Abstract
The mechanism of gamma interferon (IFN-γ) production induced by listeriolysin O (LLO), a cytolytic virulence factor of Listeria monocytogenes, was analyzed with special reference to the involvement of macrophage-derived cytokines in spleen cells of mice. LLO purified from the culture supernatant of L. monocytogenes was capable of inducing a high level of IFN-γ when its cytolytic activity was blocked by cholesterol treatment. The IFN-γ-inducing ability of LLO was not dependent on possibly contaminating lipopolysaccharide. Depletion of CD11b+ cells resulted in a profound decrease in IFN-γ production in response to LLO stimulation. Negative selection also suggested the contribution of DX5+ cells in IFN-γ production. Reverse transcription-PCR revealed that expression of interleukin-12 (IL-12) p35 and p40 was induced by LLO but that the IL-18 mRNA level in the CD11b+ fraction of spleen cells was unchanged. There was no change in the expression of the IFN-γ-inducing cytokine genes in the CD11b− fraction. Neutralization of IL-12 and IL-18 in culture abolished the IFN-γ production almost completely. Spleen cells from IL-12- or IL-18-deficient mice never produced IFN-γ after stimulation with LLO. These results clearly indicated that LLO, a well-known virulence factor of L. monocytogenes, is capable of inducing IFN-γ from NK cells through induction of IL-12 and IL-18 from macrophages. LLO appeared to play essential roles, not only as a bacterial virulence factor but also as a bacterial modulin in the immune response of the host.
Listeria monocytogenes is a gram-positive pathogenic bacterium responsible for serious infection in immunocompromised individuals and pregnant women (8, 10). The virulence of this bacterium can be attributed to intracellular parasitism subsequent to the entry into host phagocytes. L. monocytogenes is able to escape from the phagosome into host cell cytosol (28, 33). Escape from the phagosome is largely mediated by listeriolysin O (LLO), the essential determinant of pathogenicity (6, 19, 29).
LLO is a 56-kDa protein encoded by the hly gene, which is located in the virulence gene cluster of the L. monocytogenes chromosome (28). LLO is a member of a large family of thiol-activated, cholesterol-binding, pore-forming cytolysins, including streptolysin O (SLO), perfringolysin (PFO), and pneumolysin, produced by gram-positive bacteria Streptococcus pyogenes, Clostridium perfringens, and Streptococcus pneumoniae, respectively (2, 22, 31). It has been revealed that LLO is the major virulence factor of L. monocytogenes, because an LLO-negative mutant strain (mutant hly) did not show the ability for intracellular growth and was completely nonvirulent in mice (6, 21). Moreover, expression of LLO, PFO, or SLO in Bacillus subtilis resulted in escape from the phagosome and subsequent growth of the bacteria in the cytosol (4, 30).
In the experimental infection of mice with L. monocytogenes, it has been shown that various inflammatory cytokines including interleukin-1 (IL-1) (14), IL-6 (17, 18), tumor necrosis factor alpha (TNF-α) (13, 23), IL-12 (35), and gamma interferon (IFN-γ) (24) are expressed at the early phase of infection and play important roles in host defense. Among these cytokines, the pivotal role of IFN-γ in the defense of mice against primary infection has been well documented, since antilisterial resistance was enhanced by in vivo administration of recombinant IFN-γ (5) and inhibited by administration of an anti-IFN-γ antibody (24) or in mice that do not produce IFN-γ (12) and that lack the IFN-γ receptor (7).
It appears that various components of L. monocytogenes are involved in IFN-γ induction in the infected host. In our previous study, the expression of endogenous IFN-γ was observed at the initial stage only after infection with a virulent strain, not after infection with an avirulent strain not producing LLO (37), suggesting that LLO directly contributes to cytokine production. In fact, we have observed that the production of IL-1 was induced by purified LLO in thioglycolate-elicited peritoneal macrophages (36, 39) and that IFN-γ production was induced in spleen cells after stimulation with purified LLO or viable bacteria (25, 37). These findings strongly suggested that LLO plays an essential role not only as a bacterial virulence factor but also as a bacterial modulin in the cyokine response of the infected host.
In the present study, we analyzed the mechanism of LLO-induced IFN-γ production in spleen cells in vitro with special reference to macrophage-derived cytokines.
MATERIALS AND METHODS
Experimental animals.
Female C3H/HeN and C57BL/6 mice (Japan SLC, Hamamatsu, Japan), raised and maintained under specific-pathogen-free conditions, were used at 7 to 9 weeks of age. IL-12-deficient (IL-12 KO) and IL-18-deficient (IL-18 KO) mice were kindly provided by H. Okamura (Hyogo Medical Collage, Nishinomiya, Japan) and K. Kawakami (University of the Ryukyus, Nishihara, Japan), respectively.
Bacterial strains and growth condition.
L. monocytogenes EGD, a virulent strain, was used throughout the study. The bacteria were grown in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.) at 37°C for 7 h, washed repeatedly, suspended in phosphate-buffered saline (PBS) with 10% [wt/vol] glycerol, and stored at −80°C in small aliquots until used.
Reagents.
