Abstract
Cell-mediated immunity is the major protective mechanism against Cryptococcus neoformans. Delayed swelling reactions, i.e., delayed-type hypersensitivity (DTH), in response to an intradermal injection of specific antigen are used as a means of detecting a cell-mediated immune (CMI) response to the antigen. We have found previously that the presence of an anticryptococcal DTH response in mice is not always indicative of protection against a cryptococcal infection. Using one immunogen that induces a protective anticryptococcal CMI response and one that induces a nonprotective response, we have shown that mice immunized with the protective immunogen undergo a classical DTH response characterized by mononuclear cell and neutrophil infiltrates and the presence of gamma interferon and NO. In contrast, immunization with the nonprotective immunogen results in an influx of primarily neutrophils and production of tumor necrosis factor alpha (TNF-α) at the DTH reaction site. Even when the anticryptococcal DTH response was augmented by blocking the down-regulator, CTLA-4 (CD152), on T cells in the mice given the nonprotective immunogen, the main leukocyte population infiltrating the DTH reaction site is the neutrophil. Although TNF-α is increased at the DTH reaction site in mice immunized with the nonprotective immunogen, it is unlikely that TNF-α activates the neutrophils, because the density of TNF receptors on the neutrophils is reduced below control levels. Uncoupling of DTH reactivity and protection has been demonstrated in other infectious-disease models; however, the mechanisms differ from our model. These findings stress the importance of defining the cascade of events occurring in response to various immunogens and establishing the relationships between protection and DTH reactions.
Cell-mediated immunity is a very important protective mechanism against a number of microbial pathogens, including the fungus Cryptococcus neoformans (26, 39). A standard means of assessing a cell-mediated immune (CMI) response is to perform a delayed-type hypersensitivity (DTH) test, which is done by injecting the antigen of interest intradermally and then determining if there is a delayed induration or swelling reaction at the site (11, 54). The level of DTH reactivity is determined in humans and guinea pigs by the diameter of induration 48 h after antigen injection or in mice by the amount of ear or footpad swelling 24 h after antigen injection. From a histological viewpoint, classical DTH reactions have been defined as having predominantly mononuclear cell infiltrates 24 to 48 h after antigen injection (11, 54). In the mouse model, DTH reaction sites generally have significant numbers of neutrophils in addition to the mononuclear cells (11). T helper 1 (Th1) cells or activated CD4+ T cells are considered to be the pivotal cellular component of CMI responses (10) and thus drive the DTH reaction. The activated CD4+ Th1 cells specifically recognize the inducing antigen presented on antigen-presenting cells and respond by producing interleukin 2 (IL-2) and gamma interferon (IFN-γ) (3, 38). DTH reactions are considered to be reliable in vivo correlates of CMI responses to the antigen(s) used to elicit the reaction; however, in several infectious-disease models, such as listeriosis, tuberculosis, and cryptococcosis, investigators have found that DTH reactivity can occur without being associated with protection against the infectious agent (9, 10, 24, 31, 42, 46, 47, 53).
We have previously shown that anticryptococcal CMI responses as measured by DTH reactivity to a cryptococcal culture filtrate antigen, CneF, can be induced in the mouse by two different cryptococcal immunogens (4, 42). The anticryptococcal DTH reactivity stimulated by subcutaneous immunization with CneF in complete Freund's adjuvant (CneF-CFA) is associated with protection against a challenge infection with C. neoformans. In contrast, the anticryptococcal DTH reactivity induced by another immunogen, heat-killed C. neoformans cells in CFA (HKC-CFA) or HKC alone, given by the same route as CneF-CFA is not associated with any protection against a challenge with viable cryptococci (4, 33, 42). Neither HKC-CFA nor CneF-CFA induces demonstrable anticryptococcal antibodies (42).
The protective immunogen induces activated CD4+ T cells that can be demonstrated in the lymph nodes draining the immunization site during the afferent phase of the immune response 18 h after immunization (4). When CneF is injected into a surrogate DTH reaction site (a gelatin sponge implanted in the mouse back) in mice immunized with CneF-CFA, a classical DTH reaction ensues by 24 to 36 h after challenge (6, 15, 16). The cellular infiltrate consists of CD4+ T cells with an activated phenotype (CD45RBlow), neutrophils, and macrophages (4, 5). Increased levels of the chemokines MIP-1α and TCA-3 and of the cytokines IL-2, IFN-γ, and tumor necrosis factor alpha (TNF-α) are present at the DTH reaction site in mice immunized with the protective immunogen (7, 15, 16). CD8+ T cells do not play a role in the anticryptococcal DTH response induced by immunization with CneF-CFA (35; J. W. Murphy, unpublished data).
In contrast to the immune response induced by CneF-CFA, the immune response induced by HKC-CFA or HKC alone has a different T-cell profile (35, 42, 44). HKC-CFA or HKC induces both CD4+ and CD8+ T cells that are associated with anticryptococcal DTH reactivity of the mice (35). In addition, unconventional T cells that can directly bind to and kill C. neoformans cells in vitro are increased by immunization with HKC-CFA or HKC (43, 44).
Protection or clearance of the organism results from the cellular and molecular events at the site of the organism in the tissue, and in the cryptococcosis model, those events generally are similar to the events occurring at the site of a DTH reaction (1, 5, 6, 15, 16, 20-23, 28, 29). Consequently, by studying the events at a DTH reaction site, we can obtain data that are predictive of the happenings at the site of infection. It is essential to understand the protective and nonprotective mechanisms in order to develop effective immunoreplacement or immunoaugmenting therapies or vaccines. Considering that activated CD4+ T cells and IFN-γ production are required for protection against C. neoformans and are key elements of a classical DTH reaction (1, 4, 7, 18, 20, 21, 27, 28, 36, 40, 50, 55), we hypothesized that HKC-CFA immunization, which induces delayed footpad swelling responses but no protection, does not stimulate a CMI response that results in classical DTH reactivity. To test this hypothesis, we have used the gelatin sponge implantation model to compare the components at the DTH reaction site in the mice that are protected against C. neoformans with the components at the DTH reaction site in mice immunized with the nonprotective immunogen, HKC-CFA.
MATERIALS AND METHODS
Mice.
Female inbred CBA/J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and maintained in the animal facility at the University of Oklahoma Health Sciences Center. Three mice per group at 7 to 12 weeks of age were used for each experiment.
Maintenance of endotoxin-free conditions.
All experiments were performed under conditions that minimized endotoxin. Only purchased endotoxin-free plasticware or glassware baked for 3 h at 180°C was used. All reagents used in the experiments were tested for endotoxin and were not used if the endotoxin level was detectable by the Limulus amebocyte chromogenic assay (Whittaker Bioproducts Inc., Walkerville, Md.) (the minimal detectable level of endotoxin was 0.01 ng of endotoxin/ml).
Cryptococcal antigens.
The cryptococcal culture filtrate antigen (CneF) used for immunization and injection of footpads and sponges was prepared from C. neoformans isolate 184A as described previously (5). Briefly, a defined growth medium was inoculated with 109 yeast cells/liter of medium, and the culture was incubated for 5 days at 30°C. The supernatant from the culture was collected with a Millipore (Bedford, Mass.) OM-141 Pellicon tangential-flow system and a 0.45-μm-pore-size cassette. The culture filtrate was passed over a 30,000-molecular-weight-cutoff cassette in a Pellicon system, and the retentate was washed extensively with sterile endotoxin-free physiologic saline solution (saline) and concentrated 10-fold. The concentrated retentate, designated CneF, was filter sterilized and stored at −20°C until it was used. The CneF preparation used in these studies had a protein concentration of 0.106 mg/ml as determined by the bicinchoninic acid assay (Pierce Chemical Co., Rockford, Ill.) and a carbohydrate concentration of 6.9 mg/ml as determined by the phenol-sulfuric acid assay (17). HKC were prepared by heating isolate 184A for 1 h at 60°C. HKC were washed three times in saline before being used to immunize mice. The washed HKC were assessed for viability by plating them on Sabouraud's agar.
