Abstract
Listeria monocytogenes is an intracellular gram-positive human pathogen that invades eucaryotic cells. Among the surface-exposed proteins playing a role in this invasive process, internalin belongs to the family of LPXTG proteins, which are known to be covalently linked to the bacterial cell wall in gram-positive bacteria. Recently, it has been shown in Staphylococcus aureus that the covalent anchoring of protein A, a typical LPXTG protein, is due to a cysteine protease, named sortase, required for bacterial virulence. Here, we identified in silico from the genome of L. monocytogenes a gene, designated srtA, encoding a sortase homologue. The role of this previously unknown sortase was studied by constructing a sortase knockout mutant. Internalin was used as a reporter protein to study the effects of the srtA mutation on cell wall anchoring of this LPXTG protein in L. monocytogenes. We show that the srtA mutant (i) is affected in the display of internalin at the bacterial surface, (ii) is significantly less invasive in vitro, and (iii) is attenuated in its virulence in the mouse. These results demonstrate that srtA of L. monocytogenes acts as a sortase and plays a role in the pathogenicity.
Gram-positive bacteria are surrounded by a cell wall envelope containing attached polypeptides and polysaccharides that may interact with host cells and play a role in the virulence of pathogenic species (34). In gram-positive bacteria, several distinct mechanisms of cell wall attachment and display of surface proteins have been recently described (reviewed in reference 5). The only surface proteins known to be covalently linked to the cell wall are the LPXTG proteins, exemplified by protein A of Staphylococcus aureus (34). In a pioneer work, Schneewind et al. described a cysteine protease of S. aureus, designated sortase, which is responsible for the covalent attachment of protein A, and identified the biochemical processes allowing the anchoring of protein A to the cell wall (27). Very recently, the three-dimensional structure of the sortase of S. aureus was determined by nuclear magnetic resonance spectroscopy, thus identifying a catalytic domain responsible for the transpeptidation reaction (17). After synthesis in the bacterial cytoplasm, surface protein precursors are translocated across the membrane and the NH2-proximal leader peptide is removed by leader peptidase. The COOH-terminal sorting signal is first cleaved by sortase between the threonine and glycine residues of the LPXTG motif. Then, the enzyme covalently links the carboxyl of the threonine to the cell wall peptidoglycan by amide linkage (34, 35, 43, 44, 45). The sorting signal consists of a conserved LPXTG motif followed by a membrane-spanning hydrophobic domain and a tail mostly composed of positively charged residues (18, 28, 42). Notably, it was recently shown that in the gram-positive human pathogen Listeria monocytogenes, the surface proteins were also cleaved between the threonine and the glycine residue of the LPXTG motif and amide-linked to the peptidoglycan (7), strongly suggesting that this cell wall sorting mechanism is shared by all gram-positive bacteria.
In S. aureus, a mutant lacking the srtA gene encoding sortase is defective in the anchoring of surface proteins and accumulated precursor proteins with uncleaved C-terminal sorting signals. As a result, the assembly and display of surface adhesins is abolished and causes a reduction in the ability of sortase mutants to establish animal infections (26). In another gram-positive bacterium, the human commensal Streptococcus gordonii, it has been very recently shown that inactivation of srtA encoding sortase altered the expression of specific anchored surface proteins containing the canonical LPXTG motif, ultimately decreasing the ability of bacteria to colonize the oral mucosa in the mouse (3). These observations prompted us to search for a gene encoding a sortase homologue in L. monocytogenes and to test the effect of the gene disruption on surface protein anchoring and on bacterial virulence.
L. monocytogenes is a ubiquitous food-borne gram-positive bacterium, responsible for life-threatening infections in humans and animals (11). It is a facultative intracellular pathogen able to enter and multiply in both professional (25) and nonprofessional phagocytes such as epithelial cells (12, 13) or hepatocytes (8, 14, 48). The major steps of the intracellular parasitism of L. monocytogenes have been deciphered (see references 6 and 47 for reviews). After entry, bacteria rapidly lyse the phagosomal membranes and gain access to the cytosol, where they spread to adjacent cells by an actin-based motility process. Lysis of the phagosome results mainly from listeriolysin O, a sulfhydryl-activated hemolysin active at acidic pHs. Actin assembly is mediated by the surface protein ActA. The interaction of L. monocytogenes with host cells is a key event in the pathogenesis of listeriosis. This process involves a number of surface proteins, including internalin (or InlA), InlB, and ActA. InlA is an 800-amino-acid protein synthesized as a precursor bearing an N-terminal signal peptide and a C-terminal sorting signal with an LPXTG motif (20). It is required for entry into the human enterocyte-like cell line Caco-2 (9, 12) and other cell lines expressing its cellular receptor, the adhesion molecule E-cadherin (21, 31). The in vivo relevance of this molecule for the development of an infectious process has been addressed only very recently (22, 39).
In this work, we identified in silico a gene encoding a sortase-like protein, designated srtA, from the recently completed sequence of the genome of L. monocytogenes (15). We constructed a knockout mutant of this gene. The phenotypic analysis of the mutant strain revealed that srtA of L. monocytogenes acts as a sortase involved in processing and anchoring of internalin and therefore is involved in bacterial virulence.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
Brain heart infusion (BHI) (Difco Laboratories, Detroit, Mich.) and Luria-Bertani (Difco Laboratories) broth and agar were used to grow Listeria and Escherichia coli strains, respectively. We used the reference strain of L. monocytogenes EGD belonging to serovar 1/2a, recently sequenced (15). Wild-type bacteria were transformed by electroporation as previously described (37). Strains harboring plasmids were grown in the presence of the following antibiotics: for pCR derivatives, kanamycin (50 μg ml−1); for pAUL-A derivatives, erythromycin (150 μg ml−1 [E. coli] and 5 μg ml−1 [L. monocytogenes]).
Genetic manipulations.
Total DNA from Listeria cells was prepared as described (38). Standard techniques were used for plasmid DNA preparation, fragment isolation, DNA cloning and restriction analysis (41). Restriction enzymes and ligase were purchased from New England Biolabs and used as recommended by the manufacturer. DNA was amplified with Taq DNA polymerase (Promega) for 35 cycles of 60 s at 95°C, 60 s at 55°C, and 90 s at 72°C in a Gene Amp System 9600 thermal cycler (Perkin-Elmer, Branchburg, N.J.). Nucleotide sequencing was carried out with Taq DiDeoxy terminators and by the DyePrimer Cycling Sequence protocol developed by Applied Biosystems (Perkin-Elmer) with fluorescently labeled dideoxynucleotides and primers, respectively. Fluorescently labeled primers were purchased from Life Technologies, Paisley, Scotland. Labeled extension products were analyzed on an ABI Prism 310 apparatus (Applied Biosystems, Perkin-Elmer). Protein and nucleotide databases were searched using the programs BLASTP and BLASTN (National Center for Biotechnology Information, Los Alamos, N.Mex.), available via the Internet. Protein sequences were aligned by the program CLUSTAL W.