Gentamicin reagent solution was purchased from Gibco-BRL Life Technologies Inc. (Rockville, Md.). Lipopolysaccaride (LPS) derived from Escherichia coli O55:B5 was purchased from Difco Laboratories. Polymyxin B was purchased from Nacalai Tesque (Kyoto, Japan). The cytokine-specific neutralizing antibodies for IL-1α (rat, 40508.11), IL-1β (rat, 30311.11), IL-12 p70 (goat, polyclonal), and TNF-α (goat, polyclonal) were purchased from Genzyme/Techne (Minneapolis, Minn.). The IL-18-specific neutralizing antibody (rat, 93-10C) was purchased from Medical & Biological Laboratories (Nagoya, Japan). As control antibodies, normal rat immunoglobulin (IgG) (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) and normal goat IgG (Genzyme/Techne) were used. A mouse anti-LLO monoclonal antibody (7D10.E12) was established in our laboratory by fusion of NS-1 cells and spleen cells from mice hyperimmunized with purified LLO. The specificity of the hybridoma culture supernatant for LLO has been confirmed by the absence of reactivity to the culture supernatants from a number of nonhemolytic strains of listeriae.
Purification of LLO.
LLO was purified according to the method of Kayal et al. (15) with some modifications. In brief, 40 ml of overnight culture of L. monocytogenes was inoculated into 2 liters of fresh BHI broth, and the bacteria were cultured for 4 h. Bacteria were harvested by centrifugation, washed once with RPMI 1640 medium (Gibco-BRL), and transferred to 500 ml of RPMI 1640 medium. After incubation at 37°C for 2.5 h, the supernatant was collected and concentrated by ultrafiltration using Ultra Free-15 filter units (cutoff value, 30 kDa; Millipore, Bedford, Mass.). The concentrated supernatant was passed through a PD-10 column which had been equilibrated with 20 mM bis-Tris propane (Nacalai Tesque), pH 6.7, and loaded on a DEAE-Sephadex column (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) equilibrated at pH 6.7. The effluent fractions were pooled and used as LLO. The purity of the fraction was determined by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) analysis and Western blotting using an anti-LLO monoclonal antibody. The level of contaminating endotoxin was monitored by a Limulus color KY test (Wako Pure Chemical, Osaka, Japan). To inhibit the cytolytic activity, LLO (500 nM) was treated with 10 μg of cholesterol/ml for 30 min on ice. In some experiments, LLO was heated at 94°C for 15 min and the hemolytic and IFN-γ-inducing activities were assayed.
Hemolytic activity.
Hemolytic activity was measured by hemoglobin release from sheep erythrocytes (SRBC) as described previously (3). LLO was twofold diluted with PBS and incubated with an equal volume of 1% SRBC for 30 min at 37°C. The supernatant was collected, and the degree of hemolysis was evaluated by measuring the absorbance of hemoglobin (415 nm) released from SRBC. One hemolytic unit was defined as the amount of the toxin required for 50% hemolysis.
Cell preparation.
Spleens were aseptically removed from 3 to 7 normal mice and squeezed between two microslide glasses to prepare the single-cell suspension. Cells were pooled and treated with 0.83% ammonium chloride in 170 mM Tris-HCl (pH 7.65) to lyse erythrocytes. Cells were washed with RPMI 1640 and suspended at 5 × 106 cells/ml in culture medium consisting of RPMI 1640, 10% heat-inactivated fetal bovine serum (Gibco-BRL), and gentamicin (5 μg/ml).
To deplete NK cells, macrophages, and dendritic cells (DCs) from whole spleen cells, cells were suspended at 5 × 107 cells/ml in PBS containing 0.5% bovine serum albumin and 2 mM EDTA and treated with anti-NK (DX5) MicroBeads (Miltenyi Biotec, Gladbach, Germany), anti-CD11b (Mac-1) MicroBeads, and anti-CD11c MicroBeads, respectively. After incubation for 15 min at 5°C, cells were washed and the cell populations were depleted by magnetic cell sorting (MACS) system with an LD depletion column (Miltenyi Biotec) according to the manufacturer's instructions. The recovered cells were suspended at 5 × 106 cells/ml in culture medium. To isolate macrophages, spleen cells treated with anti-CD11b (Mac-1) MicroBeads were passed and CD11b+ cells were eluted from an MACS LS separation column (Miltenyi Biotec). The recovered cells were suspended at 2.5 × 106 cells/ml in culture medium. The purity of a negatively or positively selected cell population, i.e., the degree to which spleen cells had been removed, was assessed by fluorescence-activated cell sorter analysis (BD PharMingen, San Diego, Calif.) by using a secondary antibody conjugated with fluorescein isothiocyanate (BD PharMingen). The levels of depletion for DX5+ cells, CD11b+ cells, and CD11c+ cells were 85 (2.1% before depletion to 0.3% after depletion), 99 (10.3 to 0.1%), and 90% (3.0 to 0.3%), respectively.
LLO-induced IFN-γ production.
Spleen cells were plated in a 96-well tissue culture plate at 106 cells/well and stimulated for 24 h with various doses of LLO which had been treated with or without cholesterol. The supernatant was collected, and the concentration of IFN-γ was measured. In some experiments, cells were stimulated with LLO in the presence of antibodies (5 μg/ml) against various kinds of cytokines including IL-1α, IL-1β, IL-12 p70, IL-18, and TNF-α and the effects on IFN-γ production were determined.
The concentration of IFN-γ was determined by a sandwich enzyme-linked immunosorbent assay (ELISA) using a combination of nonlabeled and biotin-labeled rat anti-mouse IFN-γ monoclonal antibodies (XMG1.2; Endogen, Woburn, Mass.).