Immunization to induce anticryptococcal CMI responses.
Induction of the anticryptococcal CMI response was performed by immunizing CBA/J mice with CneF or HKC emulsified in CFA or HKC alone in saline (35, 42, 44). Briefly, 0.1 ml of a 1:1 emulsion of CneF in CFA or HKC in CFA was injected subcutaneously (s.c.) at each of two sites at the base of the tail. Thus, each mouse given CneF-CFA received 10.6 μg of protein and 690 μg of carbohydrate from CneF. We have previously found, using 10-fold-increasing numbers of HKC, that injection by the s.c. route of 107 HKC into otherwise-untreated mice induces the maximal level of footpad swelling when the mice are footpad tested with CneF (Murphy, unpublished). Consequently, each animal in the HKC-CFA- and HKC-immunized groups received a total of 107 HKC. For negative control animals, mice were injected with saline-CFA or saline and otherwise were treated the same as the immunized mice.
Assessment of the anticryptococcal DTH response.
Seven days after immunization, the left hind footpad was injected with 30 μl of saline and the right hind footpad was injected with 30 μl of CneF (3.18 μg of protein and 207 μg of carbohydrate). The footpads were measured with a spring-loaded caliper before injection and 24 h after injection of saline or CneF. The increase in footpad swelling was calculated by subtracting the difference in swelling between the 0- and 24-h measurements of the saline-injected footpads from the difference in swelling between the 0- and 24-h measurements of the CneF-injected footpads.
Anti-CTLA-4 antibody treatment.
To boost the DTH response of immunized mice, the animals were injected intraperitoneally with 100 μg of hamster anti-mouse CTLA-4 immunoglobulin G (IgG; clone UC10-4F10-11) in 0.4 ml of saline on the day before immunization or saline-CFA or saline treatment (33). As controls, mice in each group were injected with hamster IgG (Cappel, Weschester, Pa.) in place of the anti-CTLA-4 IgG. The anti-CTLA-4 IgG or control IgG treatment was continued every day after initiation until 0.7 mg of anti-CTLA-4 IgG or control IgG was given. On the second day of anti-CTLA-4 IgG or IgG treatment, the mice were immunized with either 107 HKC or CneF-CFA or given saline-CFA or saline as described above. Sponges were implanted in the mice, removed, and processed as described below. Single-cell suspensions made from the sponges were stained for flow cytometry as described below.
Sponge implantation and injection with antigen.
Gelatin sponges (Gelfoam sterile absorbable gelatin sponge; Upjohn, Kalamazoo, Mich.) were surgically implanted under aseptic conditions (5). Briefly, the sponges were cut into 17- by 18- by 10-mm blocks before being rehydrated with sterile Hank's balanced salt solution (HBSS) containing 100 U of penicillin/ml and 100 mg of streptomycin/ml. Three days after immunization or control treatment, the mice were anesthetized, and two sponges per animal were implanted s.c. through an incision on the animal's shaved back. On day 4 after implantation, one sponge in each mouse was injected with 0.1 ml of CneF and the other was injected with 0.1 ml of saline.
Sponge removal and disaggregation.
The mice were euthanized before the sponges were removed. Fluid was expressed from the sponges and stored at −70°C until it was used for cytokine and nitrite analysis. Individual sponges were placed into Stomacher bags (Tekmar, Cincinnati, Ohio) with an enzyme solution (400 U of collagenase/ml; Sigma, St. Louis, Mo.) and then homogenized with three 10-s pulses on a Stomacher 80 Lab Blender (Tekmar) at 15-min intervals (14, 15). During the 15-min intervals, the sponge homogenates were incubated at 37°C. After the sponges were disaggregated, the sponge homogenates were filtered through 390-μm-pore-size nylon screens followed by passage through 140-μm-pore-size nylon screens and washed extensively with HBSS. Red blood cells in the cell preparations were lysed by treatment with Tris-NH4Cl (17 mM Tris, 139.7 mM NH4Cl), and the remaining sponge cells were washed with HBSS. Viable cells were counted with a hemacytometer using the trypan blue dye exclusion method.
Differential analysis of sponge-infiltrating cells.
After the sponge cells were counted and resuspended in RPMI 1640 medium plus 10% fetal bovine serum to 1.5 × 105 cells/ml, 0.2 ml of the cell suspension was cytocentrifuged onto clean glass slides. The cells were stained with Harleco Wright Stain (EM Diagnostic Systems, Gibbstown, N.Y.) followed with Diff-Quick solutions I and II (Baxter, Miami, Fla.) and visualized by light microscopy. To determine the percentage of neutrophils, macrophages, and lymphocytes present in the sponges in each treatment group, each leukocyte subset was counted in at least 200 cells per sample.
Flow cytometric analyses of surface markers on sponge-infiltrating cells.
To determine the type of leukocytes infiltrating the sponges 24 h after sponge injection, cells were either double-stained with anti-CD4-fluorescein isothiocyanate (FITC; Caltag) and anti-CD45RB-phycoerythrin (PE; Caltag) or with fluorochrome-labeled, isotype-matched controls to detect activated T lymphocytes (30, 32); triple-stained with CD4-PE (Caltag), F4/80-TriColor (Caltag), and GR-1-FITC (Caltag) or with the fluorochrome-labeled isotype-matched control antibodies to detect CD4+ lymphocytes, macrophages (F4/80+ cells), and granulocytes (GR-1+ cells); or triple-stained with GR-1-APC (B-D Pharmingen, San Diego, Calif.), TNF receptor I (TNFRI) monoclonal antibody (B-D Pharmingen) followed by anti-hamster IgG labeled with TriColor (Caltag), and TNFRII-PE (Caltag) to detect granulocytes expressing TNFRI or TNFRII. After disaggregation of the sponges, cells from each sponge were counted and resuspended in HBSS at approximately 107/ml. One hundred microliters of the cell suspension was plated into wells of U-bottom 96-well microtiter plates. Cells collected from the sponges were treated with anti-CD16/32 (HB197; American Type Culture Collection, Manassas, Va.) for 30 min on ice to block Fc receptors. The cells were then washed in wash buffer (phosphate-buffered saline, 0.1% NaN3, and 0.1% bovine serum albumin). After the wash buffer was removed, fluorochrome-labeled antibodies were added to the cells, and the mixture was incubated for 30 min on ice in the dark. The labeled cells were washed twice with wash buffer and fixed with 1% paraformaldehyde in phosphate-buffered saline plus 0.1% NaN3. After being fixed and filtered through an 80-μm-pore-size nylon screen, 50,000 to 100,000 cells were analyzed with a FACSCalibur flow cytometer and WinMDI 2.8 software. The percentage of fluorochrome-positive cells in each sponge was multiplied by the total number of cells recovered from the sponge to get the total number of fluorochrome-positive cells.
RNase protection assay.
To measure the relative amounts of IFN-γ or TNF-α mRNA in the sponges, the sponges were removed from the immunized mice 18 h after injection of CneF or saline into the sponges. Total RNA was isolated from each sponge using TriReagent (Molecular Research Center, Cincinnati, Ohio) and frozen at −70°C until it was assayed. The RNase protection assay (RiboQuant Multi Probe RNase Protection Assay; PharMingen, San Diego, Calif.) was performed according to the manufacturer's instructions. Briefly, 10 μg of total RNA was hybridized for 12 to 16 h at 56°C with [32P]UTP-labeled riboprobes generated from a murine template, CK-3b (PharMingen). Unhybridized RNA was digested with RNase A and T1. The RNases were then digested by proteinase K treatment. Protected RNA (RNA to which the probe bound) was isolated by phenol-chloroform extraction and sodium acetate-ethanol precipitation. Samples were electrophoresed on a 6% acrylamide-7 M urea gel. The densities of bands were quantified using a PhosphorImager (Storm 840; Molecular Dynamics, Sunnyvale, Calif.) and analyzed using ImageQuant 4.0 software. The sponge RNA was normalized to L32 (ribosomal subunit protein) RNA.
