Abstract
Mycobacterium tuberculosis remains a major cause of pulmonary infection worldwide. Attachment of M. tuberculosis organisms to alveolar macrophages (AMs) represents the earliest phase of primary infection in pulmonary tuberculosis. In this study fibronectin (Fn), an adhesive protein, is shown to bind M. tuberculosis organisms and facilitates attachment of M. tuberculosis to murine AMs. A monoclonal antibody (MAb) specific to the heparin binding domain (HBD) of Fn decreases 125I-Fn binding to M. tuberculosis; whereas MAbs specific to either the cell binding domain (CBD) or the gelatin binding domain (GBD) have no effect on Fn binding to M. tuberculosis. In the presence of exogenous Fn (10 μg/ml) M. tuberculosis attachment to AMs increased significantly from control levels (means ± standard errors of the means) of 11.5% ± 1.1% to 44.2% ± 4.2% (P < 0.05). Fn-enhanced attachment was significantly decreased from 44.2% ± 4.2% to 10.8% ± 1.2% (P < 0.05) in the presence of anti-Fn polyclonal antibodies. The attachment is also inhibited in the presence of MAbs specific for the HBD and CBD, whereas MAbs specific to GBD did not affect the attachment. Further, an Fn cell binding peptide, Arg-Gly-Asp-Ser (RGDS), decreased the attachment from 44.2% ± 4.2% to 15.3% ± 1.2% (P < 0.05), whereas addition of a control peptide, Arg-Gly-Glu-Ser (RGES) did not affect the attachment (40.5% ± 1.8%). These results suggest that Fn-mediated attachment of M. tuberculosis can occur through the binding of Fn to the AM via the CBD and to M. tuberculosis organisms via the HBD.
Tuberculosis remains a major health problem throughout the world (11, 38). The initial infection with Mycobacterium tuberculosis typically occurs in the alveolar spaces of the lung. Attachment of the tubercle bacillus to alveolar macrophages (AMs) is a crucial step in the establishment of infection, as the organism first survives and replicates in AMs as an intracellular pathogen. Previous studies have shown that M. tuberculosis organisms can attach and enter macrophages by specific cell surface receptors, including complement receptors (CR1 and CR3), the C2a component of complement, mannose receptor, transferrin receptor, CD14 scavenger receptor, and an unknown receptor that is inhibited by β-glucan (12, 40).
Fibronectin (Fn) is known to mediate attachment of several different microorganisms to host cells (17). Fn is an extracellular matrix protein with two similar subunits joined near the C-terminal end. This heterodimeric glycoprotein contains multiple binding domains and possesses binding properties to different ligands such as heparin, collagen, and fibrin (52). Fn binds to a wide variety of microorganisms in a ligand receptor-mediated manner (17). For instance, previous in vitro studies have shown that Fn binds to Escherichia coli, Streptococcus pyogenes, Salmonella enterica serovar Dublin, and Candida albicans (6, 14, 33, 39). The binding sites of Fn play an integral role in the recognition of microorganisms. For example, Fn binds Staphylococcus aureus via the N-terminal domain and Treponema pallidum via the cell binding domain (CBD) of Fn (36, 47). Similarly, in vitro, Fn facilitates the attachment of Pneumocystis carinii to AMs via the CBD (34) that is known to interact with integrins on the cell surface. Other studies indicate that microbial attachment may occur through different binding sites on Fn, such as the C-terminal domain (24, 45, 51).
Prior studies have shown that mycobacteria interact with Fn. Fn attachment proteins (FAP) are surface proteins that are present in a variety of mycobacterial species, including Mycobacterium tuberculosis (1), Mycobacterium avium (43), Mycobacterium leprae (44), and Mycobacterium vaccae (37). The FAP are a family of highly homologous proteins (37). The FAP from M. leprae bound to the carboxy-terminal heparin binding chymotryptic fragment of Fn (44). Antibodies directed against FAP significantly inhibit attachment of mycobacteria to host epithelial cells (23). Additionally, M. tuberculosis secrete an Fn-binding protein known as the antigen 85 complex (49). This complex is composed of three proteins, Ag85A, Ag85B, and Ag85C. The antigen 85 functions as a mycolyl transferase (3). Disruption of the gene for Ag85A results in diminished growth of M. tuberculosis in cultured macrophages (2).
In this study, we demonstrated that Fn can mediate attachment of M. tuberculosis to murine AMs. The data suggest that Fn interacts with M. tuberculosis via the heparin binding domain (HBD) of Fn. Fn-enhanced attachment of M. tuberculosis to murine AMs was decreased by the addition of monoclonal antibodies (MAbs) to either HBD or cell binding domain. Further, Fn-mediated attachment of M. tuberculosis to AMs was blocked by the tetrapeptide sequence of the CBD, RGDS (Arg-Gly-Glu-Ser), suggesting a possible role for the CBD of Fn in the mediation of Fn attachment to AMs.
