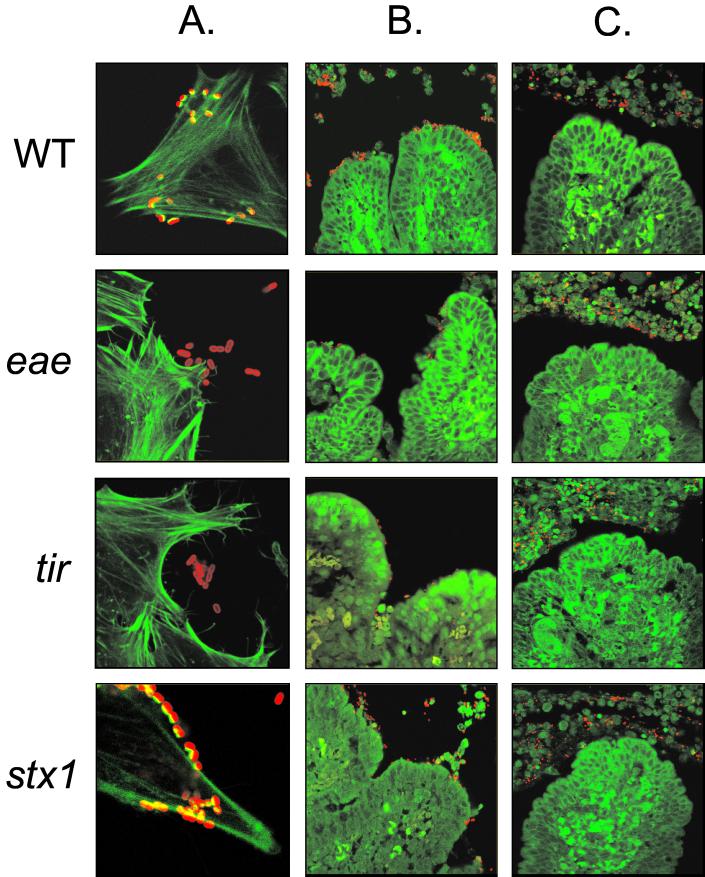
Confocal laser scanning microscopy showing PMK5 and isogenic eae, tir, and stx mutants interacting with HeLa cells or intestinal mucosa. (A) FAS of HeLa cells incubated with PMK5 and the isogenic mutants indicates that the eae and tir mutants are incapable of nucleating F-actin under the sites of bacterial adhesion. Magnification, ×1,000. (B and C) Sections of mid-ileal mucosa from inoculated loops stained for F-actin and bacteria. The images in column B show rare microcolonies formed by the wild type and the stx mutant. Microcolonies were not detected with the eae and tir mutants; however, small numbers of bacteria could occasionally be seen on the ileal mucosa. Column C contains typical fields showing an almost complete absence of bacteria on the intestinal epithelium and numerous bacteria and neutrophils in the gut lumen. Magnification (B and C), ×630. Green, F-actin stained with Oregon green 514-phalloidin; red, bacteria detected with rabbit anti-O103 typing serum and anti-rabbit immunoglobulin-Alexa568.
Enteropathogenic responses to PMK5 eae, tir, and stx1 mutants.
Defined mutations were made in the PMK5 eae, tir, and stx1 genes by allelic exchange using positive-selection suicide plasmids. Insertions in eae and tir were shown to result in a lack of intimate attachment to HeLa cells in vitro and absence of ability to nucleate F-actin under sites of bacterial adhesion in FAS tests (Fig. 3A). We were unable to detect intimin in lysates of the PMK5 eae mutant by Western blotting using antiserum raised against the conserved domain of intimin (data not shown). A deletion in the gene encoding the Stx1 catalytic A subunit was created by overlapping PCR and shown to abolish cytotoxicity of PMK5 culture supernatants for Vero cells (data not shown). Deletion of stx1 did not influence the ability of PMK5 to adhere to cultured epithelial cells or the ability to condense F-actin at sites of bacterial attachment (Fig. 3A).