IFN-γ ELISA.
The levels of IFN-γ in fluid obtained from sponges were determined by enzyme-linked immunosorbent assay (ELISA) using commercially available paired monoclonal antibodies for IFN-γ (PharMingen) according to our previously described method (40). As a standard and positive control, dilutions of recombinant mouse IFN-γ (Genzyme, Cambridge, Mass.) were used. The minimal detectable limit for the assay was 128 pg/ml, and the upper limit of detection was 4,000 pg/ml.
NO assessment with the nitrite assay.
The levels of nitrite as an indicator of the NO concentrations in the sponge fluids were measured according to the protocol of Ding et al. (13) as modified by Moshage et al. (37). Initially, we measured nitrite in the sponge fluid with and without nitrate reductase in the assay, and we found no differences in the results. Consequently, in all subsequent assays with sponge fluid, we did not include nitrate reductase. Briefly, the assay was done as follows: zinc sulfate (1 M) was added to fluid obtained from the sponges to equal a 1/20 (vol/vol) ratio of zinc sulfate to sponge fluid to precipitate the protein. After centrifugation at 1,000 × g for 15 min at room temperature, 50 μl of the supernatant that contained the nitrite was removed and added to the reaction tube. To the 50 μl of supernatant was added 50 μl of Griess reagent (a 1:1 mixture of 2% sulfanilamide and 5% H3PO4 solution to 0.2% naphthylethylenediamine dihydrochloride solution). The samples were incubated for 10 to 20 min at room temperature and read at 570 nm on a spectrophotometer. Sodium nitrite (Mallinckrodt Chemical Works, St. Louis, Mo.) was used as a standard. The assay had a minimal detectable limit of 1.56 μM nitrite. Supernatants from lipopolysaccharide-stimulated RAW 264.7 macrophages served as a positive control for the assay.
Data presentation and statistical analysis.
Data collected from the saline-injected footpad or sponge was subtracted from the data collected from the CneF-injected footpad or sponge for each mouse, and then the means and standard errors of the means (SEM) were determined for each group. Analysis of variance with a Newman-Keuls posttest was used to analyze the data. A P value of ⩽0.05 was considered to be significant.
RESULTS
Footpad swelling responses and cellular infiltrates into sponges in mice immunized with the protective immunogen or the nonprotective immunogen.
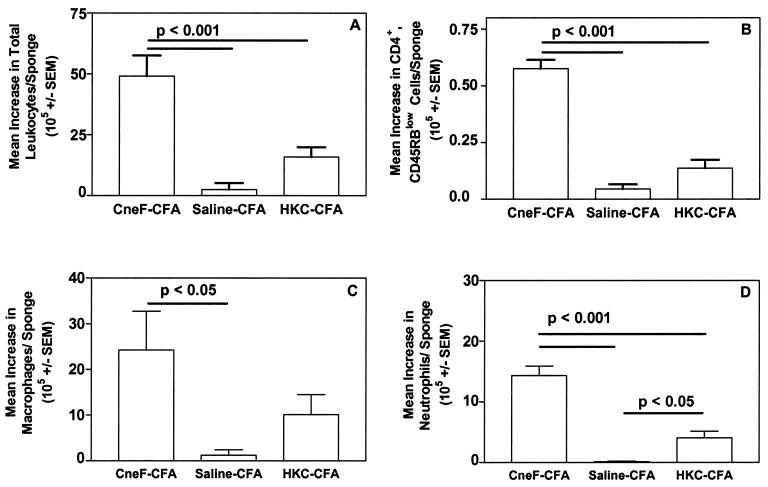
Eight days after immunization with the protective immunogen, CneF-CFA, mice had an average footpad thickness response of 22 × 10−3 ± 1.1 × 10−3 in. to CneF, whereas 8 days after immunization with the nonprotective immunogen, HKC-CFA, mice had an average footpad swelling response of 11 × 10−3 ± 1.0 × 10−3 in. to CneF. Mice treated with saline-CFA as a control had a mean footpad thickness in response to CneF of 1.0 × 10−3 ± 1.0 × 10−3 in. In previous studies in which we have made similar observations, the CneF-CFA-immunized mice survived a challenge infection with C. neoformans significantly longer than did HKC-CFA- or HKC-immunized mice or saline-CFA- or saline-treated mice (4, 33, 42). The reduced level of footpad swelling in response to CneF in mice immunized with HKC compared to that in mice immunized with CneF-CFA was not the cause of the lack of protection against a C. neoformans challenge infection (33). The footpad swelling responses induced by HKC could be augmented to the level of footpad swelling responses in the CneF-CFA-immunized mice by treating the HKC-immunized mice with anti-CTLA-4, yet the mice with elevated responses were no more protected against a C. neoformans infection than saline- or saline-CFA-treated mice (33). The leukocyte populations infiltrating into C. neoformans-infected tissues are the cells responsible for eliminating the cryptococci, and those leukocytes would be expected to be similar to the leukocyte populations infiltrating into CneF-injected sponges. Considering that CneF-CFA induces a protective response and HKC-CFA or HKC does not (4, 33, 41), we predicted that the leukocyte numbers and populations infiltrating the DTH-reactive sponges in the mice given the protective immunogen, CneF-CFA, and the nonprotective immunogen, HKC-CFA or HKC, would differ. We found that the total number of leukocytes infiltrating the DTH-reactive sponges in the mice immunized with the protective immunogen, CneF-CFA, were significantly greater than the total numbers of leukocytes infiltrating the DTH-reactive sponges in the mice immunized with the nonprotective immunogen, HKC-CFA, or infiltrating the CneF-injected sponges in mice treated with saline-CFA (P < 0.001) (Fig. 1A). The numbers of leukocytes in the sponges in the HKC-CFA- and saline-CFA-treated mice were not significantly different from one another at the 95% confidence level, even though we routinely observed a higher mean number of cells in the CneF-injected sponges from the HKC-CFA-immunized mice than in antigen-injected sponges from the saline-CFA-treated mice.
FIG. 1.
CneF-injected sponges in mice immunized with the protective immunogen, CneF-CFA, contained significantly higher numbers of leukocytes (A), activated CD4+ T cells (B), macrophages (C), and neutrophils (D) than CneF-injected sponges in mice immunized with the nonprotective immunogen, HKC-CFA. On day 0, mice were immunized with CneF-CFA (protective immunogen), saline-CFA (control immunogen), or HKC-CFA (nonprotective immunogen). Two sponges were implanted s.c. into the back of each mouse on day 3. On day 7, one sponge was injected with saline and the other was injected with CneF. Sponge cells were recovered 24 h after injection, and the total number of viable leukocytes per sponge (A) was determined by hemacytometer counts using trypan blue dye exclusion. The number of activated CD4+ T cells (B) was determined by flow cytometric analysis, and the numbers of macrophages (C) and neutrophils (D) were determined by differential counts. The data shown were derived by subtracting the number of cells in the saline-injected sponge from the number of cells in the CneF-injected sponge for each animal and calculating the mean of the difference in cells and the SEM for each group of mice. Data from two independent experiments were combined, with three mice per group per experiment. Where there are significant differences, the bars indicate the groups compared. P values are shown above the bars.