Reverse transcription-PCR (RT-PCR) for cytokine mRNA.
Cells were plated in a 24-well tissue culture plate at 2.5 × 106 cells/well and stimulated for 6 h with 50 nM LLO which had been treated with cholesterol. Total cellular RNA was extracted with an RNeasy Mini Kit (Qiagen Inc., Valencia, Calif.), and cDNA was reverse transcribed from 0.2 μg of total RNA using a random primer as previously described (38). PCR was performed by using KOD-plus DNA polymerase (TOYOBO, Osaka, Japan) and specific primer sets for cytokines. The sequences of primers for each cytokine and the sizes of PCR products are shown in Table 1.
TABLE 1.
Primer sequences and the sizes of PCR products
| Cytokine and oligonucleotide | Sequence (5′-3′) | Product size (bp) |
|---|---|---|
| IFN-γ | ||
| Sense | AGCGGCTGACTGAACTCAGATTGTAG | 244 |
| Antisense | GTCACAGTTTTCAGCTGTATAGGG | |
| IL-1α | ||
| Sense | CTCTAGAGCACCATGCTACAGAC | 309 |
| Antisense | TGGAATCCAGGGGAAACACTG | |
| IL-6 | ||
| Sense | TGGAGTCACAGAAGGAGTGGCTAAG | 155 |
| Antisense | GCAAGAGACACAGTCCTGGG | |
| IL-12 p35 | ||
| Sense | CTGCATCAGCTCATCGATGG | 618 |
| Antisense | CAGAAGCTAACCATCTCCTGGTTT | |
| IL-12 p40 | ||
| Sense | TCCGGAGTAATTTGGTGCTTCACA | 396 |
| Antisense | ACTGTACAACCGCAGTAATACGG | |
| IL-18 | ||
| Sense | ACTGTACAACCGCAGTAATACGG | 546 |
| Antisense | AGTGAACATTACAGATTTATCCC | |
| TNFα | ||
| Sense | GGCAGGTCTACTTTGGAGTCATTGC | 308 |
| Antisense | ACATTCGAGGCTCCAGTGAATTCCA | |
| β-actin | ||
| Sense | TGGAATCCTGTGGCATCCATGAAAC | 349 |
| Antisense | TAAAACGCAGCTCAGTAACAGTCCG |
The PCR cycle consisted of 94°C for 15 s, 55°C for 30 s, and 68°C for 60 s. Samples were amplified for 23 to 33 cycles. The most appropriate number of amplification cycles for each cytokine was determined by preliminary experiments. The reaction was terminated by heating at 68°C for 7 min. The PCR product (5 μl) was electrophoresed on a 2% agarose gel and stained with ethidium bromide. The bands of the PCR product were visualized on a UV transilluminator.
Statistical analysis.
The statistical significance of the data was determined by Student's t test, and a P value of <0.05 was taken as significant.
RESULTS
Purification of LLO and hemolytic activity.
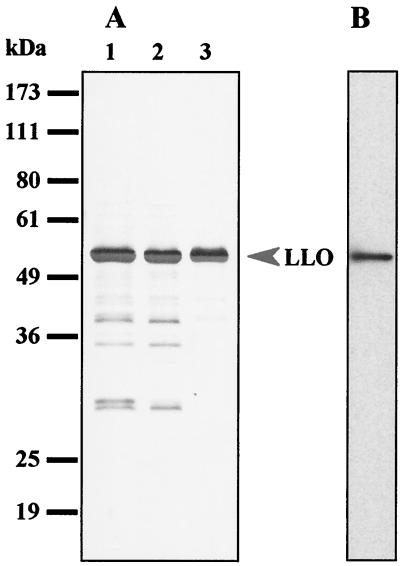
To enrich LLO in the starting sample for purification, L. monocytogenes grown in BHI broth was washed and transferred to RPMI 1640 medium. Bacteria were allowed to release LLO in RPMI medium, and then the medium was concentrated by ultrafiltration. SDS-PAGE analysis revealed that LLO was enriched in the concentrated medium (Fig. 1A, lane 1). Then buffer was replaced with 20 mM bis-Tris propane adjusted to pH 6.7, the isoelectric point of LLO (Fig. 1A, lane 2), and the fraction passed through a DEAE-Sephadex column was recovered as an LLO preparation. Purified LLO gave a single band with an approximate molecular mass of 56 kDa (Fig. 1A, lane 3), which was consistent with the reported molecular weight for LLO (11, 20). The purified LLO was detected also as a single band by immunoblotting using an anti-LLO monoclonal antibody (Fig. 1B). Densitometric analysis showed that the purity of this preparation was approximately 95%. It is known that the hemolytic activity of thiol-activated cytolysins is enhanced by treatment with a reducing agent (1). However, the purified LLO appeared to have been recovered in a fully activated form because the hemolytic activity was not altered in the presence or absence of reducing agents. The relative hemolytic activity of this preparation was determined to be approximately 50,000 hemolytic units/mg.
FIG. 1.
Purification of LLO from culture supernatant of L. monocytogenes EGD. (A) Pattern of silver staining after SDS-PAGE of concentrated supernatant from a 2.5-h culture in RPMI 1640 (lane 1), the fraction in which the buffer was exchanged by passage through a PD-10 column (lane 2), and the effluent fraction from a DEAE-Sephadex column (lane 3). Positions of molecular mass markers are indicated on the left. (B) Immunoblot analysis of LLO. The effluent fraction of a DEAE-Sephadex column was subjected to SDS-PAGE and immunoblotting to identify the LLO molecule by using an anti-LLO monoclonal antibody.