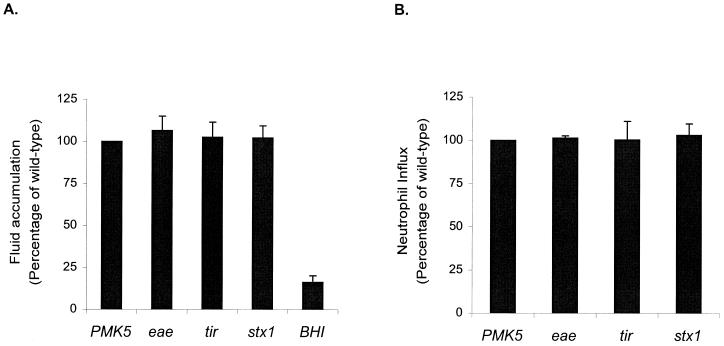
Enteropathogenic responses to the PMK5 and isogenic eae, tir, and stx mutants were tested in triplicate ligated loops in three ca. 28-day-old conventional calves (Fig. 4). To account for interanimal variation, neutrophil influx and the volume of fluid accumulated were expressed as a percentage of the responses to the wild-type strain in the same calf. The eae, tir, and stx1 mutations did not significantly impair neutrophil infiltration or fluid secretion compared to those with wild-type PMK5 (P values, >0.05).
FIG. 4.
Enteropathogenic responses to E. coli PMK5 and isogenic eae, tir, and stx1 mutants in the mid-ileum of ca. 28-day-old conventional calves. (A) Fluid accumulation. To correct for interanimal variation, the mean V/L ratio for each mutant strain was expressed as a percentage of the mean V/L ratio for the wild type in the same calf. The mean percentage plus SEM from three independent experiments is shown. (B) Neutrophil influx. The total 111In activity in the contents and mucosa of test loops was corrected for loop length and expressed as a ratio to the activity in negative control loops. Neutrophil influx for the mutant strains was then calculated as a percentage of the influx for the wild type, and the mean of the three experiments plus SEM was derived.
Confocal laser scanning microscopy of infected ileal mucosa.
To confirm that the eae and tir mutations impaired the ability of PMK5 to interact with the bovine intestinal epithelium, sections of ileal mucosa from ligated loops in 28-day-old calves were stained for bacteria and F-actin and examined by confocal microscopy. Relatively few bacteria could be seen in association with the mucosa, even in loops inoculated with the wild-type strain. Figure 3B shows rare microcolonies of wild-type bacteria that were detected on villus tips. No such microcolonies were detected in loops inoculated with the eae and tir mutants. Figure 3C shows typical fields, in which very few bacteria are associated with the ileal mucosa and most bacteria are associated with neutrophils in the intestinal lumen. Analysis of the lumenal infiltrate at high magnification revealed that some, but not all, bacteria were located within neutrophils (data not shown).
DISCUSSION
We report the use of a ligated ileal loop assay to characterize enteropathogenic responses to STEC in the bovine intestine. An STEC strain isolated from a case of HUS (PMK5; serotype O103:H2) induced significant secretory and inflammatory responses during the 12-h assay. The elevated levels of 111In activity in the contents and mucosa of PMK5-inoculated loops correlated with the presence of an inflammatory infiltrate similar to that seen in calves infected with bovine virulent STEC (29, 46). The recruitment of neutrophils in response to PMK5 appears to be specific, since injection of similar numbers of E. coli O157:H7 cells did not induce significant enteropathogenic responses. Confocal microscopy of midileal mucosa from PMK5-inoculated loops revealed small foci of bacteria on the intestinal epithelium, similar to those detected by immunohistochemical staining of ileal mucosa from E. coli O157:H7-infected neonatal calves (8) and O157:H7-infected ileal loop mucosa (data not shown). These data, combined with observations that STEC colonizes the bovine small intestine in vivo (7, 8, 29) and can induce A/E-lesion formation on bovine distal ileum both in vivo and in vitro (7, 8, 47), indicate that the ligated ileal loop assay is physiologically relevant and can be used to dissect bacterial and host factors influencing enteropathogenic responses.