It is well established that classical DTH reactions in other antigen systems contain predominately mononuclear cells consisting of activated CD4+ T cells, naive T lymphocytes, and macrophages (49). In the mouse model, it is common to observe neutrophils in addition at DTH reaction sites (11). Although we have previously shown by differential counts that lymphocytes, monocytes, and neutrophils are significantly increased in CneF-injected sponges in mice immunized with CneF-CFA compared to equivalent cell populations in control sponges (4, 6, 15, 16), we have not evaluated the subpopulations of leukocytes in sponges in HKC-CFA-immunized mice.
Considering that activated CD4+ T cells are pivotal cells in classical DTH responses (10), we first determined the numbers of T cells that were CD4+ and that had a phenotypic marker characteristic of activated T cells. The density of CD45RB is known to be low on activated CD4+ T cells (30, 32), so we assessed the numbers of CD45RBlow CD4+ cells as an indicator of activated cells. We found that the mean number of activated CD4+ T cells in the DTH-reactive sponges in CneF-CFA-immunized mice was significantly greater than in the antigen-injected sponges of saline-CFA- or HKC-CFA-immunized mice (P < 0.001) (Fig. 1B). The numbers of activated CD4+ T cells in the antigen-injected sponges in HKC-CFA-immunized mice were not significantly different from the numbers of activated CD4+ T cells in antigen-injected sponges in saline-CFA-treated mice (Fig. 1B).
Macrophages, determined by differential analysis, in the DTH-reactive sponges from mice immunized with CneF-CFA were present in significantly greater numbers than in the saline-CFA- or the HKC-CFA-treated groups (P < 0.05) (Fig. 1C). DTH-reactive sponges in the mice immunized with HKC-CFA did not contain significantly more macrophages than the antigen-injected sponges in the control mice (Fig. 1C).
As we had observed before in CneF-CFA-immunized mice 24 h after injection of sponges with CneF (5, 6, 15, 16), neutrophils, enumerated by differential analysis, were significantly increased in DTH-reactive sponges over the numbers of neutrophils in sponges in saline-CFA-immunized control mice (P < 0.001) (Fig. 1D). Neutrophil populations were significantly increased in the CneF-injected sponges in the HKC-CFA-immunized mice over the neutrophil numbers in the CneF-injected sponges in mice treated with saline-CFA (P < 0.05); however, the neutrophil numbers in the CneF-injected sponges in HKC-CFA-immunized mice were significantly lower than in the DTH-reactive sponges in mice immunized with CneF-CFA (P < 0.001) (Fig. 1D).
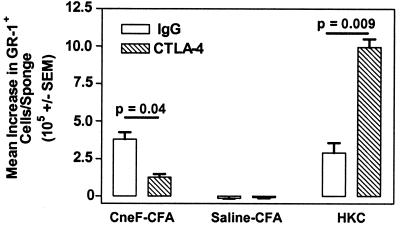
Blockade of CTLA-4 in HKC-immunized mice did not induce an increase in protection even though it boosted the footpad reactivity to CneF (33). To evaluate whether blockade of CTLA-4 changed the leukocyte populations infiltrating into the CneF-injected sponges, we treated mice with anti-CTLA-4 IgG or hamster IgG as a control during the induction phase of the anticryptococcal immune response in mice treated with HKC or CneF-CFA. Groups of control mice injected with either saline or saline-CFA in place of the immunogen and treated with anti-CTLA-4 IgG or hamster IgG were included. In this experiment, and as we have previously published (33), blocking of CTLA-4 boosted the DTH responses compared to those in the IgG-treated mice (data not shown), but anti-CTLA-4 IgG treatment compared to IgG treatment of mice had no effect on the numbers of CD4+ cells or F4/80+ cells (mainly macrophages) that infiltrated into the CneF-injected sponges in mice immunized with HKC (data not shown). In contrast, the numbers of granulocytes (GR-1+ cells) that infiltrated the CneF-injected sponges in the HKC-immunized mice treated with anti-CTLA-4 IgG were significantly increased compared to the numbers of granulocytes that infiltrated the CneF-injected sponges in mice immunized with HKC and treated with control IgG (P = 0.009) (Fig. 2). Blockade of CTLA-4 had a different effect on the numbers of granulocytes infiltrating the DTH-reactive sponges in CneF-CFA-immunized mice. The numbers were reduced from control levels in the DTH-reactive sponges in the mice that were given the protective immunogen (P = 0.04) (Fig. 2). We have previously shown that CneF-CFA-immunized mice treated with anti-CTLA-4 IgG display increased protection against a challenge with viable C. neoformans compared to the IgG-treated, CneF-CFA-immunized controls (33).
FIG. 2.
Blockade of CTLA-4 at the time of immunization with HKC results in a significant increase in the numbers of neutrophils that infiltrate the CneF-injected sponges. The experimental design is similar to that described in the legend to Fig. 1, but the mice in this experiment were treated intraperitoneally with either anti-CTLA-4 IgG or control IgG 1 day before immunization, the day of immunization, and days 1 through 5 after immunization with HKC, CneF-CFA, or saline-CFA. Cells were collected from the sponges and stained with GR-1-FITC 24 h after injection of the sponges with saline or CneF. Flow cytometric analysis was done on the cells collected from the sponges.
IFN-γ levels in DTH-reactive sponges in mice immunized with the protective or the nonprotective immunogen.
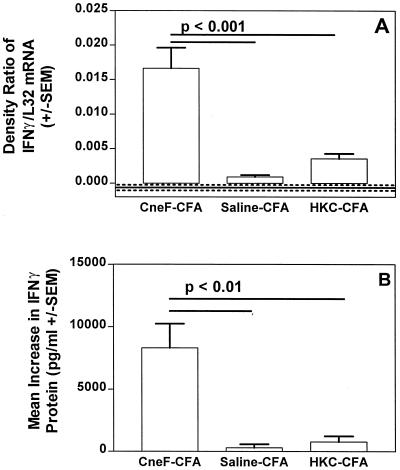
When activated T cells encounter the antigen that induced them, they are stimulated to produce cytokines, such as IL-2 and IFN-γ (3, 38). Having found that we had large numbers of activated CD4+ T cells in the DTH-reactive sponges in the CneF-CFA-immunized mice compared to the numbers in the DTH-reactive sponges in the HKC-CFA-immunized mice, we predicted that the IFN-γ levels in the antigen-injected sponges from the two different treatment groups would be different. To assess this, we measured both the level of IFN-γ mRNA in the sponge cells 18 h after antigen or saline injection into the sponges and the concentration of IFN-γ protein in fluid taken from the sponges 24 h after injection of the sponges with antigen or saline. As we predicted, the DTH-reactive sponges in the mice given the protective immunogen, CneF-CFA, had significantly higher levels of IFN-γ mRNA (P < 0.001) and protein (P < 0.01) than those found in the DTH-reactive sponges in HKC-CFA-immunized mice or the CneF-injected sponges in the saline-CFA-treated mice (Fig. 3).
FIG. 3.
CneF-injected sponges in mice immunized with the protective immunogen, CneF-CFA, contained significantly higher levels of IFN-γ mRNA and significantly higher concentrations of IFN-γ protein than CneF-injected sponges in mice immunized with the nonprotective immunogen, HKC-CFA. The experimental design was similar to that described in the Fig. 1 legend. (A) Sponges were removed 18 h after injection of antigen or saline, and RNA was extracted as described in Materials and Methods. The bars show the ratio of IFN-γ to L32 for the antigen-injected sponges. The error bars indicate the SEM. The solid horizontal line shows the mean ratio of IFN-γ to L32 for the saline control sponges, and the dotted lines indicate the SEM. (B) Sponges were removed 24 h after antigen or saline injection, and fluid was collected from each sponge for IFN-γ protein measurements by ELISA. The values shown are the mean values derived from each group by substracting the value of the saline-injected sponge from the value of the CneF-injected sponge for each mouse in the group and then calculating the mean. Where there are significant differences, horizontal bars indicate the groups compared. The P values are shown above the bars.