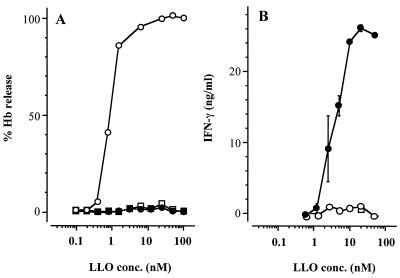
The hemolytic activity of LLO was completely blocked by pretreatment with a small amount of cholesterol or by heating at 94°C for 15 min (Fig. 2A). The hemolytic activity of our LLO preparation was also stable at a pH lower than 6.7 at 37°C (data not shown), which was consistent with the previous report that acidic pH was optimal for the hemolytic activity of LLO (11).
FIG. 2.
Hemolytic and IFN-γ-inducing activities of purified LLO. (A) LLO (500 nM) was treated without (open circle) or with cholesterol (10 μg/ml; solid circle) or with heating at 94°C for 15 min (open square). The samples were diluted and mixed with 1% SRBC. The suspension was incubated for 30 min, and the percent release of hemoglobin (Hb) was measured. (B) Spleen cells (106 cells) were stimulated with LLO samples which had been treated as described above. The level of IFN-γ production was determined by ELISA. Shown is a representative result of three similar experiments. Data are means ± standard errors for three determinations.
Cytolytic and IFN-γ-inducing activities of purified LLO.
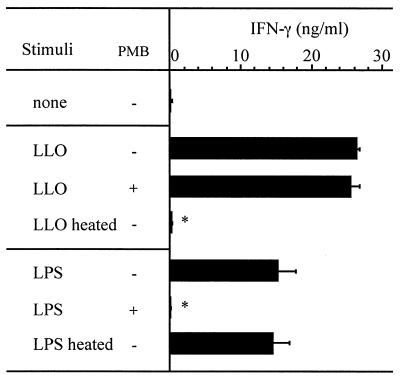
When spleen cells were stimulated with LLO pretreated with cholesterol, a high level of IFN-γ was produced in the culture supernatant in a dose-dependent manner (Fig. 2B). The absence of IFN-γ-inducing activity in an LLO sample not treated cholesterol appears to be due to the high level of cytolytic activity. The level of contaminating LPS in this preparation was 50 pg per mg of protein. This small amount of LPS in the LLO preparation did not seem to be involved in the cytokine production. As expected, LLO-induced production of IFN-γ was not affected, even in the presence of 0.5 μg of polymyxin B/ml, which completely abolished IFN-γ production from spleen cells stimulated with 1,000 pg of LPS/ml. Furthermore, the IFN-γ-inducing ability of LLO was impaired by heating at 94°C for 15 min, while LPS-induced IFN-γ production was not (Fig. 3). These results indicated that the IFN-γ-inducing activity was not attributable to a small amount of LPS in the LLO preparation.
FIG. 3.
Effect of polymyxin B (PMB) and heating on IFN-γ-inducing activity of LLO and LPS. Spleen cells were stimulated with cholesterol-pretreated LLO (20 nM) or LPS (1 ng/ml) for 24 h, and the level of IFN-γ was determined by ELISA. The effects of the addition of 0.5 μg of PMB/ml and heating at 94°C for 15 min were examined. Shown is a representative result of two similar experiments. Data are means ± standard errors for three determinations. ∗, significant difference from untreated group (P < 0.01).
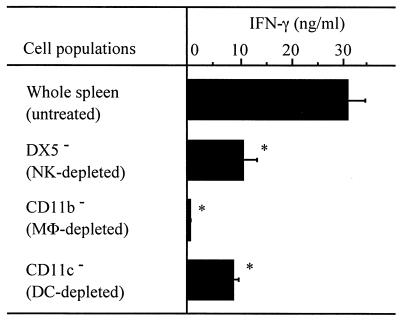
Involvement of various populations of cells in LLO-induced IFN-γ production.
In our previous report, NK cells and macrophages were shown to be necessary for LLO-induced IFN-γ production by negative selection using an antiasialo GM1 antibody and a Sephadex G-10 column (25). Again, to determine the role of NK cells, macrophages, and also DCs in LLO-induced IFN-γ production, we depleted each population of cells by using the MACS system and MicroBeads conjugated with antibodies for DX5 (NK cells), CD11b (mainly macrophages), and CD11c (mainly DCs). Negatively selected cells were stimulated with 20 nM LLO pretreated with cholesterol for 24 h, and the titer of IFN-γ in the culture supernatant was determined. After depletion of DX5+ or CD11c+ cells, the LLO-induced IFN-γ production was decreased significantly (Fig. 4). In repeated experiments, DX5+ or CD11c+ cells were depleted at efficiencies of at most 90%; however, the reduction in IFN-γ response suggested the involvement of both types of cells. CD11b+ cells could be eliminated almost completely by anti-CD11b MicroBeads, and the IFN-γ production in response to LLO was lost completely (Fig. 4).
FIG. 4.