Construction of a srtA deletion mutant of L. monocytogenes.
A 2,042-bp DNA fragment comprising the srtA gene (orf 1592.1) was amplified by PCR from L. monocytogenes (EGDwt) genomic DNA using the following primer pair: primer 1 (5′-GATATTCTGGGTATGCGTCTCGTACATCAAACGAA-3′) and primer 2 (5′-GCAACTTCTGTACTTGCCATATGCACTTTTGTTTTTCC-3′). Oligonucleotides were synthesized by Genset (Paris, France). The AmpliTaq DNA polymerase of Thermus aquaticus from Perkin-Elmer was used. The amplified double-stranded DNA fragments were first cloned into the pCR cloning vector using the Invitrogen TA cloning kit (Invitrogen Corporation, San Diego, Calif.). The recombinant plasmids pCR -rtA was cut with EcoRI and KpnI restriction enzymes (Biolabs NEN, Beverly, Mass.). The fragment comprising the srtA gene was purified on Tris-acetate-EDTA-agarose gels and subcloned into the EcoRI-KpnI sites of the thermosensitive shuttle vector pAUL-A (24) to give plasmid pAUL -srtA. The srtA deletion mutant of L. monocytogenes (EGDΔsrtA) was constructed by deleting a 57-bp internal fragment of srtA gene, corresponding to two natural NheI sites present within the sortase coding sequence, that removed residues 105 to 123 of SrtA. The resulting plasmid pAUL -ΔsrtA was introduced into EGD by electroporation, and chromosomal integration of the mutated gene was performed by allelic replacement, as described previously (16, 24). The construction was confirmed by PCR sequence analysis of chromosomal DNA from the mutant.
Insertion of a kanamycin resistance cassette into the NheI site of ΔsrtA.
A promoterless aphA3 gene, conferring resistance to kanamycin (46), was inserted into the NheI site of the ΔsrtA mutant gene carried by plasmid pAUL -ΔsrtA. The following pair of primers was used to amplify the aphA3 gene: primer 1 (5′-CTA GCTAGCTAGGTGACTAACTAG-3′) and primer 2 (5′-CTA GCTAGCTAGCTTGGGTCATTATTCCC-3′) (the NheI restriction sites are in italics). The resulting plasmid, pAUL -ΔsrtA::aphA3, was introduced into EGD by electroporation, and chromosomal integration of the mutated gene was performed by allelic replacement (see above).
Complementation.
For complementation, the entire srtA gene and its promoter region (452 bp upstream from the ATG) were introduced in the chromosome of EGDΔsrtA by using the integrative plasmid pAT113, a Tn1545 derivative allowing random insertion in the chromosome of L. monocytogenes (references 1 and 23 and references therein). The recombinant plasmid pCR -srtA (carrying the srtA gene and its promoter region [see above]) was cut with EcoRI restriction enzyme (Biolabs NEN). The 1.8-kb EcoRI fragment comprising the srtA gene was purified on TAE-agarose gels and subcloned into the EcoRI site of the integrative plasmid pAT113, to give plasmid pAT113 -srtA. Plasmid pAT113-srtA was then electroporated into EGDΔsrtA and selection on erythromycin (8 μg ml−1). One insertion of the recombinant plasmid that occurred in a region of the chromosome that did not interfere with any known biological activity of L. monocytogenes (not shown) was chosen for further analyses.
Reverse transcriptase PCR (RT-PCR).
Total RNA was extracted from L. monocytogenes (EGD) grown overnight in BHI broth at 37°C. The following pairs of primers were used to amplify the mRNAs: srtA primer 1 (5′-AGGAGGAATCATATGTTAAAGAAAACAA-3′) and primer 2 (5′-CCGCTGTCTTTTTTTCCTCATTAT-3′), orf 1589.1 primer 1 (5′-TCTGAAACGTTTAGGCGTCAAT-3′) and primer 2 (5′-CCGAAGAAGTCACCAAAAATCT-3′), and orf 1590.1 primer 1 (5′-GGAAGAAGCTGGATTTAAGCTG-3′) and primer 2 (5′-GCCAACTTCGGGTAAATGACTA-3′).
The procedure used was that described in the instructions for the SuperScript One-step RT-PCR System (Life Technologies). Prior to RT-PCR, total RNA samples were incubated for 1 h at 37°C with RNase-free DNase I (Boehringer, Mannheim, Germany) to eliminate any DNA contamination, and DNase was heat inactivated by incubation at 80°C for 15 min.
SDS-PAGE and Western blot analysis.
Proteins from culture supernatants and total bacterial extracts were prepared as follows. Fractions of bacterial cultures at different optical densities at 600 nm (OD600) were centrifuged. Supernatants were passed through a 0.22-μm-pore-size filter (Millipore, Bedford, Mass.) and further concentrated with ultrafree Biomax columns with a cutoff of 30 kDa. Samples were finally suspended in 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (130 mM Tris-HCl, pH 6.8; 1% SDS; 7% 2-β-mercaptoethanol; 7% sucrose; 0.01% bromophenol blue). The bacterial pellets were suspended in cold water, and bacteria were disrupted using a Fastprep FP120 apparatus (Ozyme; Bio101, Carlsbad, Calif.) with three pulses of 30 s at a speed of 6.5. After a centrifugation of 3 min at 10,000 rpm, lysates were collected and suspended in 1× SDS-PAGE sample buffer. Electrophoresis and Western blotting were carried out as described previously (23) in SDS-8% polyacrylamide minigels (Mini Protean II; Bio-Rad). Loading in each well corresponds to 100 μl of bacterial culture (adjusted to a final OD600 of 1). Nitrocellulose sheets were probed either with anti-internalin monoclonal antibody (MAb) L7.7, K18.4, or C20.4 (30) or with polyclonal ActA antibody, obtained from P. Cossart (Pasteur Institute, Paris, France), with an anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibody. Antibodies were used at a final dilution of 1:1,000. Antibody binding was revealed by adding 0.05% diaminobenzidine-tetrahydrochloride (Sigma) and 0.03% hydrogen peroxide (Sigma).
Protein sequencing.