The absence of enteropathogenic responses to E. coli O157:H7 may be explained by the age-related susceptibility of calves to these bacteria (5, 8). E. coli O157:H7 has been reported to produce colonic edema and diarrhea only in colostrum-deprived neonatal calves <36 h old (8). Infection of 5-day-old gnotobiotic calves with O157:H7 is asymptomatic (63). The reason PMK5 elicits stronger enteropathogenic responses than E. coli O157:H7 in bovine midileal ligated loops remains obscure. A human O103:H2 STEC formed extensive A/E lesions in the colons of gnotobiotic piglets with foci of neutrophils in the lamina propria and lumen (28), and O103:H2 STEC is associated with enteric disease in goats (15); however, to our knowledge the pathogenesis of O103:H2 STEC strains in calves has not been tested. Baker et al. (2) reported considerable variation in the virulence of O157:H7 strains of human and bovine origin in a gnotobiotic-piglet model, and it is possible that PMK5 possesses traits that are absent in the O157:H7 strain tested.
We sought to determine if the enteropathogenic responses to E. coli PMK5 required intimin and/or A/E-lesion formation by testing defined eae and tir mutants in the loop model. Intimin is required for colonization of colostrum-deprived neonatal calves and piglets by E. coli O157:H7, and eae null mutants fail to induce enteritis in such animals (7, 14, 39, 59). However in bovine ligated ileal loops, an eae mutant of PMK5 induced inflammatory and secretory responses similar to those induced by the wild-type strain. We have reported similar findings from an infant rabbit model of REPEC infection (37). While REPEC O103:H2 intimin is required for colonization of the rabbit intestine and the induction of diarrhea, infiltration of inflammatory cells still occurred in response to a defined eae mutant without destroying the brush border or general architecture. Thus, infiltration of neutrophils is probably not sufficient to induce diarrhea in the infant rabbit model. Previous studies using anti-CD18 antibody administered to STEC-infected rabbits had indicated that the host inflammatory response is implicated in the induction of diarrhea (18). Further evidence suggesting that intimin is not essential for induction of the enteropathogenic responses is provided by the finding that eae-negative STEC strains are associated with diarrhea in humans (16, 19) and calves (62). In addition, EPEC eae is not required for activation of NF-κB in epithelial cells, which in turn initiates transcription of the gene encoding IL-8 (52).
There is evidence for a direct role for intimin in stimulating mucosal inflammatory responses to C. rodentium in mice (30). Intimin drives pronounced Th1 immune responses in the murine gut and the purified cell-binding domain of intimin (Int280) augments mitogen-stimulated T-cell proliferation in a concentration-dependent manner (30). The Int280 domain binds not only to Tir but also to β1 integrins on T cells (23), and it is possible that this triggers signaling events that result in murine colonic hyperplasia. Our results indicate that host- and/or strain-specific differences between the animal models of A/E E. coli infections exist and that factors in addition to intimin are involved in PMK5-induced intestinal inflammatory responses in calves. However, we cannot exclude the possibility that in the intact host intimin facilitates the delivery of factors chemotactic to neutrophils by enabling the bacteria to colonize the intestinal epithelium. The notion that factors encoded outside the LEE may influence STEC pathogenesis is supported by the finding that Stx-minus STEC can impair epithelial barrier function and ion transport in monoloyers of T84 cells in the absence of A/E-lesion formation (35).