Determination of cell populations required for LLO-induced IFN-γ production. Spleen cells were treated with anti-NK (DX5) MicroBeads, anti-CD11b (Mac-1) MicroBeads, or anti-CD11c MicroBeads, followed by depletion of the cells bound to beads by using an MACS LS separation column. The negatively selected cells were adjusted to 5 × 106 cells/ml and stimulated with 20 nM LLO pretreated with cholesterol for 24 h. The level of IFN-γ was determined by ELISA. Shown is a representative result of three similar experiments. Data are means ± standard errors for three determinations. ∗, significant difference from untreated group (P < 0.01). MΦ, macrophage.
LLO-induced cytokine gene expression in macrophages.
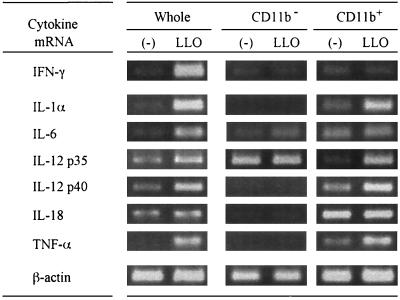
The above result indicated that CD11b+ cells, mainly macrophages, were essential for IFN-γ production from spleen cells stimulated with LLO. In the next experiment, we prepared whole spleen cells, spleen cells depleted of CD11b, and positively selected CD11b+ cells using MACS. Positive selection of CD11b+ cells by MACS resulted in enrichment from 10.3% in the whole spleen cells to 86.7% in the preparation of CD11b+ cells according to the profile of fluorescence-activated cell sorter analysis. Each cell population was stimulated with LLO pretreated with cholesterol for 6 h. The total cellular RNA was extracted, and cytokine-specific mRNA expression was examined by RT-PCR (Fig. 5). When whole spleen cells were stimulated with LLO, and expression of IFN-γ, IL-1α, IL-6, IL-10, IL-12 p35, IL-12 p40, and TNF-α mRNAs was induced. The expression of IL-18 mRNA appeared to be constant irrespective of stimulation with LLO. When CD11b− cells were stimulated with LLO, expression of the genes of most of the cytokines was not induced. The expression of IL-12 p35 mRNA was shown to be constitutive, but IL-12 p40 mRNA expression was not detected even after stimulation with LLO. In contrast, expression of the mRNA of all cytokines, except for IFN-γ, could be observed in CD11b+ cells after stimulation with LLO. Expression of both IL-12 p35 mRNA and IL-12 p40 mRNA was induced in CD11b+ cells. These results indicated that both CD11b+ and CD11b− cells are required for LLO-induced IFN-γ production. It was also suggested that CD11b+ cells were capable of producing biologically active IL-12 along with the constitutive production of IL-18. These two cytokines from CD11b+ cells appeared to be important for the final induction of IFN-γ in spleen cells.
FIG. 5.
Expression of mRNA for various cytokines after stimulation with LLO. Whole spleen cells, CD11b− cells, and CD11b+ cells were stimulated with 50 nM cholesterol-pretreated LLO for 6 h. Total RNA was extracted and subjected to RT-PCR for detection of cytokine mRNA for IFN-γ, IL-1α, IL-6, IL-12 p35, IL-12 p40, IL-18, TNF-α, and β-actin. Shown is a representative result of two similar experiments.
LLO-induced IFN-γ production from spleen cells treated with a cytokine-specific neutralizing antibody.
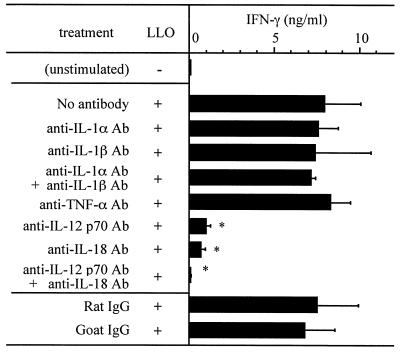
To determine the role of cytokines derived from CD11b+ cells in LLO-induced IFN-γ production, we examined the effect of neutralizing antibodies for each cytokine. After stimulation with 20 nM LLO treated with cholesterol in the presence of 5 μg of a cytokine-specific antibody/ml for 24 h, the titer of IFN-γ in culture supernatant was determined by ELISA. When anti-IL-12 p70 and/or anti-IL-18 antibodies were added, LLO-induced IFN-γ production was significantly affected, whereas other antibodies against IL-1α, IL-1β, and TNF-α had no effect on LLO-induced IFN-γ production (Fig. 6).
FIG. 6.
Effect of neutralization of various cytokines on IFN-γ production after stimulation with LLO. Spleen cells were stimulated with 20 nM cholesterol-pretreated LLO in the absence or presence of an antibody specific for IL-1α, IL-1β, TNF-α, IL-12 p70, or IL-18. The level of IFN-γ was determined by ELISA. Shown is a representative result of two similar experiments. Data are means ± standard errors for three determinations. ∗, significant difference from untreated group (P < 0.01).
LLO-induced IFN-γ production by spleen cells from IL-12 KO and IL-18 KO mice.