TNF-α mRNA levels in DTH-reactive sponges in mice immunized with the protective or nonprotective immunogens.
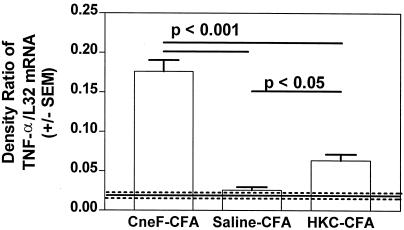
IFN-γ and TNF-α have been shown to activate the cytotoxicity of neutrophils (51, 52), so we also assessed the level of mRNA for TNF-α in the sponges. RNase protection studies of the RNA samples from the sponges showed as expected that DTH-reactive sponges from the mice immunized with the protective immunogen contained significantly more message for TNF-α than the CneF-injected sponges from saline-CFA-treated mice (P < 0.001) (Fig. 4). Although TNF-α mRNA levels in the DTH-reactive sponges in the CneF-CFA-immunized mice were significantly higher than the TNF-α mRNA levels in the CneF-injected sponges in mice immunized with the nonprotective immunogen (P < 0.001), the TNF-α mRNA levels in the reactive sponges in mice given the nonprotective immunogen were significantly greater than TNF-α mRNA levels in sponges in saline-CFA-treated control mice (P < 0.05) (Fig. 4). The levels of TNF-α protein that we measured by ELISA in sponges from the three treatment groups of mice (data not shown) paralleled the TNF-α mRNA levels shown in Fig. 4.
FIG. 4.
CneF-injected sponges in mice immunized with the protective immunogen, CneF-CFA, contained significantly higher levels of TNF-α mRNA than CneF-injected sponges in mice immunized with the nonprotective immunogen, HKC-CFA. The experimental design was similar to that described in the Fig. 1 legend. The sponges were removed 18 h after injection of antigen or saline, and RNA was extracted as described in Materials and Methods. The data are expressed in the same manner as described in the Fig. 3A legend. Where there are significant differences, bars indicate the groups compared. The P values are shown above the bars.
NO in DTH-reactive sponges in mice immunized with the protective or nonprotective immunogens.
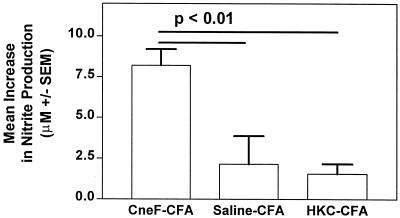
IFN-γ can stimulate macrophages to become more active and more effective killers of microorganisms (26). Macrophages activated with IFN-γ have been shown to kill C. neoformans more effectively than nonactivated macrophages (18, 36). IFN-γ-activated macrophages produce more NO than nonactivated macrophages (13), and NO can kill C. neoformans (2). Consequently, when IFN-γ and macrophages are in the same vicinity, as they are in the DTH-reactive sponges in mice immunized with CneF-CFA, one might expect to have activated macrophages that are capable of producing high levels of NO. In contrast, one would predict that in the DTH-reactive sponges in HKC-CFA-immunized mice, where macrophage numbers and IFN-γ levels are not significantly increased over controls, the levels of NO would be equivalent to control levels. To assess whether this line of reasoning would hold true, we measured nitrite as an indicator of NO production in the sponge fluids 24 h after antigen or saline injection. We found, as expected, that the DTH-reactive sponges (CneF-injected sponges in CneF-CFA-immunized mice) which also had increased numbers of macrophages and IFN-γ levels above control levels (CneF-injected sponges in saline-CFA-treated mice) had significantly higher levels of nitrite than the sponges that had lower numbers of macrophages and lower levels of IFN-γ (CneF-injected sponges in saline-CFA-treated mice or CneF-injected sponges in HKC-CFA-treated mice) (P < 0.01) (Fig. 5).
FIG. 5.
CneF-injected sponges in mice immunized with the protective immunogen, CneF-CFA, contain significantly higher concentrations of NO than CneF-injected sponges in mice immunized with the nonprotective immunogen, HKC-CFA. The experimental design was the same as that described in the Fig. 1 legend. The sponges were removed 24 h after injection with CneF or saline, and the fluid was collected from each sponge for nitrite measurements. Where there are significant differences, bars indicate the groups compared. The P values are shown above the bars.
Densities of TNFRI and TNFRII on granulocytes in DTH-reactive and control sponges in mice immunized with HKC-CFA.
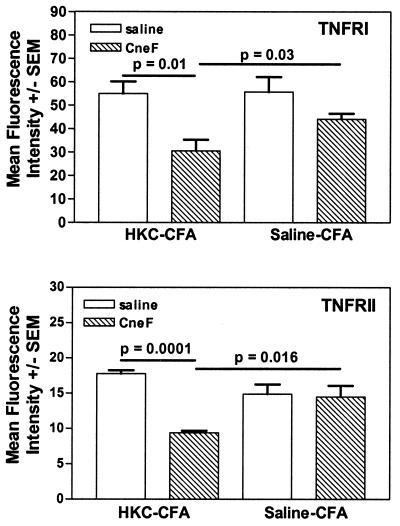
Neutrophils, which make up the major component of the granulocyte population, can kill C. neoformans (8, 12), and neutrophils subjected to IFN-γ and TNF-α have been shown to have increased cytotoxicity (51, 52). Considering these facts and the fact that there is an increase in neutrophils and TNF-α at the site of antigen injection in mice immunized with HKC-CFA (Fig. 1D and Fig. 4, respectively), one would expect clearance of C. neoformans to be enhanced in mice immunized with HKC-CFA. Yet this was not the case (4, 33, 42). We have previously found that mice immunized with HKC-CFA show no better protection against a challenge with viable C. neoformans than saline-CFA-treated control mice, and in some cases, cryptococcosis in HKC-CFA-immunized mice was exacerbated compared to that in control mice (4, 33, 42), suggesting the granulocytes infiltrating into an infection site are not very active against C. neoformans. TNF-α would be expected to activate neutrophils through TNFRI and/or TNFRII; therefore, we determined the densities of TNFRI and TNFRII on the GR-1+ cells (granulocytes, which in this case are predominantly neutrophils, as shown by differential analysis) in the sponges from mice immunized with HKC-CFA and in sponges from mice treated with saline-CFA. As we had observed in Fig. 1, the only leukocyte population significantly increased over controls was the granulocyte population (GR-1+ cells) (data not shown). The percentages of TNFRI- or TNFRII-positive granulocytes (GR-1+ cells) in the saline-injected and CneF-injected sponges were similar; however, the fluorescence intensities of TNFRI and TNFRII on the GR-1+ cells were reduced in the CneF-injected sponges compared to the fluorescence intensities of TNFRI and TNFRII, respectively, on GR-1+ cells in the saline-injected sponges (Fig. 6, top panel and bottom panel, respectively). Furthermore, the fluorescence intensities of TNFRI and TNFRII on GR-1+ cells in the CneF-injected sponges from HKC-CFA-immunized mice were significantly less than the fluorescence intensities of TNFRI and TNFRII, respectively, in the CneF-injected sponges from saline-CFA-treated mice (P = 0.03 for TNFRI [Fig. 6, top panel]; P = 0.016 for TNFRII [Fig. 6, bottom panel]).
FIG. 6.
