Abstract
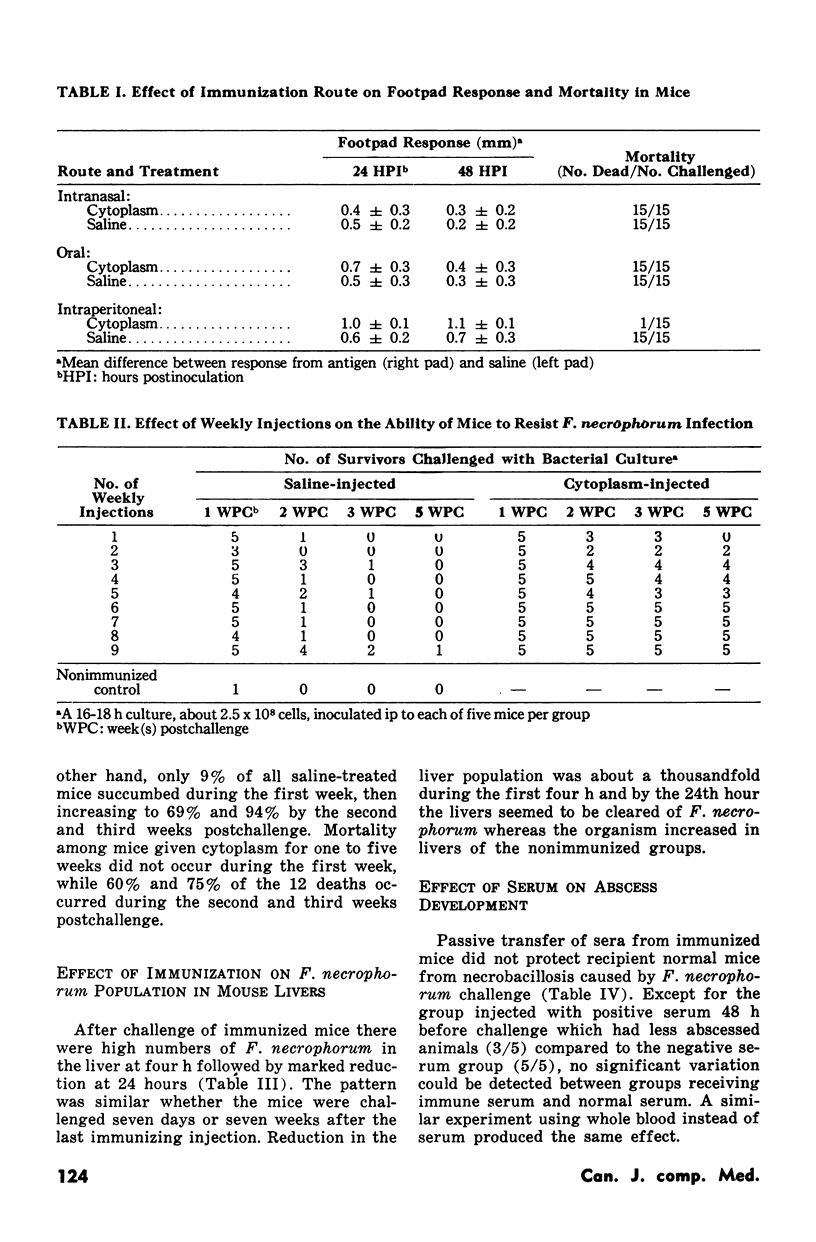
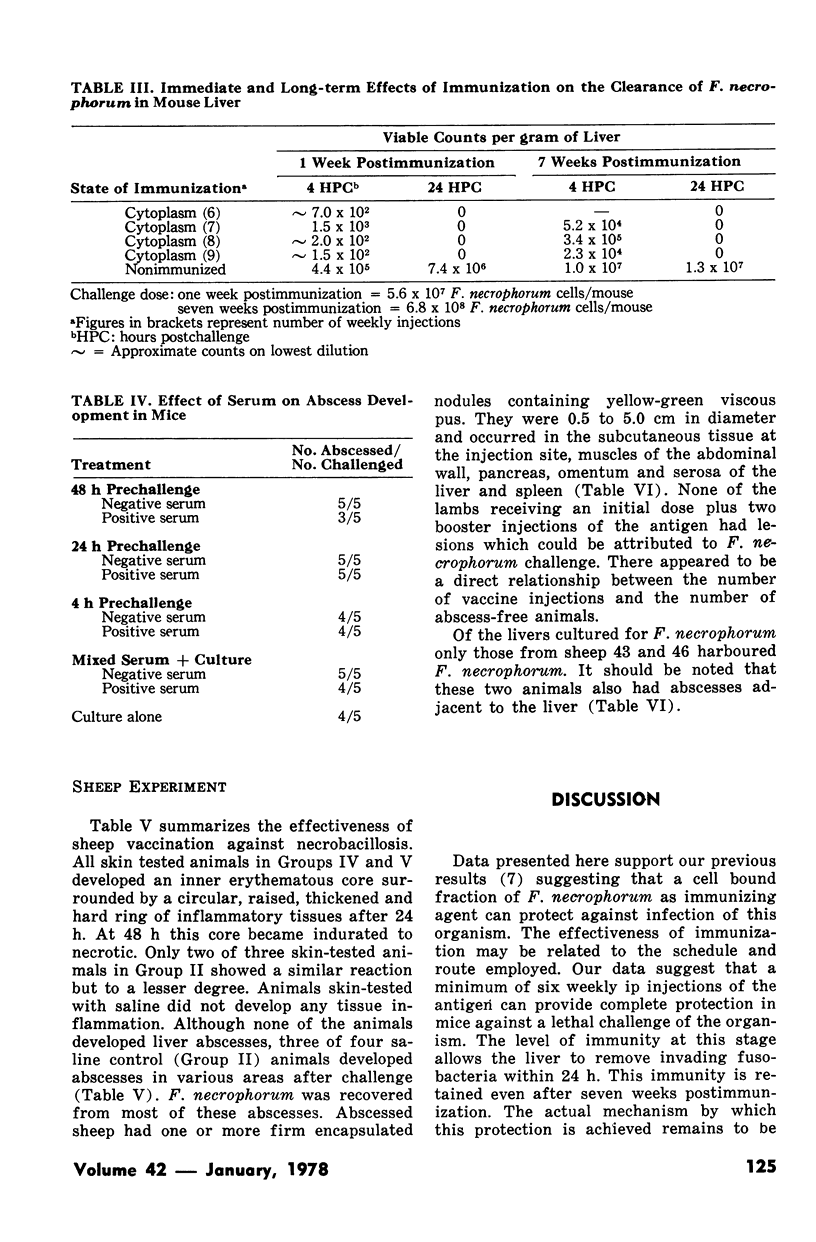
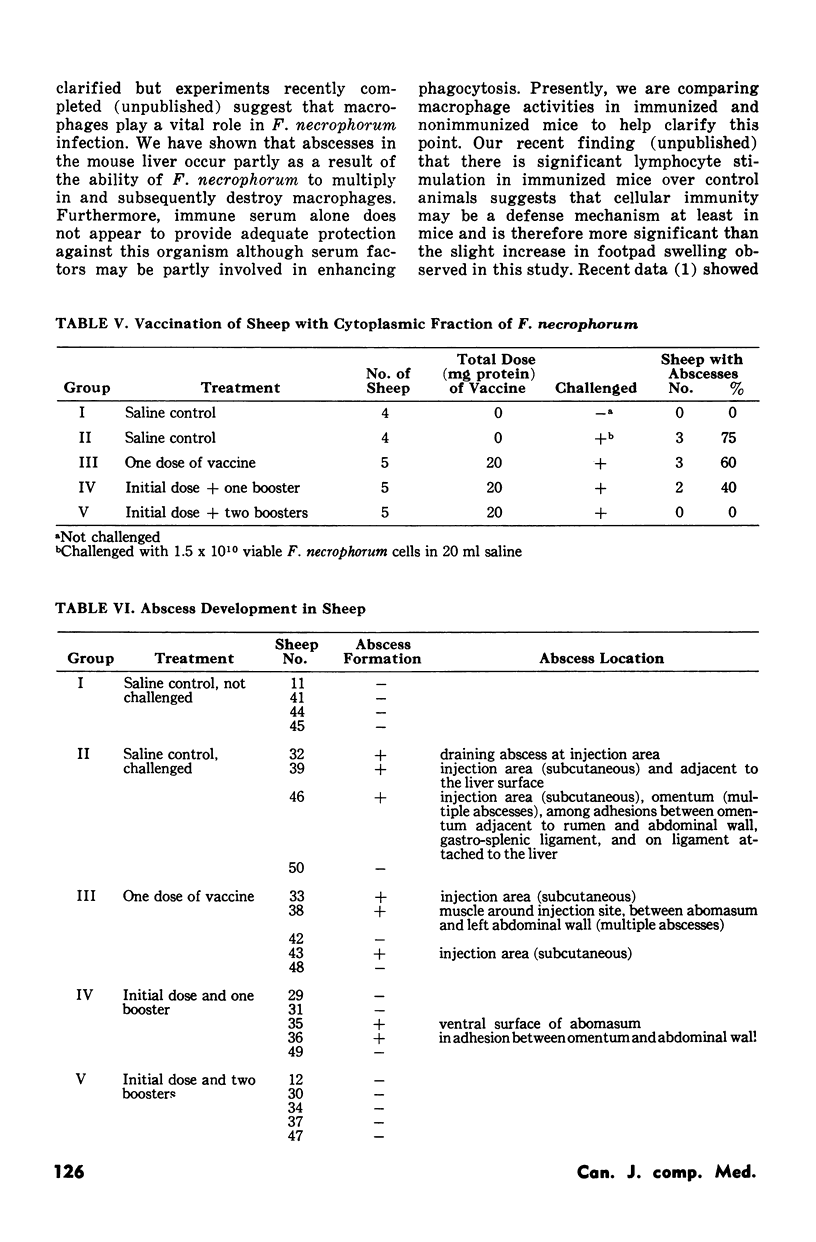
Experiments employing recently developed mouse models indicated that intraperitoneal immunization with the cytoplasm (intracellular fraction) of Fusobacterium necrophorum protected the animals from a lethal challenge of the pathogen. The critical immunization schedule needed to achieve complete protection involved six weekly intraperitoneal doses of the intracellular antigen. Livers of immunized mice were cleared of infecting fusobacterial within 24 hours whereas those of nonimmunized mice harboured increasing numbers of hte bacteria. Sera from both groups did not protect recipient mice form developing liver abscesses after challenge. Sheep immunized intraperitoneally with 20 mg of cytoplasmic protein given in three doseases were protected against the development of abscesses induced by F. necrophorum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe P. M., Lennard E. S., Holland J. W. Fusobacterium necrophorum infection in mice as a model for the study of liver abscess formation and induction of immunity. Infect Immun. 