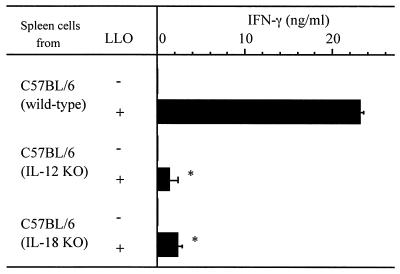
To confirm the involvement of IL-12 and IL-18 in LLO-induced IFN-γ production, we employed IL-12 KO and IL-18 KO mice. Spleen cells prepared from wild-type, IL-12 KO, and IL-18 KO mice from the same C57BL/6 background were stimulated with LLO pretreated with cholesterol. Then, the titer of IFN-γ in culture supernatant was determined. In spleen cells from both groups of KO mice, the level of IFN-γ production induced by LLO was significantly lower than that observed in the wild type (Fig. 7). This result provided evidence that both IL-12 and IL-18 were essential for IFN-γ production in response to LLO.
FIG. 7.
IFN-γ production after stimulation of cells from IL-12 and IL-18 KO mice with LLO. The spleen cell suspension was prepared from C57BL/6 wild-type, IL-12 KO, and IL-18 KO mice and was stimulated with 20 nM cholesterol-pretreated LLO for 24 h. The level of IFN-γ was determined by ELISA. Shown is a representative result of two similar experiments. Data are means ± standard errors for three determinations. ∗, significant difference from wild type (P < 0.01).
DISCUSSION
In a series of experiments, we have reported that LLO, the major virulence factor of L. monocytogenes, is highly capable of inducing various cytokines including IFN-γ in mice both in vivo and in vitro (25, 36, 37, 39). LLO secreted by viable L. monocytogenes appeared to play an important role in the induction of IFN-γ, which is essential for the primary defense of the infected host.
In this study, we purified LLO from culture supernatant of L. monocytogenes to examine the mechanism of IFN-γ induction by LLO in spleen cells. LLO purified in this study has been recovered in a fully active form even in the absence of a reducing agent, and the LLO preparation was highly toxic to the cells. When cytolytic LLO was applied to spleen cells without cholesterol treatment, there was no IFN-γ production induced. Therefore, we had to block the membrane-damaging activity by treatment with cholesterol prior to the assay. After stimulation with purified LLO pretreated with cholesterol, spleen cells produced a high level of IFN-γ (Fig. 2). This may occur in an in vivo situation because serum contains cholesterol and there is a possibility that the LLO bound to the phagosomal membrane containing cholesterol becomes a molecule similar to that obtained by cholesterol treatment.
Though LLO preparation showed a purity above 98% after silver staining, the possibility of IFN-γ induction by a contaminating substance should be ruled out. From the results on the effect of polymyxin B and heating, we could rule out the possible contribution of contaminating LPS (Fig. 3). In a previous study, we reported that the culture supernatant from a strain of L. monocytogenes not producing LLO was not capable of inducing IFN-γ (25). Therefore, it is unlikely that IFN-γ induction in this study totally depends on some bacterial product other than LLO.
We examined the role of the cell population in LLO-induced IFN-γ production. DX5+ cells (mainly NK cells) appeared to be the source of IFN-γ in LLO-induced IFN-γ production by spleen cells because the level of IFN-γ in the culture supernatants was decreased significantly when this population was eliminated. This result was consistent with previous reports demonstrating that NK cells produced IFN-γ in response to IL-12 induced at the early phase of L. monocytogenes infection (35). However, the level of IFN-γ in the culture supernatant was decreased to the basal level when CD11b+ cells were depleted. These results strongly suggested that not only IFN-γ-producing cells, including NK cells, but also CD11b+ cells (mainly macrophages) were required for LLO-induced IFN-γ production. Recently, Ohteki et al. reported that DCs produced IFN-γ in response to IL-12, which was produced by DCs themselves at an initial stage of L. monocytogenes infection (26). Because the IFN-γ production was not completely inhibited by depletion of either DX5+ cells or CD11c+ cells (Fig. 4), it appeared that DCs as well as NK cells are the source of IFN-γ. It remains to be determined whether DCs produce IL-12 and IL-18 in response to LLO. Moreover, it was shown that both IL-12 and IL-18 are important in LLO-induced IFN-γ production by spleen cells because the level of IFN-γ in the culture supernatants decreased significantly when these cytokines were neutralized by addition of cytokine-specific neutralizing antibodies (Fig. 6). Convincing support for the result of this assay using neutralizing antibodies was provided by the use of IL-12 KO and IL-18 KO mice (Fig. 7). It is unlikely that NK cells were stimulated directly with LLO for induction of IFN-γ, and IFN-γ production appeared to be induced in response to these cytokines from macrophages stimulated with LLO. We confirmed that small amounts of IL-12 and IL-18 could synergistically induce IFN-γ production by NK cells isolated from spleen cells (data not shown).
We compared the production of IL-12 and IL-18 in response to LLO by CD11b+ cells with that by CD11b− cells. The expression of these cytokines by CD11b+ cells isolated from spleen cells, except for IL-18, was strongly enhanced after stimulation with LLO (Fig. 5). In contrast, the CD11b− cell population showed a constitutive expression of IL-12 p35 mRNA but the expression of other cytokine mRNAs, including those of IL-12 p40 and IL-18, was diminished even in the presence of LLO, compared to those expressed by whole spleen cells stimulated with LLO in Fig. 5. These results clearly indicated that, in spleen cells stimulated with LLO, only CD11b+ cells (mainly macrophages) were able to produce both biologically active IL-12 p70 and IL-18, which were indispensable for LLO-induced IFN-γ production.