EGDΔsrtA mutant strain was grown in RPMI 1640 synthetic medium containing glucose (3% final concentration) overnight with agitation at 37°C. After centrifugation, cell supernatants were filtered through a 0.22-μm-pore-size Millipore filter and further concentrated with ultrafree columns with a cutoff of 30 kDa (Millipore). Denatured proteins were separated by SDS-PAGE and then transferred electrophoretically onto polyvinylidene difluoride membranes (Millipore). Protein bands were visualized by staining the membrane with amido black. To identify the protein bands corresponding to internalin, a part of the membrane was cut and tested like a classical Western blot with anti-InlA MAb L7.7. For N-terminal sequencing, the two major protein bands detected by the MAb were cut from the polyvinylidene difluoride membrane and sequenced on an Applied Biosystems Procise Sequencer (J. d'Alayer, Laboratoire de Séquençage des Protéines, Institut Pasteur, Paris).
Protein solubility in hot SDS.
In L. monocytogenes, cell wall-anchored internalin is largely insoluble in hot SDS unless the peptidoglycan had been first digested enzymatically. In contrast, the membrane-anchored protein ActA is almost completely extractable in hot SDS without any prior treatment (7). We used this assay to determine the solubility of internalin in hot SDS in the srtA mutant. Briefly, L. monocytogenes EGD and srtA mutant were grown with agitation at 37°C in BHI medium. Two milliliters of each culture (at an OD600 of 1) were collected and centrifuged for 20 min at 6,000 rpm at 4°C (in an Eppendorf centrifuge). The bacterial pellets were washed in phosphate-buffered saline and then resuspended in 100 μl of 4% SDS-0.5 M Tris-HCl, pH 8. The suspension was boiled for 10 min and then centrifuged in an Eppendorf centrifuge at 10,000 rpm for 5 min. The supernatant (100 μl) and pellet (resuspended in 100 μl of 4% SDS-0.5 M Tris-HCl, pH 8) fractions were precipitated with 10% ice-cold trichloroacetic acid; the precipitate was washed in 70% acetone and dried. Each sample was finally resuspended in 20 μl of SDS-PAGE sample buffer and denatured by boiling for 10 min prior to loading onto SDS-8% polyacrylamide gels. Ten microliters were loaded per well (corresponding to 1 ml of bacterial culture at an OD600 of 1).
Invasion assays. (i) Culture of cell lines.
The human colon carcinoma cell line Caco-2 (ATCC HTB37) and the human hepatocellular carcinoma cell line HepG-2 (ATCC HB 8065, kindly provided by S. Dramsi and P. Cossart, Institut Pasteur, Paris) were propagated as previously described (8) in Dulbecco's modified Eagle's medium (25 mM glucose) (Gibco). Cells were seeded at 8 × 104 cells cm−2 in 24-well tissue culture plates (Falcon). Monolayers were used 24 h after seeding.
(ii) Invasion assays.
The invasion assays were carried out essentially as described previously (33). Briefly, cells were inoculated with bacteria at a multiplicity of infection of approximately 100 bacteria per cell. They were incubated for 1 h to allow the adherent bacteria to enter and were then washed three times with RPMI and overlaid with fresh Dulbecco's modified Eagle's medium containing gentamicin (10 mg liter−1) to kill extracellular bacteria. At 2 and 4 h, cells were washed three times and processed for counting of infecting bacteria. For that, cells were lysed by adding cold distilled water. The titer of viable bacteria released from the cells was determined by spreading onto BHI plates. Each experiment was carried out in triplicate and repeated three times.
Virulence in the mouse.
Specific-pathogen-free female Swiss mice (Janvier, Le Geneset St. Isle, France), 6 to 8 weeks old, were used. Bacteria were grown for 18 h in BHI broth, centrifuged, washed once, appropriately diluted in 0.15 M NaCl, and inoculated intravenously (i.v.) (0.5 ml) via the lateral tail vein. Groups of five mice were challenged i.v. with various doses of bacteria, and mortality was observed for 10 days. The virulence of strains was estimated by the 50% lethal dose (LD50) using the Probit method and by the bacterial survival in tissues. Bacterial growth was monitored in organs after i.v. inoculation of 106 bacteria. Groups of five mice were sacrificed by day 1, 2, 3, 4, and 7. Organs (spleen, liver, and brain) were aseptically removed and separately homogenized in 0.15 M NaCl. Bacterial counts in organ homogenates were determined at various intervals on BHI agar plates, as described previously (1).
RESULTS
Sequence analysis and transcription of the srtA gene of L. monocytogenes.
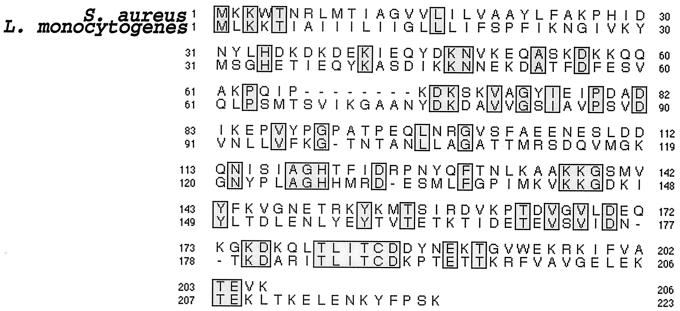
The Blast search (NCBI Blastp 2 version) of the very recently completed sequence of the genome of L. monocytogenes EGD-e serovar 1/2a (15), using the amino acid sequences of SrtA from S. aureus (27) and S. gordonii (3), led us to identify a single open reading frame (ORF) encoding a sortase-like protein of 222 amino acids (orf 1592.1). The protein shares 32% identity over its entire sequence with the sortase from S. gordonii and 27% with that of S. aureus, with a perfect conservation of the consensus motif around the putative active site cysteine (Fig. 1). When the 222-amino-acid sequence was used to scan the Listeria genome database, no additional paralogous sortase with ≥25% overall identity could be found. When only the TLXTC consensus motif was used, a second ORF (orf 812.1) encoding another putative sortase-like protein of 246 residues was found. However, in spite of a good conservation of the consensus motif (TLSTC), this ORF showed only a very low overall sequence conservation, with 21 and 16.5% identity with the sortases from S. aureus and S. gordonii, respectively. Therefore, we decided to focus in the present work on the study of the 222-amino-acid protein encoded by orf 1592.1. According to its hydropathic profile, this sortase homologue belongs to the same class of enzymes as S. aureus SrtA, which contain an N-terminal segment of hydrophobic amino acids that functions both as a signal sequence and a stop-transfer signal for membrane anchoring (17). Therefore, this gene will be referred to as srtA and the corresponding protein will be referred to as SrtA in the rest of this work for simplification.
