Abstract
Dendrites are specialized extensions of the neuronal soma that contain components of the cellular machinery involved in RNA and protein metabolism. Several dendritically localized proteins are associated with the precursor-mRNA (pre-mRNA) splicing complex, or spliceosome. Although some spliceosome-related, RNA-binding proteins are known to subserve separate cytoplasmic functions when moving between the nucleus and cytoplasm, little is known about the pre-mRNA splicing capacity of intact dendrites. Here, we demonstrate the presence and functionality of pre-mRNA-splicing components in dendrites. When isolated dendrites are transfected with a chicken δ-crystallin pre-mRNA or luciferase reporter pre-mRNA, splicing junctions clustered at or near expected splice sites are observed. Additionally, in vitro synaptoneurosome experiments show that this subcellular fraction contains a similar complement of splicing factors that is capable of splicing chicken δ-crystallin pre-mRNA. These observations suggest that pre-mRNA-splicing factors found in the dendroplasm retain the potential to promote pre-mRNA splicing.
Keywords: neurons, translation, extranuclear, noncanonical
The molecular properties of dendrites have been extensively examined since the discovery of components of the protein synthetic machinery in the dendroplasm, including ribosomes and membranous constituents of the endoplasmic reticulum and Golgi apparatus (1, 2). A more detailed analysis of ribosomal particles suggests that some serine-arginine (SR) proteins associate with ribosomes in the cytoplasm and may be involved in the translational regulation of associated mRNAs (3). These SR proteins, as well as select heterogeneous nuclear ribonucleoproteins (hnRNPs) (4), are known to constitutively move between the nucleus and cytoplasm in nonneuronal cells where they have roles in posttranscriptional gene expression separate from their characteristic activities in nuclear precursor mRNA (pre-mRNA) splicing as core parts of the spliceosome. The spliceosome is a multimegadalton complex of ribonucleoproteins and small nuclear RNAs (snRNAs) that catalyzes the ATP-dependent removal of introns and ligation of exons in nuclear pre-mRNA (5, 6). Some auxiliary constituents of the spliceosome [e.g., SMN (7) and SAM68 (8)] are found throughout the neuronal cytoplasm, and their localization extends into the dendrites. Although it is clear that these cytoplasmic RNA-binding proteins (RBPs) have critical roles in the postsplicing regulation of the intracellular transport, stability, nonsense-mediated decay (NMD), and translation of cellular mRNAs (9, 10), it is not known whether they retain the ability to assemble pre-mRNA splicing-competent complexes in the dendritic milieu. Unspliced or incompletely spliced pre-mRNAs are often sequestered in the nucleus, yet removal of introns by splicing, in many cases, is not essential for mRNA export from the nucleus (11-16). It is believed that some viral mRNAs and alternatively spliced mRNAs are likely exported to the cytosol as intron-retaining transcripts (4).
To determine the functional capacity of intact dendrites to authentically splice pre-mRNAs, we have used several localization assays to determine the presence or absence of other pre-mRNA-splicing factors in the dendroplasm. These splicing factors include proteins associated with the initiation and commitment phases of pre-mRNA splicing as well as RBPs associating with the pre-mRNA at steps just before and after the first critical transesterification reaction. Although pre-mRNA splicing requires these components, their localization itself is suggestive of but not demonstrable evidence for dendritic pre-mRNA splicing. More directly, we have introduced either of two pre-mRNA constructs into intact, isolated neuronal dendrites. Clonal analysis of the spliced products suggests that splicing at canonical as well as atypical and cryptic sites can occur with these mRNA constructs. As a proxy for the postsynaptic sites within the dendritic arbor, we also show that synaptoneurosome (SN) fractions contain similar elements of the pre-spliceosome complex and are capable of generating a similar array of spliced pre-mRNA products. Importantly, when spliced, the ensuing translation of some of the dendritically spliced mRNAs by the intrinsic translational apparatus in intact dendrites generates a detectable protein.
Methods
Immunohistochemical Detection of Splicing Proteins in Neurons. Embryonic day 18 rat hippocampal neurons were cultured as described (17) and, 8-10 days later, were processed with the following primary antibodies that have been used extensively: anti-Sm proteins [clone Y12 (18)], anti-SF2 [clone 96 (19)], anti-SC-35 [clone SC-35 (20)], anti-SR [clone 1H4G7 (21)], and anti-U2AF65 [clone 222-6 (22)]. Cells were incubated in goat anti-mouse Qdot 525 (QDot, Hayward, CA). Neurons were sequentially labeled with a polyclonal anti-microtubule-associated protein 2 (MAP2) antibody or a second primary antibody and then incubated with a Cy5 or Qdot 605 secondary antibody. Images were obtained as described in Supporting Methods, which is published as supporting information on the PNAS web site.
Generation of Spliceosome-Related RBP Constructs. cDNAs encoding rat Magoh, RNPS1, SF1/mBBP, USAF65, UAP56, and Y14 were isolated from whole rat brain by RT-PCR by using Pfu Turbo. Primers were designed by using previously reported mouse or human GenBank sequences (see Supporting Methods).
Ca2+-Phosphate Transfection of DNA Constructs into Primary Neurons. Hippocampal neurons were transfected as described (23).
In Situ Hybridizatin of U1 RNA. In situ hybridization (ISH) was performed as described with a probe complementary to base pairs 20-70 of the U1 RNA (24). Quantitative analysis of ISH signal was obtained by using metamorph software.