The expression of IL-18 mRNA in splenic CD11b+ cells was constitutive, but there was no expression in the CD11b− cell population. In mice infected with L. monocytogenes, the expression of IL-18 mRNA appeared to be constitutive in the spleen (data not shown). It was reported that PU.1, a transcription factor expressed exclusively by myeloid cells and B cells, was involved in the constitutive expression of IL-18 mRNA in RAW264.7 cells (16). These observations, taken together, led to the suggestion that the constitutive expression of IL-18 mRNA in spleen cells was attributable to macrophages. However, the expression of IL-18 by splenic CD11b+ cells was not affected by stimulation with LLO, whereas the neutralizing antibody to IL-18 affected LLO-induced IFN-γ production by spleen cells. A possible explanation is that LLO may increase IL-18 production by increasing cleavage of pro-IL-18 to the mature form by activating caspase-1 as well as LPS (32).
LLO was shown to be capable of inducing the expression of various cytokine genes, including IL-1α, IL-6, IL-10, IL-12 p35, IL-12 p40, and TNF-α genes, by CD11b+ cells. According to several reports on the promoter analysis of these cytokine genes, the activation of NF-κB, a transcription factor, was required for the expression of IL-12 p35 (34), IL-12 p40 (27, 34), and TNF-α (9) by stimulation with microbial components. Though the signaling pathway for LLO to induce an IL-12 response in CD11b+ cells has not yet been determined, it is probable that LLO activates NF-κB, because we have observed in a preliminary study that the induction of IL-12 p40 expression by stimulation with LLO was prevented by treatment with MG132, an inhibitor of the degradation of IκB (K. Tsuchiya, unpublished data). Furthermore, Kayal et al. reported that LLO induces up-regulation of adhesion molecules and chemokines on endothelial cells via activation of NF-κB (15).
In this study, we showed that purified LLO could induce the expression of various cytokine genes by CD11b+ cells in vitro. Among the cytokines, it became clear that IL-12 and IL-18 play an essential role in IFN-γ induction by spleen cells stimulated with LLO. While various cell wall components are known to be highly capable of inducing cytokines, little is known about the potential activity of protein toxins. The present results may have provided the details of a novel aspect of LLO as the possible ligand for stimulating innate immunity.
Acknowledgments
This study was supported by the “Research for the Future” Program from The Japan Society for the Promotion of Science and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- 1.Alouf, J. E. 1999. Introduction of the family of the structurally-related cholesterol-binding cytolysins (sulphydryl-activated toxins), p. 443-456. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial toxins, 2nd ed. Academic Press, London, United Kingdom.
- 2.Alouf, J. E., and M. W. Palmer. 1999. Streptolysin O, p. 457-475. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial toxins, 2nd ed. Academic Press, London, United Kingdom.
- 3.Baba, H., I. Kawamura, C. Kohda, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, S. Ichiyama, and M. Mitsuyama. 2001. Essential role of domain 4 of pneumolysin from Streptococcus pneumoniae in cytolytic activity as determined by truncated proteins. Biochem. Biophys. Res. Commun. 281:37-44. [DOI] [PubMed] [Google Scholar]
- 4.Bielecki, J., P. Youngma, P. Connelly, and D. A. Portnoy. 1990. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature 345:175-176. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., A. Nakane, and T. Minagawa. 1989. Recombinant murine gamma interferon induces enhanced resistance to Listeria monocytogenes infection in neonatal mice. Infect. Immun. 57:2345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cossart, P., M. F. Vicente, J. Mengaud, F. Baquero, J. C. Perez-Diaz, and P. Berche. 1989. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57:3629-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai, W. J., W. Bartens, G. Kohler, M. Hufnagel, M. Kopf, and F. Brombacher. 1997. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-γ receptor-deficient mice. J. Immunol. 158:5297-5304. [PubMed] [Google Scholar]
- 8.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foxwell, B., K. Browne, J. Bondeson, C. Clarke, R. de Martin, F. Brennan, and M. Feldmann. 1998. Efficient adenoviral infection with IκBα reveals that macrophage tumor necrosis factor α production in rheumatoid arthritis is NF-kB dependent. Proc. Natl. Acad. Sci. USA 95:8211-8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gellin, B. G., and C. V. Broome. 1989. Listeriosis. JAMA 261:1313-1320. [PubMed] [Google Scholar]
- 11.Geoffroy, C., J. L. Gaillard, J. E. Alouf, and P. Berche. 1987. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect. Immun. 55:1641-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFNγ. Immunity 3:109-117. [DOI] [PubMed] [Google Scholar]
- 13.Havell, E. A. 1987. Production of tumor necrosis factor during murine listeriosis. J. Immunol. 139:4225-4231. [PubMed] [Google Scholar]
- 14.Havell, E. A., L. L. Moldawer, D. Helfgott, P. L. Kilian, and P. B. Sehgal. 1992. Type I IL-1 receptor blockade exacerbates murine listeriosis. J. Immunol. 148:1486-1492. [PubMed] [Google Scholar]
- 15.Kayal, S., A. Lilienbaum, C. Poyart, S. Memet, A. Israel, and P. Berche. 1998. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-κB and upregulation of adhesion molecules and chemokines. Mol. Microbiol. 31:1709-1722. [DOI] [PubMed] [Google Scholar]
- 16.Kim, Y., H. Kang, S. Paik, K. Pyun, K. L. Anderson, B. E. Torbette, and I. Choi. 1999. Roles of IFN consensus sequence binding protein and PU.1 in regulating IL-18 gene expression. J. Immunol. 163:2000-2007. [PubMed] [Google Scholar]
- 17.Kopf, M., H. Bauman, G. Freer, M. Freudenberg, M. Iamers, T. Kishimoto, R. Zinkernagel, H. Bluethmann, and G. Kohler. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339-342. [DOI] [PubMed] [Google Scholar]
- 18.Liu, Z., R. J. Simpson, and C. Cheers. 1994. Role of IL-6 in activation of T cells for acquired cellular resistance to Listeria monocytogenes. J. Immunol. 152:5375-5380. [PubMed] [Google Scholar]
- 19.Marquis, H., H. G. Bouwer, D. J. Hinrichs, and D. A. Portnoy. 1993. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect. Immun. 61:3756-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mengaud, J., M. F. Vicente, and P. Cossart. 1989. Transcriptional mapping and nucleotide sequence of the Listeria monocytogenes hlyA region reveal structural features that may be involved in regulation. Infect. Immun. 57:3695-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michel, E., K. A. Reich, R. Favier, P. Berche, and P. Cossart. 1990. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol. Microbiol. 4:2167-2178. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell, T. J. 1999. Pneumolysin: structure, function and role in disease, p. 476-495. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial toxins, 2nd ed. Academic Press, London, United Kingdom.
