Abstract
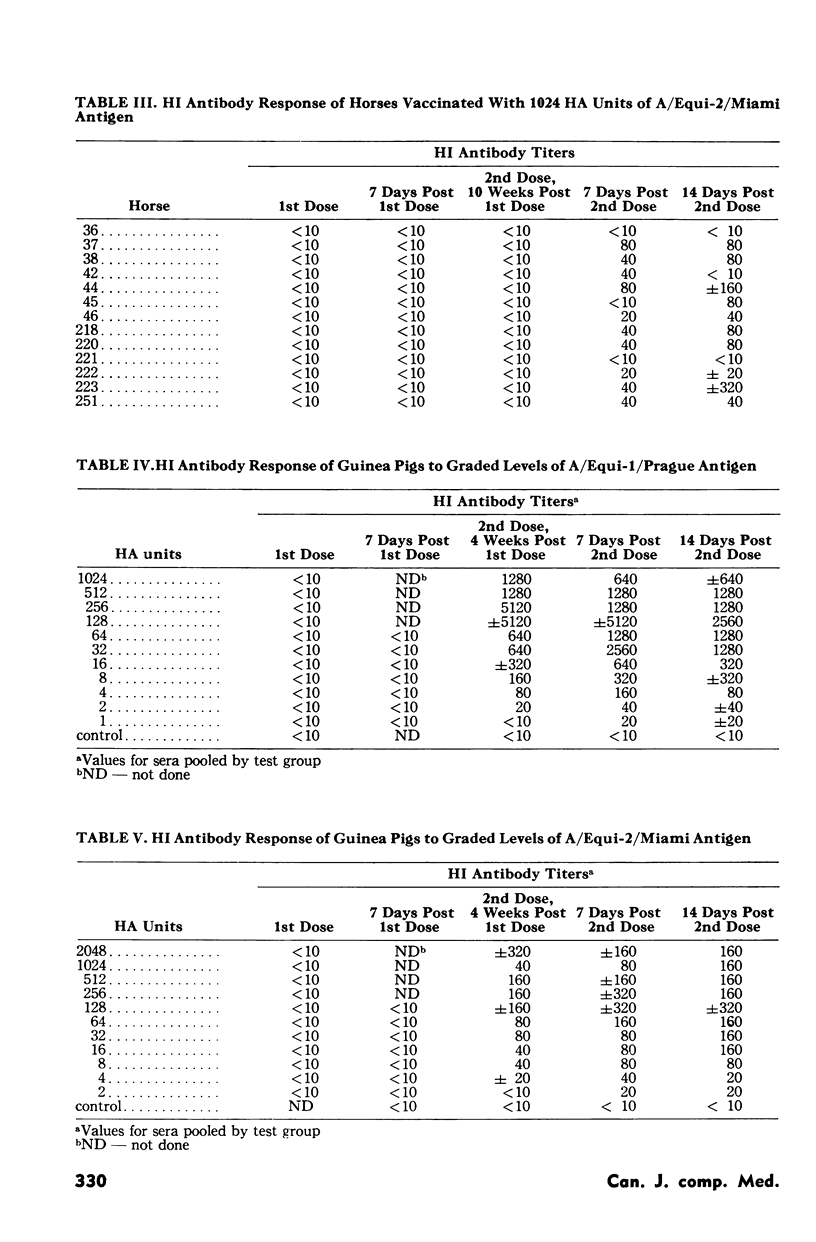
An inactivated, aluminum hydroxide adjuvant equine influenza vaccine was tested in horses and guinea pigs to determine the levels of antigen that would elicit maximum serological responses. Vaccine containing serial twofold increments of A/Equi-1/Prague and A/Equi-2/Miami strains of equine influenza virus was administered to random groupings of both types of test animals. The hemagglutination inhibition antibody response for each group was then measured. Results in horses and guinea pigs were compared to determine if the equine serological values could be related to a potency test in laboratory animals. The highest mean hemagglutination inhibition antibody response in horses occurred in groups vaccinated, respectively, with 128 or 256 hemagglutination units of A/Equi-1 and 512 or 1024 hemagglutination units of A/Equi-2 antigen. Groups vaccinated with further two- or fourfold increases in these antigens had mean hemagglutination inhibition titers that were somewhat lower than the maximum levels. When graded doses of vaccine were given to guinea pigs, their hemagglutination inhibition antibody titers reached a plateau of maximum values, similar to the serological response in vaccinated horses. Test horses remained clinically free from signs of equine influenza during the year following vaccination and no untoward post-vaccination reactions were observed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryans J. T., Doll E. R., Wilson J. C., McCollum W. H. Immunization for equine influenza. J Am Vet Med Assoc. 1966 Feb 15;148(4):413–417. [PubMed] [Google Scholar]
- DOLL E. R., BRYANS J. T. Immunization of young horses against viral rhinopneumonitis. Cornell Vet. 1963 Jan;53:24–41. [PubMed] [Google Scholar]
- DeMeio J. L., Gutekunst D. E., Beiler J. M., Paton I. M., DeSanctis A. N. The evaluation of an experimental bivalent equine influenza virus vaccine. J Am Vet Med Assoc. 1969 Jul 15;155(2):278–281. [PubMed] [Google Scholar]
- Forsyth J. R. An assessment of oil adjuvant and aqueous influenza vaccines. I. Reactions to the vaccines. J Hyg (Lond) 1967 Dec;65(4):485–495. doi: 10.1017/s0022172400046027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth J. R., Russell E. E. An assessment of oil adjuvant and aqueous influenza vaccines. II. Antibody responses to the vaccines. J Hyg (Lond) 1967 Dec;65(4):497–504. doi: 10.1017/s0022172400046039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerichs G. N., Frerichs C. C. Serological response of chickens, rabbits and guinea-pigs to equine influenza vaccines. Res Vet Sci. 1973 Mar;14(2):187–193. [PubMed] [Google Scholar]
- Kucera C. J. The case for an adjuvanted equine influenza vaccine. J Am Vet Med Assoc. 1969 Jul 15;155(2):281–284. [PubMed] [Google Scholar]
- Reisinger R. C. Comments on aqueous and adjuvanted equine influenza vaccines. J Am Vet Med Assoc. 1969 Jul 15;155(2):287–293. [PubMed] [Google Scholar]
- Shechmeister I. L., Aeschliman T., Kammlade W. G., Jr Use of sodium alginate adjuvant in immunization against equine influenza. Am J Vet Res. 1967 Sep;28(126):1373–1378. [PubMed] [Google Scholar]