Granulocytes in the CneF-injected sponges in HKC-CFA-immunized mice have less TNFRI and TNFRII on their surfaces than do granulocytes in CneF-injected sponges in saline-CFA-treated mice. The experimental design was similar to that described in the Fig. 1 legend. The sponges were removed 24 h after injection with CneF or saline. The sponge cells were collected and stained with GR-1-APC, anti-TNFRII-PE, and hamster anti-TNFRI, followed by anti-hamster IgG labeled with TriColor. The mean fluorescence intensity of the label on TNFRI or TNFRII was assessed by flow cytometric analysis. Where there are significant differences, bars indicate the groups compared. The P values are shown above the bars.
DISCUSSION
Our data show that the profile of components at the DTH reaction site in the mice immunized with the nonprotective immunogen is different than the profile of components at the DTH reaction site in mice given the protective immunogen. Although we obtained a delayed swelling response in antigen-injected footpads of mice immunized with the nonprotective immunogen, there was no evidence of the presence of a classical DTH response in those mice. In support of this statement, activated CD4+ T cells and macrophages, two cell populations that characterize a classical DTH response, were not elevated significantly above control levels in the antigen-injected sponges in the mice immunized with HKC-CFA. Furthermore, IFN-γ, a cytokine that is typically found at the site of a classical DTH response, was not present at the time of peak responsiveness in the antigen-reactive sponges in mice immunized with the nonprotective immunogen. These results contrast with the results from the DTH-reactive sponges in mice that received the protective immunogen. As we have previously observed and have confirmed here, components of a classical DTH response, i.e., lymphocytes, macrophages, and IFN-γ, appeared in the DTH-reactive sponges in mice given the protective immunogen.
The only leukocyte type that was significantly elevated above control levels in the antigen-reactive sponges in mice immunized with the nonprotective immunogen was the neutrophil population. Furthermore, when we augmented the HKC-induced anticryptococcal DTH response by blocking the down-regulator, CTLA-4, on activated T cells, we found that in the antigen-injected sponges only granulocyte numbers were elevated above control levels. Based on our observations of differential slides, neutrophils are by far the predominant leukocyte population in the CneF-injected sponges in HKC- or HKC-CFA-immunized mice. We used a stain for the differentials that would stain eosinophils; however, the number of eosinophils observed was low. Although GR-1 stains all granulocytes, the GR-1+ cells in these specimens are almost all neutrophils by their appearance in differential analysis. The numbers of infiltrating lymphocytes and macrophages, two cell populations expected in a classical CMI response, are no greater in the CneF-injected sponges in HKC-immunized mice than in the antigen-injected sponges in mice treated with saline or saline-CFA. The augmented anticryptococcal DTH response induced in mice immunized with HKC and treated with anti-CTLA-4 is not associated with enhanced protection against C. neoformans (33), so it seems that the elevated numbers of neutrophils that would be expected to be at the sites of cryptococcal antigen or organisms in the HKC-immunized mice are not sufficiently capable of limiting the growth of the organism or killing it to affect survival of the animals.
Considering that CTLA-4 is found on T lymphocytes (19), it seems logical to speculate that CTLA-4+ T cells have either a direct or indirect role in the DTH response in the mice immunized with the nonprotective immunogen. There is also other evidence that T cells are involved in the anticryptococcal DTH response in mice immunized with HKC. Mody et al. (35) reported that both CD4+ and CD8+ T cells contribute to the DTH response in mice immunized with HKC, and we have confirmed this (Murphy, unpublished). Muth and Murphy (43, 44) showed that HKC immunization of mice increases the direct inhibitory activity of T cells against C. neoformans. Results from studies of the induction phase of the anticryptococcal immune response induced by HKC-CFA show no statistically significant increases in activated CD4+ T cells above control levels in the lymph nodes draining the HKC-CFA immunization site, as well as no statistically significant increases in CD4+ T cells infiltrating the DTH-reactive sponges in mice immunized with the nonprotective immunogen (4). The latter was confirmed here (Fig. 1). It is likely that activated T cells are induced by HKC-CFA or HKC, but their numbers are below detectable levels. The evidence supporting the involvement of T cells in the anticryptococcal DTH response in HKC-immunized mice is substantial and leads one to speculate that T cells do play a role in the immune response in HKC-immunized mice but that their function differs from that of the activated T cells detectable in mice immunized with the protective immunogen. It seems unlikely that the T cells induced by HKC are Th2 cells, because we have not been able to measure anticryptococcal antibodies in mice immunized with HKC (42) and we have not been able to detect Th2 cytokines in the DTH-reactive sponges (Murphy, unpublished). It is possible that an undetectably small number of activated T cells infiltrate into the CneF-injected sponges in the HKC- or HKC-CFA-immunized mice and that those cells influence the cytokines, chemokines, and/or vascular adhesion molecules expressed at the site of antigen. These components could influence the cells that migrate into the antigen site. Further investigations are needed to establish and define a role for T cells in this nonprotective response.
The proinflammatory cytokine TNF-α was significantly elevated above control levels in the antigen-injected sponges in mice that had been immunized with the nonprotective immunogen. The elevated numbers of neutrophils with the increased levels of TNF-α would be expected to result in some protection against C. neoformans, because TNF-α can activate neutrophils to become more cytotoxic (51, 52), but protection is not elevated in HKC-immunized mice (4, 33, 42). The reason for this lack of activation of the neutrophils by TNF-α and thus the lack of protection in the HKC-CFA-immunized mice is, at least in part, due to the fact that the densities of TNF receptors, TNFRI and TNFRII, through which TNF-α would stimulate the neutrophils to become activated, were significantly reduced on the surfaces of the granulocytes in the antigen-injected sponges in HKC-CFA-immunized mice compared to granulocytes in control sponges (saline-injected sponges from the HKC-CFA-immunized mice or antigen-injected sponges from the saline-CFA-treated mice).
We and others have found that protection against C. neoformans is associated with one or more of the following: an increase in activated CD4+ T cells, IFN-γ production, TNF-α production, activated macrophages, and the presence of NO (1, 2, 4, 7, 18, 20-23, 27-29, 34, 36, 40, 50, 55). In the present study, we have demonstrated that the DTH reaction site in mice immunized with CneF-CFA, the protective immunogen, has all of these components, plus elevated numbers of lymphocytes and macrophages, the cell types associated with a classical DTH response (11, 54). Clearance of cryptococci from lungs and brain is observed when levels of lymphocytes, macrophages, IFN-γ, and NO are increased in those tissues (1, 5, 6, 15, 16, 20-23, 28, 29). Our data on events at the DTH reaction site combined with data of others from clearance of cryptococci from lungs and brains suggest that the following cascade of events occurs in the protective immune response. For induction of a protective immune response against C. neoformans, the antigen must stimulate the appropriate populations of dendritic cells, most likely myeloid dendritic cells, to migrate to lymph nodes and develop into mature antigen-presenting cells, which activate CD4+ T cells (4). Upon restimulation with cryptococcal antigens, cytokines and chemokines are produced at the site of antigen, and neutrophils, lymphocytes, and macrophages migrate into the DTH reaction site (6, 7, 15, 16). The IFN-γ and TNF-α that are produced in large amounts at the DTH reaction site would most likely activate the macrophages, which would then have a greater capability to produce reactive nitrogen (NO) and oxygen intermediates (26). Neutrophils may also be activated by IFN-γ and TNF-α to kill more effectively (51, 52). The presence of NO in the sponges indicates that the macrophages in the vicinity were indeed activated. Because C. neoformans is sensitive to NO and oxygen intermediates, the activated neutrophils and macrophages would reduce the numbers of the organisms, and the life expectancies of the animals would thus be extended.