Chicken δ-Crystallin (CDC) Pre-mRNA Transfection of Isolated Dendrites and Analysis of Splice Products. Transfection of CDC pre-mRNA into isolated dendrites was as described (25). After transfection, DHPG was added to a final concentration of 20 μM, and isolated dendrites were incubated at 37°C for 1 h. Cultures were washed, fixed in 4% paraformaldehyde/PBS, and washed in PBS. Dendrites were picked individually and reverse transcribed by using primer “sp6.” Dendrites were not pooled for PCR analysis. An initial PCR was performed by using primers sp6 and 5A and then analyzed by gel electrophoresis. Successful amplification of mRNA resulted in the presence of a major 476-bp fragment, with spliced products. The area immediately below the 476-bp band was excised, purified, and reamplified by using the 5A primer and nested primer FLAG. Samples displaying a positive splicing activity produced DNA fragments ranging in size from 125 bp to 476 bp. These fragments were subcloned and sequenced. A total of 35 separate dendrites from 8 different hippocampal cultures gave rise to the 57 sequences that were characterized in this analysis.
SN Preparation. SNs were isolated as described (26). For Western blotting, SNs or whole brain tissue was lysed in the presence of protease inhibitors. Equal amounts of protein (as determined by Bradford assay) were run on NuPAGE 10% Bis-Tris gels, transferred to poly(vinylidene difluoride) (PVDF) membrane, stained by using the antibodies listed, and visualized by using chemiluminescence.
Results
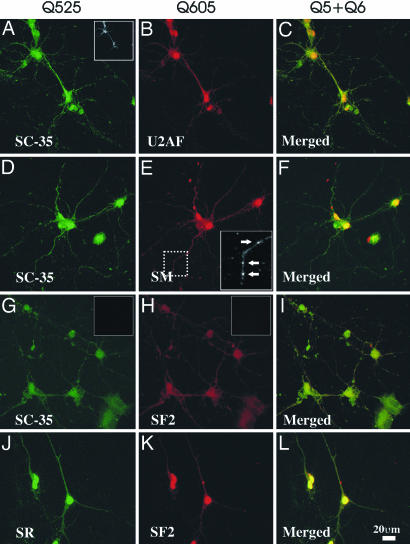
Components of the Spliceosome Machinery Are Localized to Neuronal Dendrites. Initially, we used confocal microscopy to determine the immunofluorescent localization of SC-35 domains (Fig. 1 A, D, and G). Expression in neurons was observed as a series of small puncta interspersed among diffuse grains in the perinuclear space and dendrite. These data were unexpected because little information is known about SC-35 nucleocytoplasmic shuttling. Because SC-35-identified nuclear speckles are believed to be storage sites for numerous splicing factors and SR proteins (27) (Fig. 6, which is published as supporting information on the PNAS web site), we next examined select splicing factors (SF2/ASF and USAF65) or families of factors (Sm or SR proteins) associated with the initiation and commitment steps of pre-mRNA splicing (28). Within the nucleus, each of these RBPs is known to partially colocalize with nuclear speckles (Fig. 6) (29), and we surmised that a similar overlap in expression would be present within the periphery due in part to their known ability to shuttle between the nucleus and cytosol (30-32). In these experiments, we observed high expression of USAF65 (Fig. 1B), Sm antigen (Fig. 1E), SF2 (Fig. 1 H and K), and SR proteins (Fig. 1D) in the nuclei of both neurons and glia. Furthermore, in each experiment, we observed small puncta, or granule-like structures, or, on some occasions, a more diffuse granular signal located in proximal and distal portions of dendrites and their branch points. These granule-like bodies were also observed in the perinuclear space of both neurons and glia, often juxtaposed with the nuclear envelope (data not shown). For some proteins, such as SF2, more granule-like structures were observed in the perinuclear space with reduced levels of SF2 puncta localized in proximal and distal dendrites. The merged confocal images shown in Fig. 1 C, F, I, and L confirm the partial colocalization of each of the antigens when present with speckles or with each other. The presence of the pre-spliceosome antigens in neuronal dendrites was established by colocalization with MAP2 (Fig. 1A Inset). The nucleus contains a high abundance of these splicing enzymes that is easily visible with low laser intensity (Fig. 6). However, identifying these splicing enzyme antigens in dendrites or the cell soma required an increased laser intensity, because the dendrite contains significantly lower quantities of these antigens than the nucleus. Therefore, the nucleus will invariably have an overexposed appearance, obscuring visualization of nuclear subdomains (speckles). The nuclear and cytoplasmic localization of these splicing factors is contrasted against a known nuclear antigen, histone 3 (Fig. 7 H and I, which is published as supporting information on the PNAS web site). We have not examined the localization of other splicing-associated enzymes in dendrites.
Fig. 1.
Coexistence of prespliceosome proteins in neuronal dendrites. Shown are confocal images of cultured hippocampal neurons labeled with Qdots 525/605 for detection of the following: SC-35 (A), U2AF65 (B) and merged A and B (C); SC-35 (D), Sm antigen (E), and merged D and E (F); SC-35 (G), SF2 (H), and merged G and H (I); SR proteins (J), SF2 (K), and merged J and K (L). MAP2 protein was detected in all samples by means of Cy5 immunodetection (A Inset). Qdot secondary antibodies without primary antibodies (G and H Insets) do not produce an observable signal. (Scale bars: B and E, 20 μm.)