FIG. 1.
Alignment of L. monocytogenes SrtA with S. aureus sortase. Alignment was performed using CLUSTALw. Identical residues are boxed. L. monocytogenes SrtA comprises 222 amino acids; the sortase of S. aureus comprises 206 amino acids.
We then performed the analysis of the region of the L. monocytogenes chromosome comprising srtA. A canonical AGGAGG Shine-Dalgarno sequence precedes the srtA coding sequence (7 bp upstream of the start codon). We identified a potential promoter region immediately upstream of srtA (57 bp) that is very similar to the consensus sequence of σ-70-dependent promoters (29), with a TTGCTT −35 region and a TATAAT −10 region spaced by 17 bp. Immediately downstream of srtA, a putative transcription terminator is found, with a predicted free energy of the stem-loop structure of −18.2 kcal/mol.
The genes surrounding srtA in the chromosome of L. monocytogenes encode hypothetical proteins of unknown functions. The two genes upstream of srtA encode a putative 3-methyladenine DNA glycosylase (orf 1593.1) and a hypothetical protein of unknown function (orf 1594.1), sharing 50 and 59% identity with YxlJ and YfnIJ, respectively, two hypothetical proteins of Bacillus subtilis. Strikingly, the two genes encoding these putative ORFs are located in two different regions of the B. subtilis chromosome, revealing a divergent genetic organization between the two bacterial species. The two genes downstream of srtA encode a hypothetical protein of unknown function (orf 1590.1) and a putative lipoate ligase (orf 1589.1), sharing 47 and 65% identity with YhfI and YhfJ, respectively, two hypothetical proteins encoded by consecutive genes of the B. subtilis chromosome. orf 1589.1 and orf 1590.1 are followed by a typical rho-independent transcription terminator.
We confirmed by RT-PCR (see Materials and Methods) that the srtA gene was transcribed in the wild-type strain grown in BHI medium at 37°C in exponential phase, strongly suggesting that it encodes a functional protein(s). RT-PCR also confirmed that the internal deletion in the srtA gene had no polar effect on the transcription of the two downstream genes, orf 1590.1 and orf 1589.1 (not shown).
We constructed an L. monocytogenes srtA knockout mutant by deletion of an internal fragment of srtA gene, encoding residues 105 to 123 of SrtA. The mutation was integrated in the chromosome of L. monocytogenes by allelic replacement (EGDΔsrtA) (see Materials and Methods). To confirm that the phenotypes observed were due to the ΔsrtA deletion, we constructed an srtA-complemented strain. We also constructed a mutant carrying a kanamycin resistance cassette into the deleted portion of the srtA gene (EGDΔsrtA::aphA3) to check that the deletion ΔsrtA had completely inactivated SrtA (see Materials and Methods). The properties of these two strains were compared to those of EGDΔsrtA.
SrtA acts as a sortase involved in processing and anchoring of internalin in L. monocytogenes.
The ΔsrtA mutant did not show any growth defect in BHI medium at 37°C, compared with wild-type EGD (data not shown), indicating that the mutated gene identified is not essential for bacterial multiplication. Internalin was used as a reporter protein to study the sorting defect of the srtA mutant, because it is the best-studied LPXTG protein of L. monocytogenes involved in the virulence (references 12 and 22 and references therein) against which a series of antibodies are available (30).
Truncated forms of internalin are released in the ΔsrtA mutant.
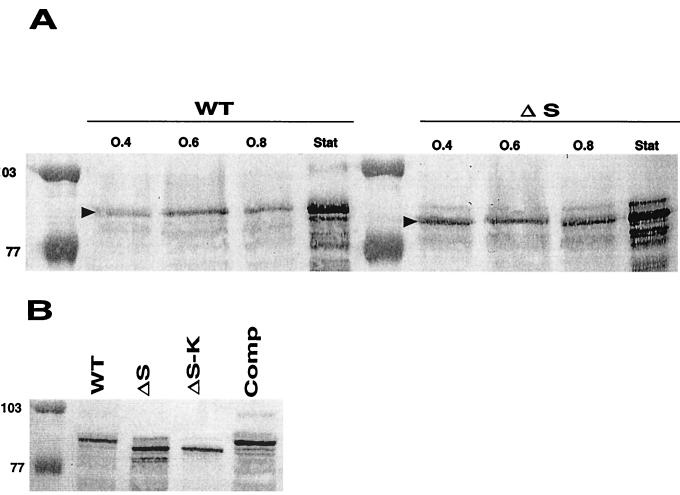
We found by Western blot analysis, using the anti-internalin MAb L7.7 (Fig. 2A), that substantial amounts of internalin were released in the culture medium during bacterial growth, as previously shown (10). These amounts of internalin released in the culture supernatant of wild-type bacteria were weak in early exponential phase and increased considerably in stationary phase. In the ΔsrtA mutant, significantly higher amounts of internalin were released as soon as the early exponential phase. At each time point of exponential growth, one major band of internalin was detected by MAb L7.7. Strikingly, this band had a lower molecular weight (MW) in the ΔsrtA mutant than that in the wild-type strain. Similar results were obtained with the two other anti-internalin MAbs tested, K18.4 and C20.4 (not shown). In stationary phase, multiple band patterns were detected with both strains. Such patterns have been previously reported and were suspected to reflect partial proteolytic degradation of the C-terminal part of internalin (12, 20, 31). Interestingly, in stationary phase a band with a higher MW was also clearly detected (Fig. 2A). This protein species has an MW compatible with that of a membrane-anchored form of the protein that would be released in the culture supernatant from lysed bacteria, as proposed earlier (20). As shown in Fig. 2B, the MW of the internalin band detected in the supernatant of the complemented strain was identical to that of wild-type EGD (Fig. 2B), confirming that the defect of internalin anchoring and processing observed was due to the ΔsrtA deletion. The band of internalin detected in the culture supernatant of the mutant strain EGDΔsrtA::aphA3 had the same MW as that detected in EGDΔsrtA.
FIG. 2.
Western blot analysis of internalin in culture supernatants of BHI-grown cells. (A) Supernatants were harvested at early (OD600 = 0.4), log (OD600 = 0.6), late log (OD600 = 0.8), and stationary (Stat) phase from EGD (WT) and EGDΔsrtA (ΔS). Loading corresponds to 100 μl of bacterial culture (adjusted to a final OD600 of 1). Internalin was detected with MAb L7.7 at a final dilution of 1/1,000 (indicated by a black arrowhead). (B) Supernatants were harvested at stationary phase from EGD (WT), EGDΔsrtA (ΔS), EGDΔsrtA::aphA3 (ΔS-K), and EGDΔsrtA complemented with the wild-type srtA gene (Comp). Internalin was detected with MAb K18.4 at a final dilution of 1/1,000. Loading corresponds to 100 μl of bacterial culture (adjusted to a final OD600 of 1). Molecular mass markers are indicated in kilodaltons to the left of the panels.