Mutations in the genes encoding intimin and its translocated receptor did not result in a complete lack of association of PMK5 with the bovine midileal epithelium. Intimin-independent association of STEC with bovine intestinal epithelia has been reported following oral inoculation of one calf with an O157:H7 eae mutant (7) and has also been reported in rabbits infected with a REPEC eae mutant (34). This indicates that accessory adhesins mediate the binding of STEC to intestinal mucosa. A number of loci have been implicated in the attachment of STEC to epithelial cells, including those for LEE-encoded EspA filaments (17), Efa (42), and Iha (56) and several other genes identified by transposon mutagenesis of E. coli O157:H7 (57).
We did not detect any role for Stx1 in the induction of inflammatory responses to PMK5 in calves. The finding that Stx does not influence enteropathogenesis is supported by reports that Stx-negative E. coli O157:H7 strains produce diarrhea and colonic edema in neonatal calves (6) and gnotobiotic piglets (60), with no obvious histopathological differences from the Stx-producing parent strain. Pruimboom-Brees et al. recently reported that cattle lack intestinal receptors for Shiga toxin (48) and concluded that this may explain why cattle are more resistant to the enterotoxic effects of Shiga toxin than are rabbits. Indeed, the authors reported that 108 to 109 50% cytotoxic doses of purified Stx failed to induce fluid accumulation in calf ligated ileal loops. Our data show that cattle are capable of raising intestinal secretory and inflammatory responses to viable STEC and confirm that Stx1 does not influence the induction of these responses. The possibility remains, however, that Shiga toxins may facilitate STEC colonization and pathogenesis in cattle by inhibiting the activation and proliferation of lymphocytes (20, 40), and this is the subject of ongoing studies. An Stx-producing O157:H7 strain colonized weaned calves better than a nonproducing strain (6); however, the strains used were not isogenic, and differences in colonization may have been due to traits other than Stx production. While in vitro evidence supports a role for Stx in neutrophil transmigration and the secretion of proinflammatory cytokines (1, 49, 58), it should be noted that EPEC, which shares many virulence factors with STEC but does not express Shiga toxin, also invokes these changes in vitro (51).
The rapid and pronounced infiltration of neutrophils into the lamina propria and gut lumen in response to E. coli PMK5 in the bovine midileum, and to other STEC strains following natural and experimental infection, indicates that cell-mediated immunity may be important in controlling STEC in the bovine intestine. The inflammatory response occurs in the absence of large numbers of bacteria on the intestinal epithelium and is not influenced by Stx or the ability of the bacteria to form A/E lesions. We propose to investigate the survival of STEC exposed to this response and to study the role of bacterial factors in this process.
Acknowledgments
This work was supported by grants from the Biotechnology and Biological Sciences Research Council, United Kingdom (T.S.W., G.F., and A.D.P.; no. 201/D10261) and the European Union (E.U. project number QLK2-2000-00600). O.M. was the recipient of a scholarship from the Ecole Nationale Vétérinaire de Toulouse.
Editor: A. D. O'Brien
REFERENCES
- 1.Acheson, D. W. K., R. Moore, S. De Breucker, L. Lincicome, M. Jacewicz, E. Skutelsky, and G. T. Keusch. 1996. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect. Immun. 64:3294-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, D. R., R. A. Moxley, and D. H. Francis. 1997. Variation in virulence in the gnotobiotic pig model of O157:H7 Escherichia coli strains of bovine and human origin. Adv. Exp. Med. Biol. 412:53-58. [DOI] [PubMed] [Google Scholar]
- 3.Blake, D. C. I., R. G. Russell, E. Santini, T. Bowen, and E. C. Boedeker. 1996. Pro-inflammatory mucosal cytokine responses to Shiga-like toxin-1 (SLT-1), p. 75-82. In G. T. Keusch and M. Kawakami (ed.), Cytokines, cholera and the gut. IOS Press, Amsterdam, The Netherlands.
- 4.Carlson, G. P., and J. J. Kaneko. 1973. Isolation of leukocytes from bovine peripheral blood. Proc. Soc. Exp. Biol. Med. 142:853-855. [DOI] [PubMed] [Google Scholar]
- 5.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Bovine infection with Shiga toxin-producing Escherichia coli, p. 261-267. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing Escherichia coli. ASM Press, Washington, D.C.