Because pre-mRNA splicing complex also employs an snRNA component, we performed ISH for U1 snRNA, which is the core snRNA of the U1 small nuclear ribonucleoprotein (snRNP). ISH revealed the presence of U1 snRNA in the nucleus as expected and more dispersed localization in the cytoplasm (Fig. 2A). Spliceosomal snRNAs are exported to the cytoplasm before maturation into functional snRNPs (33), but there are no previous reports of U1 snRNA localized along the length of dendrites (identified by MAP2; Fig. 2 C and D) with noticeable staining occurring >30 μm from the nucleus and a moderated signal appearing at dendritic branch points. Dendritic U1 signal, although somewhat diffuse, is present at significant levels in the antisense panel (Fig. 2A) as compared with background and when compared with the control ISH (Fig. 2B). In the presence of excess unlabeled antisense U1 snRNA, only slight staining was visible within the nucleus (Fig. 2B). This staining may be due to cross-hybridization with the U1 RNA gene or pseudogenes in the genome (Fig. 2B). Quantitative metamorph analysis (Fig. 2A Inset) identified densitometric differences (as a function of area and compared with background) in probe hybridization intensity between dendrites in Fig. 2 A and B. Additionally, sense controls showed little staining (data not shown).
Fig. 2.
ISH detection of U1 snRNA in dendrites. Shown is ISH with labeled antisense U1 snRNA in the absence (A) or presence (B) of excess unlabeled antisense U1 snRNA. Densitometric analysis of the ISH signals (A Inset) of ISH signal in dendrites (MAP2; C and D) provides quantitation of the signal observed in A and B.(Inset) Relative fold intensity is the y axis.
After the initial commitment to splicing, pre-mRNA processing is accompanied by a series of changes in the protein composition of the spliceosome. Three individual subcomplexes of the spliceosome [i.e., A (previously referred to as the pre-spliceosome), B*, and C] have recently been purified, and their components have been identified (6, 34-36). Subcomplex B* temporally represents the messenger RNP (mRNP) remodeling before the first transesterification reaction whereas the catalytic C subcomplex represents the splice-intermediate stage after this first chemical step. It is notable that many RBPs can be identified as components of multiple subcomplexes (6). In addition, similar to certain SR proteins and heterogeneous nuclear RNPs (hnRNPs), many of these RBPs are known to shuttle to the cytosol from the nucleus (31, 37) and have distinct roles in the cytoplasm (38, 39).
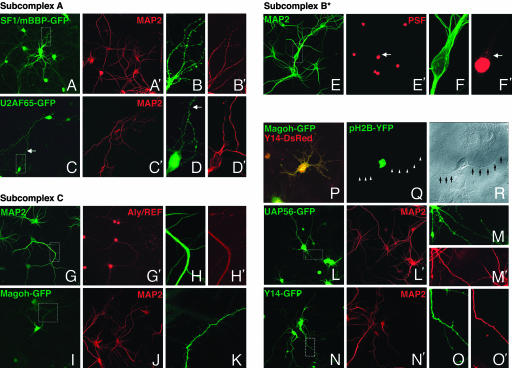
To examine a select few of the RBPs that can be associated with different spliceosome subcomplexes, we opted to engineer RBP-GFP or RBP-DsRed chimeras in addition to using well characterized antibodies for immunolocalization. For complex A, we generated SF1/mBBP-GFP (Fig. 3 A and B) and U2AF65-GFP fusion constructs (Fig. 3 C and D). For complex B*-related elements, we used a well characterized antibody to the polypyrimidine tract-binding protein-associated splicing factor (PSF, Fig. 3 E′ and F′) (40). Finally, for complex C-related factors, we used an antibody to Aly/REF (Fig. 3 G′ and H′) and GFP or DsRed chimeras containing the ORF of Magoh (Fig. 3 I and K), UAP56 (Fig. 3 L and M), Y14 (Fig. 3 N and O), and RNPS1 (data not shown). GFP or DsRed fusion constructs were transfected into primary neurons at 10-12 days in vitro. With low-resolution whole-cell images, signal can clearly be detected in dendrites. At this laser intensity, the signal in nuclei is saturated such that nuclear subdomains cannot be identified. SF1-mBBP-GFP and U2AF65-GFP showed consistent nuclear localization complemented by larger puncta as well as more dispersed granule-like structures throughout the perinuclear and dendritic cytoplasm in higher magnification images. Importantly, USAF65-GFP overexpression patterns did not differ significantly from the U2AF65 immunofluorescence obtained previously. PSF, however, showed a separate pattern of localization, with speckled nuclei distinctly visible. Low but significant cytoplasmic staining was also visible in the perinuclear space (arrow in Fig. 3 F′). Similar immunofluorescent observations of endogenous PSF levels have been shown. Of note, when Kanai et al. (40) used a PSF-GFP chimera, granular puncta were exhibited throughout the dendroplasm, suggesting that there may be some masking of the antibody epitope when part of larger macromolecules. Many splice-factor RBPs are normally sequestered as components of messenger RNP complexes. Some protease treatments are capable of unmasking a greater percentage of the endogenous protein. However, in some cases, conventional immunofluorescence may not be altogether representative of the native protein localization. Aly/REF immunofluorescence was predominantly seen in nuclei. However, a consistently low level of diffuse signal was visible interspersed throughout the dendrite. In comparison, Magoh-GFP, Y14-GFP, UAP56-GFP, and RNPS1-GFP (data not shown) were scattered in granule-like structures in the dendroplasm and, as expected, speckle-like domains in the nucleoplasm (Fig. 7). Experiments performed with anti-Magoh (mAb 21B12) or anti-Y14 (mAb 4C4) antibodies (41) showed no difference when comparing the steady-state expression pattern of these proteins in parallel cultures and the pattern of signal obtained with the overexpression of Magoh- or Y14-GFP in transfected cultures (Fig. 7 L and N). Magoh and Y14 are known to directly interact (41). When coexpressed in primary neurons, Magoh-GFP and Y14-DsRed showed significant colocalization (Fig. 3P).