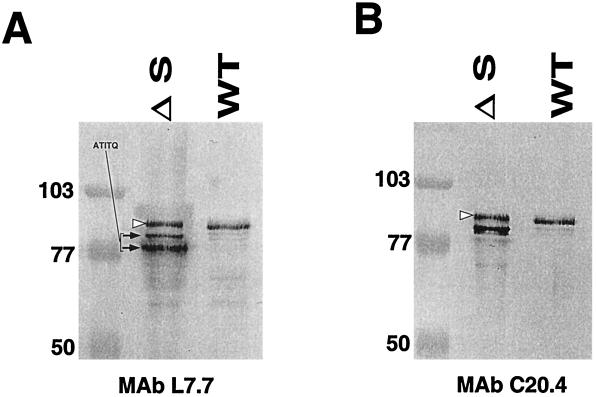
Internalin release was also evaluated in bacteria grown in RPMI synthetic medium. During stationary phase, three major forms of internalin were detected by MAb L7.7 in culture supernatants of the ΔsrtA mutant, for only one in the wild-type strain (Fig. 3A). As observed in BHI (see above), a band with a slightly higher MW than that of internalin released by the wild-type strain was detected (Fig. 3). We performed the N-terminal protein sequencing of the two lower migrating bands detected by MAb L7.7, showing that both polypeptides had the same N terminus as the mature form of wild-type internalin (ATITQ). This result indicates that, in the ΔsrtA mutant, an important fraction of internalin is processed by proteolysis at its C terminus. Moreover, MAb C20.4, a MAb directed against the portion of internalin immediately preceding the sorting signal, detected only one of the two truncated forms of internalin (Fig. 3B). These data are compatible with a proteolytic cleavage of internalin close to the natural cleavage site at the LPXTG motif, which would remove the antibody recognition site. The enzyme(s) responsible for this activity remains to be identified.
FIG. 3.
Western blot analysis of internalin in culture supernatants of RPMI-grown cells. (A) Detection with MAb L7.7. (B) Detection with MAb C20.4. Three major forms of internalin protein were detected by MAb L7.7 in the culture supernatant of the srtA mutant. The upper bands detected in the mutant are indicated by open arrowheads. The two forms with a lower apparent molecular mass than that of internalin released by the wild-type strain are indicated by arrows. Both polypeptides have the same N terminus as the mature form of wild-type internalin (ATITQ). Internalin was detected with MAb L7.7 or MAb C20.4 at final dilutions of 1/1,000. Molecular mass markers are indicated in kilodaltons to the left of the panels. ΔS, EGDΔsrtA; WT, EGD.
Internalin is solubilized by SDS treatment in the ΔsrtA mutant.
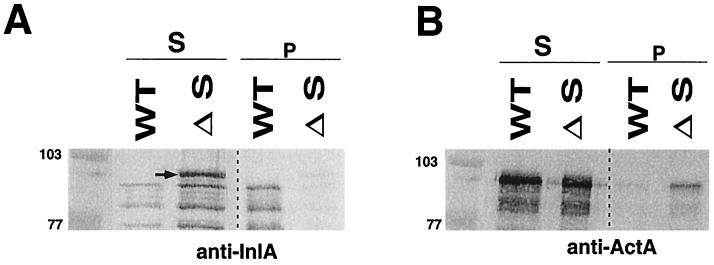
It is known that only a fraction of internalin is solubilized when wild-type bacteria are treated by SDS, whereas ActA, a protein of L. monocytogenes anchored to the bacterial membrane through a hydrophobic tail, is easily solubilized by this treatment (7). When the ΔsrtA mutant was incubated with hot SDS, we found that membrane-associated internalin was entirely solubilized, with a multiple band pattern, probably reflecting partial degradation (Fig. 4A). Interestingly, the upper protein band detected by MAb L7.7 in the mutant was higher than that detected in wild-type EGD. The size of this upper band and its solubility in SDS are compatible with that of uncleaved internalin that would be anchored to the cytoplasmic membrane through its conserved hydrophobic C-terminal tail. As expected after treatment by SDS, the membrane-anchored protein ActA was almost totally released in supernatants of the wild-type strain and the ΔsrtA mutant (Fig. 4B).
FIG. 4.
Western blot analysis of SDS-treated bacteria. In the srtA mutant (ΔS), essentially all internalin was solubilized by SDS treatment, while in the wild-type (WT) strain, only a fraction of internalin was solubilized. (A) The uppermost form of internalin released by the mutant strain is indicated by an arrow. Internalin was detected with MAb L7.7 at a final dilution of 1/1,000. (B) In the wild-type strain, as well as in the srtA mutant, the membrane-anchored protein ActA is as good as totally released in the supernatant after incubation of the bacteria in hot SDS. ActA was detected with anti-ActA polyclonal serum at a final dilution of 1/1,000. Molecular mass markers are indicated in kilodaltons to the left of the panels. Abbreviations: S, soluble fraction; p, pellet fraction.
SrtA is involved in virulence of L. monocytogenes.
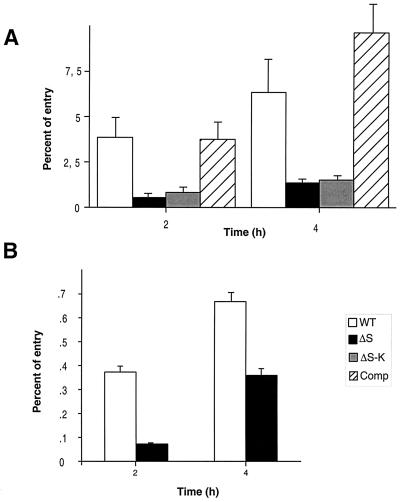
Since internalin is known to be important in the invasion of eucaryotic cells by L. monocytogenes (12), we first studied the ability of EGDΔsrtA to penetrate into and to replicate within cells in vitro. Using a multiplicity of infection of 100 bacteria/cell, we tested two different types of human cell lines, the enterocyte-like cell line Caco-2 and HepG-2 hepatocytes, previously used as model systems to study infection with L. monocytogenes (4, 13). In Caco-2 cells, invasion was strongly affected in EGDΔsrtA (Fig. 5A). After 2 h of exposure, the intracellular multiplication of mutant bacteria was only 1/20 that of wild-type EGD and ca. 1/10 after 4 h. As shown in Fig. 5A, the phenotype of mutant EGDΔsrtA::aphA3 was almost identical to that of EGDΔsrtA, demonstrating that the ΔsrtA deletion was sufficient to impair SrtA activity. As expected, intracellular multiplication of the complemented mutant was similar to that of wild-type EGD (Fig. 5A), confirming that the phenotype observed was indeed due to the inactivation of srtA. In HepG-2 cells (Fig. 5B), the ΔsrtA mutant showed a less-pronounced growth defect after 2 h of infection (one-sixth that of wild-type EGD). However, after 4 h, this defect corresponded to only a twofold reduction of bacterial counts compared to the wild-type strain.