- 7.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean-Nystrom, E. A., B. T. Bosworth, W. C. Cray, Jr., and H. W. Moon. 1997. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect. Immun. 65:1842-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis, M. J., D. C. Davies, and M. N. Hoare. 1976. A simplified apparatus for the microbiological isolation of calves. Br. Vet. J. 132:642-646. [DOI] [PubMed] [Google Scholar]
- 11.DeVinney, R., M. Stein, D. Reinscheid, A. Abe, S. Ruschkowski, and B. B. Finlay. 1999. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect. Immun. 67:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eaeA deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnenberg, M. S., C. O. Tacket, S. P. James, G. Losonsky, J. P. Nataro, S. S. Wasserman, J. B. Kaper, and M. M. Levine. 1993. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J. Clin. Investig. 92:1412-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnenberg, M. S., S. S. Tzipori, M. L. McKee, A. D. O'Brien, J. Alroy, and J. B. Kaper. 1993. Role of the eae gene of enterohaemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Investig. 92:1418-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duhamel, G. E., R. A. Moxley, C. W. Maddox, and E. D. Erickson. 1992. Enteric infection of a goat with enterohemorrhagic Escherichia coli (O103:H2). J. Vet. Diagn. Investig. 4:197-200. [DOI] [PubMed] [Google Scholar]
- 16.Dytoc, M. T., A. Ismaili, D. J. Philpott, R. Soni, J. L. Brunton, and P. M. Sherman. 1994. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect. Immun. 62:3494-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebel, F., T. Podzadel, M. Rohde, A. U. Kresse, S. Krämer, C. Deibel, C. A. Guzmán, and T. Chakraborty. 1998. Initial binding of Shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol. Microbiol. 30:147-161. [DOI] [PubMed] [Google Scholar]
- 18.Elliott, E., Z. Li, C. Bell, D. Stiel, A. Buret, J. Wallace, I. Brzuszczak, and E. O'Loughlin. 1994. Modulation of host response to Escherichia coli O157:H7 infection by anti-CD18 antibody in rabbits. Gastroenterology 106:1554-1561. [DOI] [PubMed] [Google Scholar]
- 19.Feng, P., S. D. Weagant, and S. R. Monday. 2001. Genetic analysis for virulence factors in Escherichia coli O104:H21 that was implicated in an outbreak of hemorrhagic colitis. J. Clin. Microbiol. 39:24-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferens, W. A., and C. J. Hovde. 2000. Antiviral activity of Shiga toxin 1: suppression of bovine leukemia virus-related spontaneous lymphocyte proliferation. Infect. Immun. 68:4462-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira, A. J. P., W. P. Elias, Jr., J. S. Pelayo, R. Giraldi, M. Z. Pedroso, and I. C. A. Scaletsky. 1998. Culture supernatants of Shiga toxin-producing Escherichia coli strains provoke fluid accumulation in rabbit ileal loops. FEMS Immun. Med. Microbiol. 19:285-288. [DOI] [PubMed] [Google Scholar]
- 22.Frankel, G., A. D. Phillips, L. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 23.Frankel, G., O. Lider, R. Hershkoviz, A. P. Mould, S. G. Kachalsky, D. C. A. Candy, L. Cahalon, M. J. Humphries, and G. Dougan. 1996. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to beta1 integrins. J. Biol. Chem. 271:20359-20364. [DOI] [PubMed] [Google Scholar]
- 24.Galán, J. E., C. Ginocchio, and P. Costeas. 1992. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J. Bacteriol. 174:4338-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gansheroff, L. J., and A. D. O'Brien. 2000. Escherichia coli O157:H7 in beef cattle presented for slaughter in the U.S.: higher prevalence rates than previously estimated. Proc. Natl. Acad. Sci. USA 97:2959-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin, P. M. 1995. Escherichia coli O157:H7 and other enterohaemorrhagic Escherichia coli, p. 739-761. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, New York, N.Y.