Fig. 3.
Distribution of select components of spliceosomal subcomplexes A, B*, and C. Olympus FV1000 images of primary neurons show the subcellular localization of the selected spliceosome components. For subcomplex A, we show SF1/mBBP-GFP (A) and U2AF65-GFP (B) contrasted against MAP2 (red; A′ and C′). A hatched box in the leftmost panel outlines the area highlighted for higher magnification views of SF1/mBBP-GFP (B) and U2AF65-GFP (D). For subcomplex B*, immunofluorescence of anti-PSF monoclonal antibody in whole cell (E′) and higher magnification view (F′) is shown. MAP2 immunofluorescence is shown in E and F. For subcomplex C, immunofluorescence with an anti-Aly/REF antibody in low (G′) and high (H′) magnification views is contrasted with MAP2 (G and H). GFP chimeras of Magoh, UAP56, or Y14 at the whole cell (I, L, and N) or dendritic fields (K, M, and O) show the diffusely and more concentrated puncta within the dendroplasm. MAP2 immunofluorescence is visible for both low (J, L′, and N′) and high magnification (M′ and O′) images. Overexpression of H2B-YFP is nuclear (Q). A differential interference contrast image (R) highlights dendritic morphology (arrowheads).
Three additional observations are worth noting. First, we routinely see a distinct staining pattern in nonneuronal cells in our primary cultures when compared with transfected neurons on the same coverslip. With the exception of U2AF65-GFP and Magoh-GFP, the subcellular distribution of RBP-GFP chimeras in glia was limited to nuclei with modest, if any, staining visible in the cytoplasm (Fig. 7 B-E). Second, when neurons were transfected with the pEGFP-N1 or pDsRed-N1 (data not shown) construct alone, we observed two distinct types of expression patterns that are dissimilar to any spliceosome RBP-GFP fusion construct (Fig. 7 F and G). These localization experiments provide strong evidence that a mixture of pre-mRNA-splicing factors are distributed throughout the dendritic cytoplasm of neurons and have a variant expression in nonneuronal cells.
Sequence Characterization of Spliced CDC RNA from Isolated Dendrites. To directly assess the capability of intact neuronal dendrites to splice pre-mRNAs, we used a pre-mRNA-splicing construct comprising exons 14 and 15 of the CDC gene (42) (37). This construct has been used to assess splicing in a variety of cell types (14, 43, 44) (45). In the CDC pre-mRNA, the 87-bp exon 14 and 73-bp exon 15 of the CDC gene are interrupted by a 257-bp intron (Fig. 8, which is published as supporting information on the PNAS web site). To perform these experiments, CDC pre-mRNA was transcribed, encoated with a polycationic lipid, and manually applied onto isolated dendrites that were isolated from their cognate cell somas (25, 46-48). After a period of incubation, RNA was extracted from the isolated dendrites. After reverse transcription of this RNA with a 3′ end anchor primer, the cDNA was used as template in multiple rounds of PCR by using CDC-specific primers with nested primer sets. These PCR amplicons were subcloned and sequenced to determine the splice boundaries used.
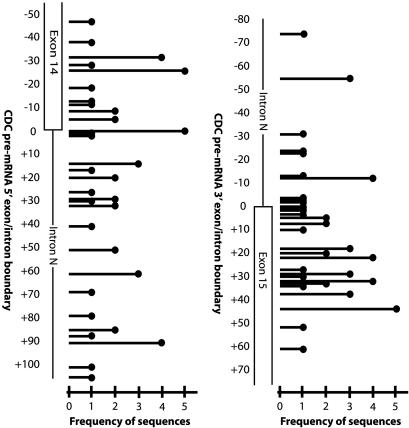
Analysis of 53 CDC pre-mRNA sequences spliced in isolated dendrites from eight independent experiments revealed clustering around several separate exon/intron sequences at the donor and acceptor splice junctions (Figs. 4 and 8). At the donor splice junction, 5 of 53 sequences adhered to the AG canonical dinucleotide pair predicted for CDC pre-mRNA splicing (42). The statistical probability of this splicing occurring randomly is <3 × 10-7. This number was calculated by determining the binomial probability of detecting this clustering over the length of the transfected RNA, given the total number of spliced RNA sequences available for analysis. Other observed 5′ exon/intron boundaries were dispersed 5′ or 3′ to the predicted CDC pre-mRNA splice site adhering to other nominal canonical, atypical (49), and cryptic splice sites (50). At the acceptor splice junction, we observed a cluster of sequences surrounding the predicted exon/intron boundary, with a single clone exhibiting the canonical 3′ GT splice junction. These data show a greater conservation of the CDC pre-mRNA donor splice site than of the acceptor site. There was no single full-length clone that contained both the 5′ and 3′ predicted CDC pre-mRNA splice junctions. In ≈50% of the transfections, no splicing of pre-mRNAs was observed.
Fig. 4.
Schematic representation of spliced CDC pre-mRNA splice junctions. A schematic of CDC-spliced pre-mRNAs shows the observed donor and acceptor splice sites, their frequency, and their position relative to the predicted exon donor and acceptor splice sites. A single line corresponds to the observed donor or acceptor site. The height of the line indicates the frequency of that specific sequence. The distance 5′ or 3′ from the predicted donor or acceptor site (e.g., the zero point) is denoted by descending and ascending numbers to the left of the schematic showing the exon/intron organization.