FIG. 5.
Invasivity assay of EGD and ΔsrtA into Caco-2 and HepG-2 cells. Invasiveness was evaluated in two different types of cell lines: Caco-2 cells (A) and HepG-2 cells (B). Cell monolayers were incubated for 1 h at 37°C with approximately 100 bacteria per cell. After washing, the cells were reincubated for 4 h in fresh culture medium containing gentamicin (10 mg liter−1). At 2 and 4 h, the cells were washed again and lysed and viable bacteria were counted on BHI plates. The percentage of entry is the number of bacteria that survived in the presence of gentamicin per number of inoculated bacteria. Values are means of three different experiments. Error bars show standard deviations. Abbreviations: WT, EGD; ΔS, EGDΔsrtA; ΔS-K, EGDΔsrtA::aphA3; Comp, EGDΔsrtA complemented with the wild-type srtA gene.
The role of SrtA in the virulence of L. monocytogenes was first studied by determining the LD50 after i.v. inoculation of Swiss mice. The LD50 of the ΔsrtA mutant was estimated at 106.4 bacteria, almost 2 logs higher than that of the parental strain (104.5 bacteria), indicating that bacterial virulence was attenuated but not completely abolished. The LD50 of the mutant EGDΔsrtA::aphA3 was slightly higher, with that of EGDΔsrtA at 107.2 bacteria. As expected, the complemented strain regained almost complete virulence, with an LD50 at 105.3.
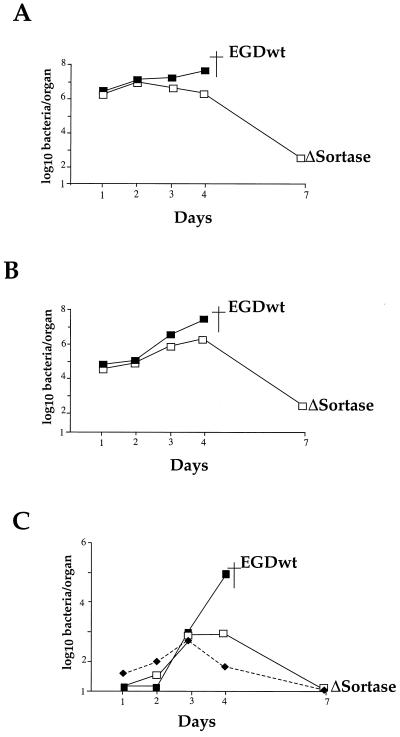
The ability of the ΔsrtA mutant to multiply in the target organs of infected mice was then evaluated by injecting mice i.v. with the normally lethal dose of 106 bacteria/animal. Earlier studies (2) have shown that upon i.v. inoculation of Swiss mice with 106 wild-type EGD organisms, bacterial growth in the spleen and liver reaches a peak at day 3, with up to 108 bacteria per organ, ultimately leading to death at day 4 to 5. At this dose, all the mice inoculated with the ΔsrtA mutant fully recovered from infection. In the spleen, bacterial multiplication of mutant bacteria was almost identical to that of the wild-type strain until day 2, and then bacteria were slowly eliminated, reaching <103 bacteria per spleen by day 7 (Fig. 6A). The kinetics of survival in the liver showed an increase of bacterial counts up to day 4, reaching ca. 107 bacteria, followed by a drop to fewer than 103 bacteria per liver by day 7 (Fig. 6B). In the brain, the growth curve of mutant bacteria was similar to that of the wild-type strain until day 3 (Fig. 6C) and paralleled the increased bacteremia. Then, mutant bacteria were completely cleared from the brain by day 7, in contrast to wild-type bacteria (Fig. 6C).
FIG. 6.
In vivo survival of the ΔsrtA mutant. The kinetics of bacterial growth was monitored in organs of mice infected with the srtA mutant, compared with EGD as a positive control. Mice were inoculated with 106 bacteria. Bacterial survival was monitored in the spleen (A), liver (B), and brain and blood (C) over a 7-day period. The counts in the blood for the srtA mutant are represented by dashed lines and black diamonds. Symbols: ▪, EGD; □, ΔsrtA. ▪†, death.
DISCUSSION
We have identified a gene, srtA, that encodes a sortase in the genome of L. monocytogenes and tested whether inactivation of this gene would have an effect on bacterial virulence. We show that an srtA knockout mutant (i) is defective in processing and anchoring of internalin, (ii) has reduced invasiveness in vitro, and (iii) is attenuated in vivo, demonstrating the role of this sortase in the pathogenicity of L. monocytogenes.
SrtA of L. monocytogenes acts as a sortase.
Earlier protein sequence comparison within sortase enzymes from several different gram-positive bacteria (43) revealed a striking conservation of the region surrounding the cysteine 184 (referring to the S. aureus sortase numbering), a residue known to be essential for enzyme activity (17, 43). A more recent survey on sortase homologues among predicted proteins from 92 bacterial genomes led to the identification of putative sortase-like proteins in most gram-positive bacterial species (36). Interestingly, in almost every gram-positive bacterium, there was usually more than one putative sortase-like protein. The physiological reasons for this apparent redundancy are at present unknown.
Here, we identified a single orf in the genome of L. monocytogenes encoding a sortase-like protein. We found that, in a ΔsrtA mutant, a significant amount of a truncated form of internalin was released in the culture supernatant in early exponential growth. The truncated forms released in stationary phase were shown to correspond to proteolysis at the C terminus of internalin, most likely close to the natural cleavage site at the LPXTG motif. Moreover, solubilization of membrane-anchored proteins by SDS treatment suggested that, in the ΔsrtA mutant, the form of internalin still associated to the cell wall was anchored to the cytoplasmic membrane through its uncleaved hydrophobic C-terminal tail.