It is clear from our studies and those of others that not all immunogens that induce a DTH response also induce a protective immune response (9, 10, 24, 31, 42, 46, 47, 53). An example of this uncoupling of the DTH response and protection is evident in the cryptococcosis model with CneF-CFA and HKC-CFA or HKC alone as immunogens (4, 33, 41). Here, we show that the infiltrating cells and the levels of cytokines and reactive nitrogen molecules at the DTH reaction sites are different in mice immunized with the protective and the nonprotective immunogens, despite the fact that both immunogens induce a delayed swelling response upon injection of specific antigen. Our findings emphasize the importance, at least in the cryptococcosis model, of establishing whether an antigen induces a classical cell-mediated immune response by determining the cell types and cytokines present at the DTH reaction site before predicting whether the response is protective. DTH and protection have been dissociated as well in other infectious-disease systems, such as listeriosis and tuberculosis models; however the mechanisms behind the dissociation are different in the different models (9, 10, 24, 31, 46, 47, 53). For example, the uncoupling of DTH and protection in the immune response to Listeria monocytogenes results from the fact that activated CD4+ cells are responsible for the DTH reaction and CD8+ cells mediate protection (45). It is also of interest to note that the T cells induced by heat-killed Listeria mediate DTH responses but not protection (31). It might be assumed that the DTH responses in the animals immunized with heat-killed Listeria are classical responses, because they are mediated by CD4+ cells (31). If that is the case, the nonprotective DTH response in Listeria would be different than the DTH response we have induced with HKC. In the Mycobacterium model, the mechanisms responsible for the uncoupling of the DTH response and protection are not completely understood (25). Granuloma formation, which is in many ways equivalent to DTH reaction development, is not necessary for protection against Mycobacterium tuberculosis (25). Both anti-M. tuberculosis protection and DTH responsiveness require activated CD4+ T cells, but protection differs from DTH responsiveness in that the former is mediated by IL-12 and IFN-γ whereas the DTH response is mediated by TNF and chemokines (48). Although CD4+ T cells are essential to the M. tuberculosis CMI response, other leukocytes, such as gamma-delta T cells, CD4+ NK cells, and CD8+ T cells, play a role in protection (26, 48). It appears that the protective mechanisms against Listeria and Mycobacterium, two intracellular bacterial pathogens, have both similarities and differences from the protective mechanism against C. neoformans, a yeast-like organism that mainly resides extracellularly. The differences and the fact that DTH can be dissociated from protection in all three situations, but for different reasons, emphasize the need to carefully define the protective and nonprotective responses of the host for each pathogen before attempting to develop immunoreplacement or immunomodulatory therapies or vaccines.
In summary, the protective anticryptococcal CMI response is associated with a classical DTH response consisting of (i) increased numbers of activated CD4+ T cells with increased production of IFN-γ upon restimulation with antigen and (ii) increased numbers of macrophages and neutrophils and elevated levels of NO compared to controls. Increased NO at the DTH reaction site in the mice immunized with the protective immunogen is a sign of the presence of activated macrophages, so enhanced killing of the organism would be expected to take place. In contrast, the nonprotective anticryptococcal DTH reaction is associated with an influx of granulocytes and elevated TNF-α levels. The granulocytes subjected to TNF-α do not appear to be activated to reduce the infection, because no protection is detectable in the mice in which such a reaction could occur. The lack of activation of the granulocytes by TNF-α could be due to the fact that granulocytes at the reaction site have reduced densities of TNFRI and TNFRII on their surfaces. Our results point to the fact that there can be delayed footpad swelling in response to the inducing antigen in immunized mice, but swelling is not indicative of a classical DTH reaction and is not indicative of protection. Furthermore, our results emphasize the need to define mechanisms of DTH and protection for each microbe.
Acknowledgments
We greatly appreciate the assistance of Jim Henthorn with the flow cytometric analyses. Flow cytometric analyses were performed at the Flow Cytometry and Cell Sorting Core Facility for Molecular Medicine and St. Francis Research Institute, University of Oklahoma Health Sciences Center.
This work was supported by Public Health Service grants AI-15716 and T32A107364 from the National Institutes of Health.
Editor: T. R. Kozel
REFERENCES
- 1.Aguirre, K., E. A. Havell, G. W. Gibson, and L. L. Johnson. 1995. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect. Immun. 63:1725-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alspaugh, J. A., and D. L. Granger. 1991. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect. Immun. 59:2291-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annie, T., T. Fong, and T. R. Mosmann. 1989. The role of IFNγ in delayed-type hypersensitivity mediated by Th1 clones. J. Immunol. 143:2887-2899. [PubMed] [Google Scholar]
- 4.Bauman, S. K., K. L. Nichols, and J. W. Murphy. 2000. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J. Immunol. 165:158-167. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan, K. L., and J. W. Murphy. 1993. Characterization of cellular infiltrates and cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect. Immun. 61:2854-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan, K. L., and J. W. Murphy. 1997. Kinetics of cellular infiltration and cytokine production during the efferent phase of a delayed-type hypersensitivity reaction. Immunology 90:189-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan, K. L., and J. W. Murphy. 1994. Regulation of cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect. Immun. 62:2930-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi, V., B. Wong, and S. L. Newman. 1996. Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J. Immunol. 156:3836-3840. [PubMed] [Google Scholar]
- 9.Chen-Woan, M., D. H. Sajewski, and D. D. McGregor. 1985. T cell cooperation in the mediation of acquired resistance to Listeria monocytogenes. Immunology 56:33-42. [PMC free article] [PubMed] [Google Scholar]
- 10.Cher, D. J., and T. R. Mosmann. 1987. Two types of murine T cell clones. II. Delayed-type hypersensitivity is mediated by Th1 clones. J. Immunol. 138:3688-3694. [PubMed] [Google Scholar]
- 11.Crowle, A. J. 1975. Delayed hypersensitivity in the mouse. Adv. Immunol. 20:197-264. [DOI] [PubMed] [Google Scholar]
- 12.Diamond, R. D., R. K. Root, and J. E. Bennett. 1972. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J. Infect. Dis. 125:367-376. [DOI] [PubMed] [Google Scholar]
- 13.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 14.Dong, Z. M., and J. W. Murphy. 1995. Intravascular cryptococcal culture filtrate (CneF) and its major component, glucuronoxylomannan, are potent inhibitors of leukocyte accumulation. Infect. Immun. 63:770-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle, H. A., and J. W. Murphy. 1997. MIP-1α contributes to the anticryptococcal delayed-type hypersensitivity reaction and protection against Cryptococcus neoformans. J. Leukoc. Biol. 61:147-155. [DOI] [PubMed] [Google Scholar]
- 16.Doyle, H. A., and J. W. Murphy. 1999. Role of the C-C chemokine, TCA3, in the protective anticryptococcal cell-mediated immune response. J. Immunol. 162:4824-4833. [PubMed] [Google Scholar]
- 17.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for the determination of sugars and related substances. Anal. Chem. 28:350-356. [DOI] [PubMed] [Google Scholar]