1976 May;13(5):1473–1478. doi: 10.1128/iai.13.5.1473-1478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D. C., Garcia M. M., McKay K. A. Assessment of various adjuvants in sphaerophorus necrophorus toxoids. Can Vet J. 1973 Oct;14(10):247–251. [PMC free article] [PubMed] [Google Scholar]
- Berg J. N., Loan R. W. Fusobacterium necrophorum and Bacteroides melaninogenicus as etiologic agents of foot rot in cattle. Am J Vet Res. 1975 Aug;36(08):1115–1122. [PubMed] [Google Scholar]
- Garcia M. M., Alexander D. C., McKay K. A. Biological characterization of Fusobacterium necrophorum. Cell fractions in preparation for toxin and immunization studies. Infect Immun. 1975 Apr;11(4):609–616. doi: 10.1128/iai.11.4.609-616.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. M., Dorward W. J., Alexander D. C., Magwood S. E., McKay K. A. Results of a preliminary trial with Sphaerophorus necrophorus toxoids to control liver abscesses in feedlot cattle. Can J Comp Med. 1974 Jul;38(3):222–226. [PMC free article] [PubMed] [Google Scholar]
- Garcia M. M., Neil D. H., McKay K. A. Application of immunofluorescence to studies on the ecology of Sphaerophorus necrophorus. Appl Microbiol. 1971 May;21(5):809–814. doi: 10.1128/am.21.5.809-814.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN R., FLINT J. C., GRINER L. A. Experimental hepatic necrobacillosis in beef cattle. Am J Vet Res. 1954 Jan;15(54):5–14. [PubMed] [Google Scholar]
- Roberts D. S., Graham N. P., Egerton J. R. Infective bulbar necrosis (heel-abscess) of sheep, a mixed infection with Fusiformis necrophorus and Corynebacterium pyogenes. J Comp Pathol. 1968 Jan;78(1):1–8. doi: 10.1016/0021-9975(68)90106-0. [DOI] [PubMed] [Google Scholar]
- Taubler J. H. Staphylococcal delayed hypersensitivity in mice. I. Induction and in vivo demonstration of delayed hypersensitivity. J Immunol. 1968 Sep;101(3):546–549. [PubMed] [Google Scholar]