In Caco-2 cells, and to a lesser extent in HepG-2 cells, invasion was diminished in the ΔsrtA mutant. These results suggest that the inactivation of srtA has altered the processing and/or cell wall presentation of one or several proteins that participate in bacterial entry in these two cell types. Remarkably, the invasion defect observed with the srtA mutant in Caco-2 cells is significantly less pronounced than that of an inlA mutant (ca. 3 logs less than wild-type [references 12 and 31 and unpublished data]). In this respect, it is worth recalling that L. monocytogenes strain LO28, which expresses a truncated internalin protein lacking its C-terminal anchor (and thus noncovalently linked to the peptidoglycan) (19), invades Caco-2 cells much less efficiently than EGD (10). In spite of that defect, LO28 is fully virulent in the mouse model (32).
The role of SrtA in virulence was demonstrated in the mouse model. The srtA mutant of L. monocytogenes was attenuated, but virulence was not abolished. In all the assays performed, complementation of the mutant strain EGDΔsrtA with the wild-type srtA allele restored wild-type properties, demonstrating that the phenotypes observed were due to the srtA mutation.
Functional implications.
Several members of the LPXTG protein family have been shown to be involved in the virulence of L. monocytogenes (1, 22, 40). Of particular interest, internalin, which is required for entry into human Caco-2 cells in vitro, had as good as no effect on bacterial virulence in the mouse model, even upon administration by the oral route (14, 22). The work by Cossart and colleagues showed that this was due to a point mutation in the mouse receptor for internalin, E-cadherin (21). The critical role of internalin-E-cadherin interaction in vivo was demonstrated very recently (22), using transgenic mice expressing human E-cadherin. In such mice, inlA mutants have a clear defect of virulence after oral infection.
The ΔsrtA deletion mutant and its derivative carrying a kanamycin resistance cassette (ΔsrtA::aphA3) shared similar in vitro and in vivo properties. However, the fact that EGDΔsrtA::aphA3 was slightly more attenuated (0.8 log) than EGDΔsrtA might suggest that the deletion ΔsrtA did completely abolish SrtA activity. Interestingly, the deleted region did not remove the conserved cysteine comprised within the putative active site of the molecule (17). However, in SrtA from S. aureus, the region corresponding to the deleted portion comprises one of the three residues (N98) proposed to constitute the catalytic triad that mediates the transpeptidation reaction, as well as all three acidic residues (E105, E108, and D112) constituting the putative calcium binding site that stimulates catalysis (located near the active site in the tertiary structure) (17). It is thus likely that, in SrtA of L. monocytogenes, the deletion of this internal region led to drastic alterations of the structural, and hence functional, organization of the molecule.
One hypothesis to account for the fact that the srtA mutant did not completely abolish bacterial virulence could be that additional sortase-like proteins of L. monocytogenes participate in the cell wall anchoring of the LPXTG protein involved in bacterial virulence (for example, orf 812.1). The presence of multiple sets of genes involved in secretion in the genomes of gram-positive bacteria (see reference 28 for a review) suggests that these organisms might have evolved several distinct pathways for surface protein transport. Another possible explanation could be that the fraction of the LPXTG proteins that remain associated with the cell wall in the srtA mutant is sufficient to promote the development of the infectious process. Ionic interactions between noncovalently attached surface proteins and other components of the cell wall (such as teichoic and/or lipoteichoic acids) might account for the conservation of invasiveness and virulence. Since we focused here on the sorting defect of the srtA mutant with respect to internalin, further analyses will be required to test the specificity of this sortase on the other LPXTG proteins expressed by L. monocytogenes. Finally, it is possible than in human infections by the oral route, a sortase-defective mutant could be more severely impaired in virulence.
Acknowledgments
We thank Pascale Cossart for the gift of anti-ActA and anti-InlA antibodies and Colin Tinsley for careful reading of the manuscript.
This work was supported by CNRS, INSERM, and University Paris V.
REFERENCES
- 1.Autret, N., I. Dubail, P. Trieu-Cuot, P. Berche, and A. Charbit. 2001. Identification of new genes involved in the virulence of Listeria monocytogenes by signature-tagged transposon mutagenesis. Infect. Immun. 69:2054-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berche, P. 1995. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb. Pathog. 18:323-336. [DOI] [PubMed] [Google Scholar]
- 3.Bolken, T. C., C. A. Franke, K. F. Jones, G. O. Zeller, C. H. Jones, E. K. Dutton, and D. E. Hruby. 2001. Inactivation of the srtA gene in Streptococcus gordonii inhibits cell wall anchoring of surface proteins and decreases in vitro and in vivo adhesion. Infect. Immun. 69:75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun, L., S. Dramsi, P. Dehoux, H. Bierne, G. Lindahl, and P. Cossart. 1997. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol. 25:285-294. [DOI] [PubMed] [Google Scholar]
- 5.Cossart, P., and R. Jonquieres. 2000. Sortase, a universal target for therapeutic agents against gram-positive bacteria? Proc. Natl. Acad. Sci. USA 97:5013-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cossart, P., and M. Lecuit. 1998. Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. EMBO J. 17:3797-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhar, G., K. F. Faull, and O. Schneewind. 2000. Anchor structure of cell wall surface proteins in Listeria monocytogenes. Biochemistry 39:3725-3733. [DOI] [PubMed] [Google Scholar]
- 8.Dramsi, S., I. Biswas, E. Maguin, L. Braun, P. Mastroeni, and P. Cossart. 1995. Entry of L. monocytogenes into hepatocytes requires expression of inIB, a surface protein of the internalin multigene family. Mol. Microbiol. 16:251-261. [DOI] [PubMed] [Google Scholar]
- 9.Dramsi, S., P. Dehoux, and P. Cossart. 1993. Common features of gram-positive bacterial proteins involved in cell recognition. Mol. Microbiol. 9:1119-1121. [DOI] [PubMed] [Google Scholar]
- 10.Dramsi, S., C. Kocks, C. Forestier, and P. Cossart. 1993. Internalin-mediated invasion of epithelial cells by Listeria monocytogenes is regulated by the bacterial growth state, temperature and the pleiotropic activator prfA. Mol. Microbiol. 9:931-941. [DOI] [PubMed] [Google Scholar]
- 11.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard, J. L., P. Berche, C. Frehel, E. Gouin, and P. Cossart. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 65:1127-1141. [DOI] [PubMed] [Google Scholar]