As with any PCR-dependent protocol, aberrant amplification of unspliced CDC pre-mRNA or spliced CDC mRNA through mis-priming, or extension of two hybridizing DNA fragments could result in a truncated DNA fragment that would mimic pre-mRNA splicing. Consequently, sequences that do not contain extension of CDC pre-mRNA sequence on both the 5′ and 3′ ends were discounted. If these data were the result of such internal priming of the CDC cDNA at regions of homology in other CDC cDNAs, then one would expect a majority of transcripts to contain three or more bases of similarity in the donor and acceptor sites at the splice junction (the number of 3′ end primer-matched bases to prime PCR) (51, 52). In contrast, two-thirds of the spliced sequences have two or fewer bases overlap, suggesting that the PCR was of high fidelity. CDC pre-mRNA transfections were also performed in the absence of dendrites or in combination with dendrites and immediately harvested for analysis. Under these conditions, we observed only unspliced pre-mRNA.
To show that pre-mRNA splicing in dendrites was not unique to the CDC pre-mRNA, we repeated experiments using a second construct containing the SV40 small tumor antigen intron. The pGL2 splicing construct contains the luciferase-coding region fused to the SV40 small T antigen intron interrupting the luciferase 3′ untranslated region (Fig. 8). This construct has been used extensively in pre-mRNA-splicing experiments as a control where it is efficiently spliced in mammalian cells. This luciferase-SV40 sequence was PCR amplified from the pGL2 plasmid with the 5′-luciferase-directed primer containing a T7 RNA polymerase promoter site so that sense RNA could be made from this construct. After transfection, splice product sequences from the luciferase-SV40 chimera were analyzed as described for the CDC pre-mRNA-splicing experiments by using specific primers. Five unique spliced sequences were detected (Fig. 8). As observed with CDC pre-mRNA, both conventional and cryptic splice donor/acceptor sites were observed. Cryptic splicing sites with the SV40 small T antigen intron 3′ to some genes have been previously observed (53, 54).
SNs Can Splice CDC Pre-mRNA. SNs have been used to demonstrate protein synthesis in dendritically enriched regions of neurons (55-57). This subcellular fraction of tissue homogenates contains liposomes of pre- and postsynaptic entities and as such is a close proxy for the postsynaptic compartment within dendrites (26). U2AF65, pan-SR, SC-35, SF2, and Sm antigens were all present in the whole-brain extract as well as in the SN fraction at differing relative abundances (Fig. 9, which is published as supporting information on the PNAS web site). Although SN preparations are never purely neuronal, if the observed differing intensities of the protein bands were a function of nuclear contamination, then one would expect the same relative abundances for each of the proteins in the SN and whole-cell lysate fractions. As seen in Fig. 9, this phenomenon is not observed.
Pre-mRNA splicing in SN fractions was performed with freshly prepared SNs supplemented with 1.5 mM ATP and the CDC pre-mRNA. Analysis of 10 spliced sequences generated from three separate experiments (Fig. 9) showed a majority of donor (8 of 10) and acceptor (8 of 10) splice junctions at or near the sequences observed with CDC pre-mRNA splicing in intact dendrites. Consistent with the ATP-dependent nature of the spliceosome and the relative absence of mitochondria in these fractions, no splice forms were generated when ATP was omitted from the SN preparation (data not shown).
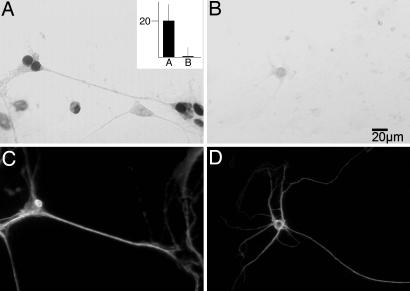
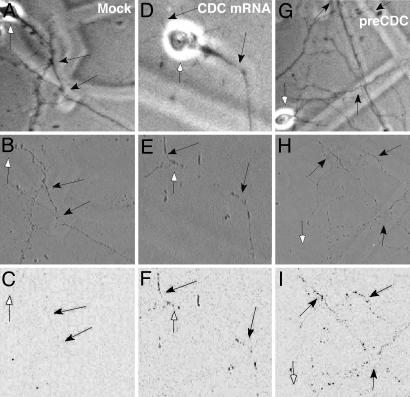
Spliced CDC RNA Can Be Translated in Isolated Dendrites. Current models of pre-mRNA metabolism have suggested a functional coupling between different processing stages, in particular pre-mRNA splicing and translation (39). Transfection of the CDC pre-mRNA into isolated dendrites involves mechanical severing of the dendrites from the cell body and removal of the soma, followed by local application of the lipid-encoated RNA to the isolated dendrites. Fig. 5 A, D, and G shows photomicrographs of primary neurons with the cell somas intact (white arrows). Fig. 5 B, E, and H shows these same microscopic fields after removal of the soma, leaving the dendrites (black arrows). Because the CDC pre-mRNA contains an in-frame FLAG tag in the second exon, this epitope should be immunohistochemically detectable if the dendritically spliced CDC pre-mRNA can be translated. Unspliced CDC pre-mRNA will not produce a translatable FLAG epitope. As a control, we have removed the exon 14 and part of the intron N of the CDC pre-mRNA, leaving intact the 3′ end of the intron and exon 15 that is fused with the FLAG epitope. Upon transfection of this RNA into isolated dendrites, no FLAG antigen is detected (Fig. 10, which is published as supporting information on the PNAS web site), showing that the second exon is not translationally competent by itself. When these dendrites are stained for FLAG expression after mock transfection (Fig. 5C) and transfection with mature CDC pre-mRNA (Fig. 5F) or unspliced CDC pre-mRNA (Fig. 5I), FLAG sequence expression levels in dendrites is similar when transfected with mature CDC RNA and CDC pre-mRNA. These data show that the CDC pre-mRNA can be translated after splicing has occurred.