- 18.Flesch, I. E., G. Schwamberger, and S. H. Kaufmann. 1989. Fungicidal activity of IFN-γ-activated macrophages. Extracellular killing of Cryptococcus neoformans. J. Immunol. 142:3219-3224. [PubMed] [Google Scholar]
- 19.Harper, K., C. Balzano, E. Roouvier, M. G. Mattei, M. F. Luciani, and P. Golstein. 1991. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J. Immunol. 147:1037-1044. [PubMed] [Google Scholar]
- 20.Hoag, K. A., M. F. Lipscomb, A. A. Izzo, and N. E. Street. 1997. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am. J. Respir. Cell Mol. Biol. 17:733-739. [DOI] [PubMed] [Google Scholar]
- 21.Hoag, K. A., N. E. Street, G. B. Huffnagle, and M. F. Lipscomb. 1995. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am. J. Respir. Cell Mol. Biol. 13:487-495. [DOI] [PubMed] [Google Scholar]
- 22.Huffnagle, G. B. 1996. Role of cytokines in T cell immunity to a pulmonary Cryptococcus neoformans infection. Biol. Signals 5:215-222. [DOI] [PubMed] [Google Scholar]
- 23.Huffnagle, G. B., G. B. Toews, M. D. Burdick, M. B. Boyd, K. S. McAllister, R. A. McDonald, S. L. Kunkel, and R. M. Strieter. 1996. Afferent phase production of TNF-α required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 157:4529-4536. [PubMed] [Google Scholar]
- 24.Hussein, S., J. Curtis, H. Akuffo, and J. L. Turk. 1987. Dissociation between delayed-type hypersensitivity and resistance to pathogenic mycobacteria demonstrated by T-cell clones. Infect. Immun. 55:564-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, C. M., A. M. Cooper, A. A. Frank, and I. M. Orme. 1998. Adequate expression of protective immunity in the absence of granuloma formation in Mycobacterium tuberculosis-infected mice with a disruption in the intracellular adhesion molecule 1 gene. Infect. Immun. 66:1666-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufmann, S. 1998. Immunity to intracellular bacteria, p. 1335. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 27.Kawakami, K., S. Kohno, J. I. Kadota, M. Tohyama, K. Teruya, N. Kudeken, A. Saito, and K. Hara. 1995. T cell-dependent activation of macrophages and enhancement of their phagocytic activity in the lungs of mice inoculated with heat-killed Cryptococcus neoformans: involvement of IFN-γ and its protective effect against cryptococcal infection. Microbiol. Immunol. 39:135-143. [DOI] [PubMed] [Google Scholar]
- 28.Kawakami, K., M. Tohyama, K. Teruya, N. Kudeken, Q. Xie, and A. Saito. 1996. Contribution of interferon-γ in protecting mice during pulmonary and disseminated infection with Cryptococcus neoformans. FEMS Immunol. Med. Microbiol. 13:123-130. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami, K., X. Wifeng, M. Tohyama, M. H. Qureshi, and A. Saito. 1996. Contribution of tumour necrosis factor-alpha (TNF-α) in host defence mechanism against Cryptococcus neoformans. Clin. Exp. Immunol. 106:468-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearney, E. R., T. L. Walunas, R. W. Karr, P. A. Morton, D. Y. Loh, J. A. Bluestone, and M. K. Jenkins. 1995. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and is inhibited by CTLA-4. J. Immunol. 155:1032-1036. [PubMed] [Google Scholar]
- 31.Koga, T., M. Mitsuyama, T. Handa, T. Yayama, K. Muramori, and K. Nomoto. 1987. Induction by killed Listeria monocytogenes of effector T cells mediating delayed-type hypersensitivity but not protection in mice. Immunology 62:241-248. [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, W. T., X. M. Yin, and E. S. Vitetta. 1990. Functional and otogenic analysis of murine CD45Rhi and CD45Rlo CD4+ T cells. J. Immunol. 144:3288-3295. [PubMed] [Google Scholar]
- 33.McGaha, T., and J. W. Murphy. 2000. CTLA-4 down-regulates the protective anticryptococcal cell-mediated immune response. Infect. Immun. 68:4624-4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mody, C. H., M. F. Lipscomb, N. E. Street, and G. B. Toews. 1990. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J. Immunol. 144:1472-1477. [PubMed] [Google Scholar]
- 35.Mody, C. H., R. Paine III, C. Jackson, G. H. Chen, and G. B. Toews. 1994. CD8 cells play a critical role in delayed type hypersensitivity to intact Cryptococcus neoformans. J. Immunol. 152:3970-3979. [PubMed] [Google Scholar]
- 36.Mody, C. H., C. L. Tyler, R. G. Sitrin, C. Jackson, and G. B. Toews. 1991. Interferon-γ activates rat alveolar macrophages for anticryptococcal activity. Am. J. Respir. Cell Mol. Biol. 5:19-26. [DOI] [PubMed] [Google Scholar]
- 37.Moshage, H., B. Kok, J. R. Huizenga, and P. L. M. Jansen. 1995. Nitrite and nitrate determination in plasma: a critical evaluation. Clin. Chem. 41:892-896. [PubMed] [Google Scholar]
- 38.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 39.Murphy, J. W. 1999. Cell-mediated immunity and medically related fungi, p. 593-621. In M. W. Cunningham and R. S. Fujinami (ed.), Effects of microbes on the immune system. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 40.Murphy, J. W. 1993. Cytokine profiles associated with induction of the anticryptococcal cell-mediated immune response. Infect. Immun. 61:4750-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy, J. W. 1998. Protective cell-mediated immunity against Cryptococcus neoformans. Res. Immunol. 149:373-386. [DOI] [PubMed] [Google Scholar]
- 42.Murphy, J. W., F. Schafer, A. Casadevall, and A. Adesina. 1998. Antigen-induced protective and non-protective cell-mediated immune components against Cryptococcus neoformans. Infect. Immun. 66:2632-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muth, S. M., and J. W. Murphy. 1995. Direct anticryptococcal activity of lymphocytes from Cryptococcus neoformans-immunized mice. Infect. Immun. 63:1637-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muth, S. M., and J. W. Murphy. 1995. Effects of immunization with Cryptococcus neoformans cells or cryptococcal culture filtrate antigen on the direct anticryptococcal activities of murine T lymphocytes. Infect. Immun. 63:1645-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.North, R. J., P. L. Dunn, and J. W. Conlan. 1997. Murine listeriosis as a model of antimicrobial defense. Immunol. Rev. 158:27-36. [DOI] [PubMed] [Google Scholar]
- 46.Orme, I. M. 1988. The immune response to the cell wall of Mycobacterium bovis BCG. Clin. Exp. Immunol. 71:388-393. [PMC free article] [PubMed] [Google Scholar]
- 47.Orme, I. M. 1988. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance, in mice inoculated with killed mycobacterial vaccines. Infect. Immun. 56:3310-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orme, I. M. 2000. Tuberculosis: recent progress in basic immunity and vaccine development. Kekkaku 75:97-101. [PubMed] [Google Scholar]
- 49.Platt, J. L., B. W. Grant, A. A. Eddy, and A. F. Michael. 1983. Immune cell populations in cutaneous delayed-type hypersensitivity. J. Exp. Med. 158:1227-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salkowski, C., and E. Balish. 1991. A monoclonal antibody to gamma interferon blocks augmentation of natural killer cell activity induced during systemic cryptococcosis. Infect. Immun. 59:486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shalaby, M. R., B. B. Aggarwal, E. Rinderknecht, L. P. Svedersky, B. S. Finkle, and M. A. Palladino, Jr. 1985. Activation of human polymorphonuclear neutrophil functions by interferon-γ and tumor necrosis factors. J. Immunol. 135:2069-2073. [PubMed] [Google Scholar]
- 52.Steinbeck, M. J., J. A. Roth, and M. L. Kaeberle. 1986. Activation of bovine neutrophils by recombinant interferon-γ. Cell. Immunol. 98:137-144. [DOI] [PubMed] [Google Scholar]
- 53.Tsukada, H., I. Kawamura, M. Arakawa, K. Nomoto, and M. Mitsuyama. 1991. Dissociated development of T cells mediating delayed-type hypersensitivity and protective T cells against Listeria monocytogenes and their functional difference in lymphokine production. Infect. Immun. 59:3589-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turk, J. L. 1975. Delayed hypersensitivity, 2nd ed., vol. 4. Elsevier/North-Holland Biomedical Press, Amsterdam, The Netherlands.
- 55.Yuan, R. R., A. Casadevall, J. Oh, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc. Natl. Acad. Sci. USA 94:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]