- 23.Nakane, A., T. Minagawa, and K. Kato. 1988. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect. Immun. 56:2563-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakane, A., A. Numata, and T. Minagawa. 1992. Endogenous tumor necrosis factor, interleukin-6, and gamma interferon levels during Listeria monocytogenes infection in mice. Infect. Immun. 60:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishibori, T., H. Xiong, I. Kawamura, M. Arakawa, and M. Mitsuyama. 1996. Induction of cytokine gene expression by listeriolysin O and roles of macrophage and NK cells. Infect. Immun. 64:3188-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohteki, T., T. Fukao, K. Suzue, C. Maki, M. Ito, M. Nakamura, and S. Koyasu. 1999. Interleukin 12-dependent interferon γ production by CD8α+ lymphoid dendritic cells. J. Exp. Med. 189:1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plevy, S. E., J. H. M. Gemberling, H. Sang, A. J. Dorner, and S. T. Smale. 1997. Multiple control elements mediate activation of the murine and human interleukin-12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol. Cell. Biol. 17:4572-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portnoy, D. A. 1996. Cellular biology of Listeria monocytogenes infection, p. 279-293. In V. L. Miller, J. B. Kaper, D. A. Portnoy, and R. R. Isberg (ed.), Molecular genetics of bacterial pathogenesis. American Society for Microbiology, Washington, D.C.
- 29.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portnoy, D. A., R. K. Tweten, M. Kehoe, and J. Bielecki. 1992. Capacity of listeriolysin O, streptolysin O, and perfringolysin O to mediate growth of Bacillus subtilis within mammalian cells. Infect. Immun. 60:2710-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossjohn, J., R. K. Tweten, J. I. Rood, and M. W. Parker. 1999. Perfringolysin O, p. 496-510. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial toxins, 2nd ed. Academic Press, London, United Kingdom.
- 32.Seki, E., H. Tsutsui, H. Nakano, N. Tsuji, K. Hoshino, O. Adachi, K. Adachi, S. Futatsugi, K. Kuida, O. Takeuchi, H. Okamura, J. Fujimoto, S. Akira, and K. Nakanishi. 2001. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1β. J. Immunol. 166:2651-2657. [DOI] [PubMed] [Google Scholar]
- 33.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite Listeria monocytogenes. J. Cell Biol. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tone, Y., S. A. J. Thompson, J. M. Babik, K. F. Nolan, M. Tone, C. Raven, and H. Waldmann. 1996. Structure and chromosomal location of the mouse interleukin-12 p35 and p40 subunit genes. Eur. J. Immunol. 26:1222-1227. [DOI] [PubMed] [Google Scholar]
- 35.Tripp, C. S., M. K. Gately, J. Hakimi, P. Ling, and E. R. Unanue. 1994. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. J. Immunol. 152:1883-1887. [PubMed] [Google Scholar]
- 36.Tsukada, H., I. Kawamura, T. Fujimura, K. Igarashi, M. Arakawa, and M. Mitsuyama. 1992. Induction of macrophage interleukin-1 production by Listeria monocytogenes hemolysin. Cell. Immunol. 140:21-30. [DOI] [PubMed] [Google Scholar]
- 37.Xiong, H., I. Kawamura, T. Nishibori, and M. Mitsuyama. 1994. Cytokine gene expression in mice at an early stage of infection with various strains of Listeria spp. differing in virulence. Infect. Immun. 62:3649-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong, H., T. Nishibori, S. Ohya, Y. Tanabe, and M. Mitsuyama. 1996. Involvement of various combinations of endogenous inflammatory cytokines in Listeria monocytogenes-induced expression of inducible nitric oxide synthase in mice. FEMS Immunol. Med. Microbiol. 16:257-266. [DOI] [PubMed] [Google Scholar]
- 39.Yoshikawa, H., I. Kawamura, M. Fujita, H. Tsukada, M. Arakawa, and M. Mitsuyama. 1993. Membrane damage and interleukin-1 production in murine macrophages exposed to listeriolysin O. Infect. Immun. 61:1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]