Fig. 5.
Protein translation from FLAG-tagged CDC pre-mRNA in isolated dendrites. Phase contrast images of whole neurons before cell body removal (A, D, and G). Shown are corresponding phase contrast images of isolated dendrites transfected with the following: no RNA (B, mock), mature CDC RNA (E), or unspliced CDC pre-mRNA (H), and subsequent immunodetection of protein translation with an anti-FLAG antibody and DAB visualization (C, F, and I, respectively). Black arrows indicate isolated, transfected dendrites before and after immunodetection of FLAG protein translation. White arrows point out areas previously occupied by cell bodies before dissection.
Discussion
In this report, we have used a primary neuronal culture system to establish the pre-mRNA-splicing capability of isolated neuronal dendrites. These data are supported by a series of localization studies examining the subcellular distribution of several spliceosome-related components. The presence of many of these RBPs in the cytosol is not completely unexpected because previous studies, many of which were done in nonneuronal cells, have noted that these factors shuttle between the nucleus and cytoplasm. In combination with their distinct, burgeoning roles in regulating other aspects of RNA metabolism, the trafficking of these RBPs to the dendroplasm may seem less than startling. However, these data do not exclude the possibility that a subfraction of these factors may yet be able to form pre-mRNA-splicing-competent complexes. Artificial pre-mRNA reporter constructs are capable of being spliced when transfected into isolated dendrites. Similarly, SN fractions containing postsynaptic sites like those found in dendrites contain a comparable diversity of pre-spliceosome components that are pre-mRNA-splicing-competent when supplemented with ATP.
One current model of mRNA splicing suggests coordination between splicing and translation by means of the association of SR protein-splicing factors, in particular SF2, and translating ribosomes (3). The data in this manuscript suggest that spliced reporter pre-mRNA can be translated in the mammalian dendritic cytoplasm after dendritic pre-mRNA splicing. The functional significance of dendritic splicing is unclear. Sequence analysis of spliced pre-mRNAs from isolated dendrites or in vitro SN fractions yielded clusters of sequences that cluster at or near the predicted CDC pre-mRNA splicing junctions, which adhere to the canonical AG/GT donor/acceptor pair concensus site. The remainder of the characterized sequences showed diverse intronic terminal dinucleotides. This dispersal of donor and acceptor sites has made finding a splicing mechanism difficult. It is the structure of the entire splice site signal that determines whether the major or minor spliceosome catalyzes the reaction. In the nucleus, the correct secondary structure of the pre-mRNA splice site is assured by the sequential binding of chaperones and accessory proteins, some in the nucleus whereas others bind in the cytoplasm, that promote the correct folding of the RNA and position splicing factors appropriately to facilitate pre-mRNA splicing (58, 59). This contextual binding of splicing factors to pre-mRNA is likely to prove to be an important factor determining efficiency in splicing and postsplicing events (37), such as protein translation, quite possibly in a transcript-specific manner. We observed slight (1- to 6-bp) differences in several of the donor/acceptor site position (Figs. 4 and 8). Thus, it is likely that some of the dendritically spliced sequences represent splicing errors rather than noncanonical or cryptic splice sites. Although there are little data on the major spliceosome error rate, the minor spliceosome error rate is estimated to be as high as 1 in 280 splice events (60).
Data in Fig. 4 for dendrite splicing of CDC pre-mRNA show that there is a clustering of splice sites at the predicted donor and acceptor sites. These data suggest a partial conservation of mechanism between the nucleus and the dendrite. This conservation likely results from an overlap in the components of the splicing complex that are shared between these subcellular domains. It should be noted that only a subset of splicing-associated proteins were assessed in this manuscript, and some may be absent from the dendritic domain. It is possible that additional splicing proteins or chaperones are required to generate conventionally spliced CDC mRNA in the dendrite. Further, it is possible that the relative abundances of the dendritically localized splicing proteins, which are distinct from that in the nucleus (Fig. 9), may be important in facilitating the conventional splicing patterns.
During several stages of mRNA processing, surveillance mechanisms were used to prevent inappropriate gene expression. Interestingly, several of the splicing factors that make up the exon junction complex (Magoh, Y14, RNPS1, Aly/REF, SRm160, and Upf3) are at the nexus linking pre-mRNA processing and several postsplicing activities, including nuclear export, NMD, and cytoplasmic localization. The spliced pre-mRNAs observed in the present study cannot result from NMD, given that, in the mammalian system, (i) there is no RNA ligation step associated with the NMD phenomena and (ii) NMD occurs through 5′ to 3′ and 3′ to 5′ exonuclease degradation of the RNA as opposed to endolytic cleavage (61). Even if an endonucleolytic cleavage had been described in the mammalian system, the generation of the observed products would have to result from two endolytic cleavages or a single endolytic cleavage, followed by exonuclease activity with subsequent ligation of the two RNA products. Therefore, the described data are more consistent with a dendritic-splicing activity rather than NMD.
The totality of the data presented provides evidence for the rudiments of pre-mRNA splicing in neuronal dendrites. As occurred with the discovery of protein synthesis in neuronal dendrites, the existence of dendritic splicing may well be followed by the elucidation of endogenous pre-mRNAs in dendrites, a finding that would provide insight into the functional significance of this phenomenon. It is intriguing to speculate, however, that such dendritic splicing may be responsible for the generation of a previously unappreciated diversity of postsynaptic responses that significantly increase the molecular complexity and functional capacity of the synapse.