- 27.Griffin, P. M., L. C. Olmstead, and R. E. Petras. 1990. Escherichia coli O157:H7-associated colitis. A clinical and histological study of 11 cases. Gastroenterology 99:142-149. [DOI] [PubMed] [Google Scholar]
- 28.Hall, G. A., C. R. Dorn, N. Chanter, S. M. Scotland, H. R. Smith, and B. Rowe. 1990. Attaching and effacing lesions in vivo and adhesion to tissue culture cells of vero-cytotoxin-producing Escherichia coli belonging to serogroups O5 and O103. J. Gen. Microbiol. 136:779-786. [DOI] [PubMed] [Google Scholar]
- 29.Hall, G. A., D. J. Reynolds, N. Chanter, J. H. Morgan, K. R. Parsons, T. G. Debney, A. P. Bland, and J. C. Bridger. 1985. Dysentery caused by Escherichia coli (S102-9) in calves: natural and experimental disease. Vet. Pathol. 22:156-163. [DOI] [PubMed] [Google Scholar]
- 30.Higgins, L. M., G. Frankel, I. Connerton, N. S. Goncalves, G. Dougan, and T. T. MacDonald. 1999. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285:588-591. [DOI] [PubMed] [Google Scholar]
- 31.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 32.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 33.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohaemorrhagic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krejany, E. O., T. H. Grant, V. Bennett-Wood, L. M. Adams, and R. M. Robins-Browne. 2000. Contribution of plasmid-encoded fimbriae and intimin to capacity of rabbit-specific enteropathogenic Escherchia coli to attach to and colonize rabbit intestine. Infect. Immun. 68:6472-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, Z., E. Elliott, J. Payne, J. Isaacs, P. Gunning, and E. V. O'Loughlin. 1999. Shiga toxin-producing Escherichia coli can impair T84 cell structure and function without inducing attaching/effacing lesions. Infect. Immun. 67:5938-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mainil, J. 1999. Shiga/verocytotoxins and Shiga/verotoxigenic Escherichia coli in animals. Vet. Res. 30:235-257. [PubMed] [Google Scholar]
- 37.Marchès, O., J.-P. Nougayrède, S. Boullier, J. Mainil, G. Charlier, I. Raymond, P. Pohl, M. Boury, J. De Rycke, A. Milon, and E. Oswald. 2000. Role of Tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect. Immun. 68:2171-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mariani-Kurkdjian, M., E. Denamur, A. Milon, B. Picard, H. Cave, N. Lambert-Zechovsky, C. Loirat, P. Goullet, P. J. Sansonetti, and J. Elion. 1993. Identification of a clone of Escherichia coli O103:H2 as a potential agent of hemolytic-uremic syndrome in France. J. Clin. Microbiol. 31:296-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKee, M. L., A. R. Melton-Celsa, R. A. Moxley, D. H. Francis, and A. D. O'Brien. 1995. Enterohaemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect. Immun. 63:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menge, C., L. H. Wieler, T. Schlapp, and G. Baljer. 1999. Shiga toxin 1 from Escherichia coli blocks activation and proliferation of bovine lymphocyte subpopulations in vitro. Infect. Immun. 67:2209-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholls, L., T. H. Grant, and R. M. Robins-Browne. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35:275-288. [DOI] [PubMed] [Google Scholar]
- 43.Oswald, E., H. Schmidt, S. Morabito, H. Karch, O. Marchès, and A. Caprioli. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pai, C. H., J. K. Kelly, and G. L. Meyers. 1986. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect. Immun. 51:16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga-toxin producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearson, G. R., K. J. Bazeley, J. R. Jones, R. F. Gunning, M. J. Green, A. Cookson, and M. J. Woodward. 1999. Attaching and effacing lesions in the large intestine of an eight-month-old heifer associated with Escherichia coli O26 infection in a group of animals with dysentery. Vet. Rec. 145:370-373. [DOI] [PubMed] [Google Scholar]