- 13.Gaillard, J. L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillard, J. L., F. Jaubert, and P. Berche. 1996. The inlAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J. Exp. Med. 183:359-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaser, P., L. Frangeul, C. Buchrieser, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couvé, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durand, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-Del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gómez-López, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueño, A. Maitournam, J. Mata Vicente, E. Ng, G. Nordsiek, S. Novella, B. de Pablos, J. C. Pérez-Diaz, R. Purcell, B. Remmel, M. Rose, C. Rusniok, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vázquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 16.Guzman, C. A., M. Rohde, T. Chakraborty, E. Domann, M. Hudel, J. Wehland, and K. N. Timmis. 1995. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect. Immun. 63:3665-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilangovan, U., H. Ton-That, J. Iwahara, O. Schneewind, and R. T. Clubb. 2001. Structure of sortase, the transpeptidase that anchors proteins to the cell wall of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 98:6056-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janulczyk, R., and M. Rasmussen. 2001. Improved pattern for genome-based screening identifies novel cell wall-attached proteins in gram-positive bacteria. Infect. Immun. 69:4019-4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonquieres, R., H. Bierne, J. Mengaud, and P. Cossart. 1998. The inlA gene of Listeria monocytogenes LO28 harbors a nonsense mutation resulting in release of internalin. Infect. Immun. 66:3420-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebrun, M., J. Mengaud, H. Ohayon, F. Nato, and P. Cossart. 1996. Internalin must be on the bacterial surface to mediate entry of Listeria monocytogenes into epithelial cells. Mol. Microbiol. 21:579-592. [DOI] [PubMed] [Google Scholar]
- 21.Lecuit, M., S. Dramsi, C. Gottardi, M. Fedor-Chaiken, B. Gumbiner, and P. Cossart. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1725. [DOI] [PubMed] [Google Scholar]
- 23.Lety, M. A., C. Frehel, I. Dubail, J. L. Beretti, S. Kayal, P. Berche, and A. Charbit. 2001. Identification of a PEST-like motif in listeriolysin O required for phagosomal escape and for virulence of Listeria monocytogenes. Mol. Microbiol. 39:1124-1140. [DOI] [PubMed] [Google Scholar]
- 24.Lingnau, A., E. Domann, M. Hudel, M. Bock, T. Nichterlein, J. Wehland, and T. Chakraborty. 1995. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect. Immun. 63:3896-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackaness, G. B. 1962. Cellular resistance to infection. J. Exp. Med. 116:381-406. [PubMed] [Google Scholar]
- 26.Mazmanian, S. K., G. Liu, E. R. Jensen, E. Lenoy, and O. Schneewind. 2000. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. USA 97:5510-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazmanian, S. K., G. Liu, H. Ton-That, and O. Schneewind. 1999. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285:760-763. [DOI] [PubMed] [Google Scholar]
- 28.Mazmanian, S. K., H. Ton-That, and O. Schneewind. 2001. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 40:1049-1057. [DOI] [PubMed] [Google Scholar]
- 29.McClure, W. R. 1985. Mechanism and control of transcription initiation in prokaryotes. Annu. Rev. Biochem. 54:171-204. [DOI] [PubMed] [Google Scholar]
- 30.Mengaud, J., M. Lecuit, M. Lebrun, F. Nato, J. C. Mazie, and P. Cossart. 1996. Antibodies to the leucine-rich repeat region of internalin block entry of Listeria monocytogenes into cells expressing E-cadherin. Infect. Immun. 64:5430-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 32.Michel, E., K. A. Reich, R. Favier, P. Berche, and P. Cossart. 1990. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol. Microbiol. 4:2167-2178. [DOI] [PubMed] [Google Scholar]
- 33.Milohanic, E., B. Pron, P. Berche, and J. L. Gaillard. 2000. Identification of new loci involved in adhesion of Listeria monocytogenes to eukaryotic cells. Microbiology 146:731-739. [DOI] [PubMed] [Google Scholar]
- 34.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarre, W. W., H. Ton-That, K. F. Faull, and O. Schneewind. 1998. Anchor structure of staphylococcal surface proteins. II. COOH-terminal structure of muramidase and amidase-solubilized surface protein. J. Biol. Chem. 273:29135-29142. [DOI] [PubMed] [Google Scholar]
- 36.Pallen, M. J., A. C. Lam, M. Antonio, and K. Dunbar. 2001. An embarrassment of sortases: a richness of substrates? Trends Microbiol. 9:97-101. [DOI] [PubMed] [Google Scholar]
- 37.Park, S. F., and G. S. Stewart. 1990. High efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 38.Poyart-Salmeron, C., P. Trieu-Cuot, C. Carlier, A. MacGowan, J. McLauchlin, and P. Courvalin. 1992. Genetic basis of tetracycline resistance in clinical isolates of Listeria monocytogenes. Antimicrob. Agents Chemother. 36:463-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pron, B., C. Boumaila, F. Jaubert, P. Berche, G. Milon, F. Geissmann, and J. L. Gaillard. 2001. Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell. Microbiol. 3:331-340. [DOI] [PubMed] [Google Scholar]
- 40.Raffelsbauer, D., A. Bubert, F. Engelbrecht, J. Scheinpflug, A. Simm, J. Hess, S. H. Kaufmann, and W. Goebel. 1998. The gene cluster inlC2DE of Listeria monocytogenes contains additional new internalin genes and is important for virulence in mice. Mol. Gen. Genet. 260:144-158. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Expression of cloned genes in Escherichia coli, p. 17.37-17.41. In C. Nolan (ed.), Molecular cloning: a laboratory manual., vol. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 42.Schneewind, O., A. Fowler, and K. F. Faull. 1995. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science 268:103-106. [DOI] [PubMed] [Google Scholar]
- 43.Ton-That, H., G. Liu, S. K. Mazmanian, K. F. Faull, and O. Schneewind. 1999. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl. Acad. Sci. USA 96:12424-12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ton-That, H., S. K. Mazmanian, K. F. Faull, and O. Schneewind. 2000. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. Sortase catalyzed in vitro transpeptidation reaction using LPXTG peptide and NH(2)-Gly(3) substrates. J. Biol. Chem. 275:9876-9881. [DOI] [PubMed] [Google Scholar]
- 45.Ton-That, H., and O. Schneewind. 1999. Anchor structure of staphylococcal surface proteins. IV. Inhibitors of the cell wall sorting reaction. J. Biol. Chem. 274:24316-24320. [DOI] [PubMed] [Google Scholar]
- 46.Trieu-Cuot, P., and P. Courvalin. 1983. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′5′-aminoglycoside phosphotransferase type III. Gene 23:331-341. [DOI] [PubMed] [Google Scholar]
- 47.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, and B. Gonzalez-Zorn. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood, S., N. Maroushek, and C. J. Czuprynski. 1993. Multiplication of Listeria monocytogenes in a murine hepatocyte cell line. Infect. Immun. 61:3068-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]