Supplementary Material
Acknowledgments
We thank Dr. R. Pittman (University of Pennsylvania, Philadelphia) for the anti-histone 3 antibody, Dr. D. Spector (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) for the pH2B-YFP construct, Dr. C. Garner (Stanford University, Palo Alto, CA) for anti-MAP2 antibody, and Dr. T. Kannanayakal for the metamorph analysis. This work was funded by National Institutes of Health Grants NS46894 (to J.G.), P30 NS047321 (to P.H.), P20 MH071705 (to P.H. and J.E.), and AG9900 and MH58561 (to J.E.).
Author contributions: J.G., K.Y.M., B.B., and J.E. designed research; J.G., K.Y.M., J.-Y.S., L.B., B.B., and J.E. performed research; J.G., K.Y.M., and J.E. contributed new reagents/analytic tools; J.G., K.Y.M., J.-Y.S., L.B., B.B., P.H., and J.E. analyzed data; and J.G., K.Y.M., J.-Y.S., L.B., B.B., P.H., and J.E. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: pre-mRNA, precursor mRNA; SR, serine-arginine; snRNA, small nuclear RNA; NMD, nonsense-mediated decay; SN, synaptoneurosome; RBP, RNA-binding protein; ISH, in situ hybridization; CDC, chicken δ-crystallin; MAP2, microtubule-associated protein 2; RNP, ribonucleoprotein; PSF, protein-associated splicing factor.
References
- 1.Torre, E. R. & Steward, O. (1996) J. Neurosci. 16, 5967-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardiol, A., Racca, C. & Triller, A. (1999) J. Neurosci. 19, 168-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanford, J. R., Gray, N. K., Beckmann, K. & Caceres, J. F. (2004) Genes Dev. 18, 755-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakielny, S. & Dreyfuss, G. (1997) Curr. Opin. Cell Biol. 9, 420-429. [DOI] [PubMed] [Google Scholar]
- 5.Staley, J. P. & Guthrie, C. (1998) Cell 92, 315-326. [DOI] [PubMed] [Google Scholar]
- 6.Jurica, M. S. & Moore, M. J. (2003) Mol. Cell 12, 5-14. [DOI] [PubMed] [Google Scholar]
- 7.Fan, L. & Simard, L. R. (2002) Hum. Mol. Genet. 11, 1605-1614. [DOI] [PubMed] [Google Scholar]
- 8.Grange, J., Boyer, V., Fabian-Fine, R., Fredj, N. B., Sadoul, R. & Goldberg, Y. (2004) J. Neurosci. Res. 75, 654-666. [DOI] [PubMed] [Google Scholar]
- 9.Lykke-Andersen, J., Shu, M. D. & Steitz, J. A. (2001) Science 293, 1836-1839. [DOI] [PubMed] [Google Scholar]
- 10.Reed, R. & Hurt, E. (2002) Cell 108, 523-531. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues, J. P., Rode, M., Gatfield, D., Blencowe, B. J., Carmo-Fonseca, M. & Izaurralde, E. (2001) Proc. Natl. Acad. Sci. USA 98, 1030-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzik, B. W., Levesque, L., Prasad, S., Bor, Y. C., Black, B. E., Paschal, B. M., Rekosh, D. & Hammarskjold, M. L. (2001) Mol. Cell. Biol. 21, 2545-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun, I. C., Herold, A., Rode, M., Conti, E. & Izaurralde, E. (2001) J. Biol. Chem. 276, 20536-20543. [DOI] [PubMed] [Google Scholar]
- 14.Jin, L., Guzik, B. W., Bor, Y. C., Rekosh, D. & Hammarskjold, M. L. (2003) Genes Dev. 17, 3075-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi, R., Qin, Y., Macara, I. G. & Cullen, B. R. (2003) Genes Dev. 17, 3011-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lund, E., Guttinger, S., Calado, A., Dahlberg, J. E. & Kutay, U. (2004) Science 303, 95-98. [DOI] [PubMed] [Google Scholar]
- 17.Buchhalter, J. R. & Dichter, M. A. (1991) Brain Res. Bull. 26, 333-338. [DOI] [PubMed] [Google Scholar]
- 18.Lerner, E. A., Lerner, M. R., Janeway, C. A., Jr., & Steitz, J. A. (1981) Proc. Natl. Acad. Sci. USA 78, 2737-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanamura, A., Caceres, J. F., Mayeda, A., Franza, B. R., Jr., & Krainer, A. R. (1998) RNA 4, 430-444. [PMC free article] [PubMed] [Google Scholar]
- 20.Fu, X. D. & Maniatis, T. (1990) Nature 343, 437-441. [DOI] [PubMed] [Google Scholar]
- 21.Neugebauer, K. M., Stolk, J. A. & Roth, M. B. (1995) J. Cell Biol. 129, 899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamore, P. D. & Green, M. R. (1991) EMBO J. 10, 207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park, Y. K., Galik, J., Ryu, P. D. & Randic, M. (2004) Neurosci. Lett. 361, 220-224. [DOI] [PubMed] [Google Scholar]
- 24.Prakash, N., Fehr, S., Mohr, E. & Richter, D. (1997) Eur. J. Neurosci. 9, 523-532. [DOI] [PubMed] [Google Scholar]
- 25.Crino, P. B. & Eberwine, J. (1996) Neuron 17, 1173-1187. [DOI] [PubMed] [Google Scholar]