- 47.Phillips, A. D., S. Navabpour, S. Hicks, G. Dougan, T. Wallis, and G. Frankel. 2000. Enterohaemorrhagic Escherichia coli O157:H7 target Peyer's patches in humans and cause attaching/effacing lesions in both human and bovine intestine. Gut 47:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pruimboom-Brees, I. M., T. W. Morgan, M. R. Ackermann, E. D. Nystrom, J. E. Samuel, N. A. Cornick, and H. W. Moon. 2000. Cattle lack vascular receptors for Escherichia coli O157:H7 Shiga toxins. Proc. Natl. Acad. Sci. USA 97:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakiri, R., B. Ramegowda, and V. L. Tesh. 1998. Shiga toxin type 1 activates tumor necrosis factor-α gene transcription and nuclear translocation of the transcriptional activators nuclear factor-κB and activator protein-1. Blood 92:558-566. [PubMed] [Google Scholar]
- 50.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 51.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Attachment of a non-invasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 64:4480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed]
- 53.Schauer, D. B., and S. Falkow. 1993. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect. Immun. 61:4654-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon, R., U. Preifer, and A. Puhler. 1983. A broad host range mobilisation system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 55.Slutsker, L., A. A. Ries, K. D. Greene, J. G. Wells, L. Hutwagner, and P. M. Griffin. 1997. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiological features. Ann. Intern. Med. 126:505-513. [DOI] [PubMed] [Google Scholar]
- 56.Tarr, P. I., S. S. Bilge, J. C. Vary, Jr., S. Jelacic, R. L. Habeeb, T. R. Ward, M. R. Baylor, and T. E. Besser. 2000. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect. Immun. 68:1400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tatsuno, I., H. Kimura, A. Okutani, K. Kanamaru, H. Abe, S. Nagai, K. Makino, H. Shinagawa, M. Yoshida, K. Sato, J. Nakamoto, T. Tobe, and C. Sasakawa. 2000. Isolation and characterization of mini-Tn 5Km2 insertion mutants of enterohemorrhagic Escherichia coli O157:H7 deficient in adherence to Caco-2 cells. Infect. Immun. 68:5943-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thorpe, C. M., B. P. Hurley, L. L. Lincicome, M. S. Jacewicz, G. T. Keusch, and D. W. K. Acheson. 1999. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect. Immun. 67:5985-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tzipori, S., F. Gunzer, M. S. Donnenberg, L. de Montigny, J. B. Kaper, and A. Donohue-Rolfe. 1995. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohaemorrhagic Escherichia coli infection. Infect. Immun. 63:3621-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tzipori, S., H. Karch, K. I. Wachsmuth, R. M. Robins-Browne, A. D. O'Brien, H. Lior, M. L. Cohen, J. Smithers, and M. M. Levine. 1987. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect. Immun. 55:3117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallis, T. S., S. M. Paulin, J. S. Plested, P. R. Watson, and P. W. Jones. 1995. The Salmonella dublin virulence plasmid mediates systemic but not enteric phases of salmonellosis in cattle. Infect. Immun. 63:2755-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wieler, L. H., E. Vieler, C. Erpenstein, T. Schlapp, H. Steinrück, R. Bauerfeind, A. Byomi, and G. Baljer. 1996. Shiga toxin-producing Escherichia coli strains from bovines: association of adhesion with carriage of eae and other genes. J. Clin. Microbiol. 34:2980-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodward, M. J., D. Gavier-Widen, I. M. McLaren, C. Wray, M. Sozmen, and G. R. Pearson. 1999. Infection of gnotobiotic calves with Escherichia coli O157:H7 strain A84. Vet. Rec. 144:466-470. [DOI] [PubMed] [Google Scholar]