- 26.Booth, R. F. & Clark, J. B. (1978) Biochem. J. 176, 365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moen, P. T., Jr., Smith, K. P. & Lawrence, J. B. (1995) Hum. Mol. Genet. 4, 1779-1789. [DOI] [PubMed] [Google Scholar]
- 28.Guth, S., Martinez, C., Gaur, R. K. & Valcarcel, J. (1999) Mol. Cell. Biol. 19, 8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamond, A. I. & Spector, D. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 605-612. [DOI] [PubMed] [Google Scholar]
- 30.Yong, J., Wan, L. & Dreyfuss, G. (2004) Trends Cell Biol. 14, 226-232. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson, M. F. (2005) Trends Genet. 21, 143-148. [DOI] [PubMed] [Google Scholar]
- 32.Huang, Y. & Steitz, J. A. (2005) Mol. Cell 17, 613-615. [DOI] [PubMed] [Google Scholar]
- 33.Will, C. L. & Luhrmann, R. (2001) Curr. Opin. Cell Biol. 13, 290-301. [DOI] [PubMed] [Google Scholar]
- 34.Hartmuth, K., Urlaub, H., Vornlocher, H. P., Will, C. L., Gentzel, M., Wilm, M. & Luhrmann, R. (2002) Proc. Natl. Acad. Sci. USA 99, 16719-16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurica, M. S., Licklider, L. J., Gygi, S. R., Grigorieff, N. & Moore, M. J. (2002) RNA 8, 426-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makarov, E. M., Makarova, O. V., Urlaub, H., Gentzel, M., Will, C. L., Wilm, M. & Luhrmann, R. (2002) Science 298, 2205-2208. [DOI] [PubMed] [Google Scholar]
- 37.Kataoka, N., Yong, J., Kim, V. N., Velazquez, F., Perkinson, R. A., Wang, F. & Dreyfuss, G. (2000) Mol. Cell 6, 673-682. [DOI] [PubMed] [Google Scholar]
- 38.Tange, T. O., Nott, A. & Moore, M. J. (2004) Curr. Opin. Cell Biol. 16, 279-284. [DOI] [PubMed] [Google Scholar]
- 39.Maquat, L. E. (2004) Nat. Rev. Mol. Cell Biol. 5, 89-99. [DOI] [PubMed] [Google Scholar]
- 40.Kanai, Y., Dohmae, N. & Hirokawa, N. (2004) Neuron 43, 513-525. [DOI] [PubMed] [Google Scholar]
- 41.Kim, V. N., Yong, J., Kataoka, N., Abel, L., Diem, M. D. & Dreyfuss, G. (2001) EMBO J. 20, 2062-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohno, M., Sakamoto, H. & Shimura, Y. (1987) Proc. Natl. Acad. Sci. USA 84, 5187-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi, Y., Hanaoka, K., Hayasaka, M., Katoh, K., Kato, Y., Okada, T. S. & Kondoh, H. (1988) Development (Cambridge, U.K.) 102, 259-269. [DOI] [PubMed] [Google Scholar]
- 44.Ohe, K., Lalli, E. & Sassone-Corsi, P. (2002) Proc. Natl. Acad. Sci. USA 99, 1146-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kondoh, H., Takahashi, Y. & Okada, T. S. (1984) EMBO J. 3, 2009-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aakalu, G., Smith, W. B., Nguyen, N., Jiang, C. & Schuman, E. M. (2001) Neuron 30, 489-502. [DOI] [PubMed] [Google Scholar]
- 47.Kacharmina, J. E., Job, C., Crino, P. & Eberwine, J. (2000) Proc. Natl. Acad. Sci. USA 97, 11545-11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Job, C. & Eberwine, J. (2001) Proc. Natl. Acad. Sci. USA 98, 13037-13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hastings, M. L. & Krainer, A. R. (2001) RNA 7, 471-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee, M. P. & Feinberg, A. P. (1997) Cancer Res. 57, 3131-3134. [PubMed] [Google Scholar]
- 51.Wu, D. Y., Ugozzoli, L., Pal, B. K., Qian, J. & Wallace, R. B. (1991) DNA Cell Biol. 10, 233-238. [DOI] [PubMed] [Google Scholar]
- 52.Liang, P. & Pardee, A. B. (1995) Curr. Opin. Immunol. 7, 274-280. [DOI] [PubMed] [Google Scholar]
- 53.Evans, M. J. & Scarpulla, R. C. (1989) Gene 84, 135-142. [DOI] [PubMed] [Google Scholar]
- 54.Huang, M. T. & Gorman, C. M. (1990) Mol. Cell. Biol. 10, 1805-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiler, I. J. & Greenough, W. T. (1993) Proc. Natl. Acad. Sci. USA 90, 7168-7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao, A. & Steward, O. (1993) J. Neurochem. 61, 835-844. [DOI] [PubMed] [Google Scholar]
- 57.Bagni, C., Mannucci, L., Dotti, C. G. & Amaldi, F. (2000) J. Neurosci. 20, RC76, 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith, C. W. & Valcarcel, J. (2000) Trends Biochem. Sci. 25, 381-388. [DOI] [PubMed] [Google Scholar]
- 59.Maniatis, T. & Tasic, B. (2002) Nature 418, 236-243. [DOI] [PubMed] [Google Scholar]
- 60.Levine, A. & Durbin, R. (2001) Nucleic Acids Res. 29, 4006-4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lejeune, F., Li, X. & Maquat, L. E. (2003) Mol. Cell 12, 675-687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.