MATERIALS AND METHODS
M. tuberculosis isolation.
The H37Ra strain of M. tuberculosis (American Type Culture Collection, Manassas, Va.) was cultured at 37°C in 5% CO2 atmosphere in dispersed form in Middlebrook 7H9 broth (Difco Laboratories, Detroit, Mich.) containing albumin, dextrose, and catalase as enrichments. Bacterial growth was monitored by a Spectronic 20D spectrophotometer (Milton Roy Company, Rochester, N.Y.) (9). To achieve a single-cell suspension, the bacterial suspension was briefly sonicated (20 W for 5 to 10 s). The suspension was then gently agitated and allowed to settle for 5 min. The top portion of the suspension containing bacteria was used in the assay. Bacterial cultures, 10 to 14 days old, were centrifuged and washed once with normal saline. The final concentration of the bacterial suspension was adjusted to 109 organisms/ml. Each batch of bacterial suspension was stained with Kinyoun stain (Midlantic Biomedical Inc., Paulsboro, N.J.) and observed under the microscope to verify the purity of the suspension. Routinely, samples of bacteria were also grown on mycobacterial 7H11 agar (Difco Laboratories) plates as stock.
Isolation and preparation of Fn.
Bovine Fn was used for the majority of studies due to its homology with human Fn and because it shares identical binding sites with human Fn (18, 21). Bovine Fn was much less expensive than human Fn and was isolated according to the method of Hynes (19) with modifications (50). Briefly, a gelatin-Sepharose column (4.8 by 30 cm; Pharmacia, LKB Biotechnology, Piscataway, N.J.) was equilibrated with 50 mM Tris-HCl (pH 7.4), and 4.0 liters of serum was applied. The column was washed with 1.0 liter of equilibration buffer (50 mM Tris-HCl [pH 7.4]) followed by 1.0 liter of 50 mM Tris-HCl (pH 7.4) with 0.5 M NaCl. Fn was eluted from the column with an elution buffer (50 mM Tris-HCl [pH 7.4], 4 M urea). The eluted fractions were collected and read in a spectrophotometer at 280 nm. The positive fractions were applied to a DEAE cellulose column (2.5 by 120 cm; Whatman Biosystems Ltd., Maidstone, Kent, United Kingdom) and equilibrated in 50 mM Tris-HCl (pH 7.4)-4 M urea. After washing the DEAE column with 500 ml of 10 mM Tris-HCl (pH 7.4)-4.5 M urea, Fn was eluted with a linear gradient of 500 ml of 10 mM Tris-Cl (pH 7.4)-4.5 M urea and 500 ml of 10 mM Tris-Cl (pH 7.4)-4.5 M urea and 0.5 M NaCl. The fractions were collected and read in a spectrophotometer at 280 nm. The positive fractions were pooled, and the Fn was dialyzed three times in 4 liters of 50 mM NaHCO3. The concentration of Fn was determined by the Lowry protein assay (28). Purity of the isolated Fn was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (25) and confirmed by Western blotting (48) using an anti-Fn antibody (Gibco-BRL, Life Technologies, Inc., Gaithersburg, Md.). For comparisons, we used commercially available human Fn in selected experiments (Gibco-BRL, Life Technologies).
125I-Fn binding assay.
The binding of 125I-Fn (ICN Chemical Co., Irvine, Calif.) to M. tuberculosis organisms was quantified as described previously (36, 53). Briefly, M. tuberculosis organisms grown in 7H9 Middlebrook broth were isolated, washed by centrifugation and resuspended to a final concentration of 109 organisms/ml of Dulbecco's modified Eagle medium (DMEM). The above mixture containing 108 M. tuberculosis organisms in 100 μl of DMEM was incubated with 125I-Fn (10 μg/ml) in the presence or absence of 0 to 5 mM Ca2+ for 60 min at 37°C in microcentrifuge tubes. For the subsequent reaction conditions the final volume was brought up to 200 μl. After the incubation, the reaction mixture was centrifuged at 12,000 × g for 20 min in an Eppendorf microcentrifuge. The pellet was washed three times with DMEM to remove free 125I-Fn. The pellet containing bound 125I-Fn to M. tuberculosis organisms was counted in a gamma counter (Beckman 5500; Beckman Instruments Corp., Fullerton, Calif.). The amount of 125I-Fn bound was then quantified.
To determine the binding mechanisms, the 125I-Fn binding assay was performed in the presence or absence of RGDS (1.0 mM) and a control peptide, RGES (1.0 mM) (Gibco-BRL, Life Technologies, Inc.) or heparin (1.0 μM) (Sigma Chemical Co., St. Louis, Mo.). The binding assay was also carried out in the presence or absence of anti-Fn MAbs directed against the HBD, CBD, and gelatin binding domain (GBD) of Fn or in the presence or absence of appropriate isotype control MAbs (Chemicon International, Temecula, Calif.). 125I-Fn and M. tuberculosis organisms were incubated with anti-Fn MAbs for 60 min. After the incubation, the samples were washed by centrifugation in DMEM to remove free 125I-Fn and unbound antibody. The fractions were counted in a gamma counter to determine the amount of bound 125I-Fn.
AM isolation.
Murine AMs were isolated by bronchoalveolar lavage from 10-week-old Swiss Webster pathogen-free mice (Harlan Sprague-Dawley, Inc. Indianapolis, Ind.) as previously described (10). Briefly, the mice were sacrificed by intraperitoneal injection of Beuthanasia-D solution (Schering-Plough Animal Health Corporation, Kenilworth, N.J.). The trachea was cannulated following a midline neck incision, and the lungs were lavaged 10 times with 1.0 ml of 0.9% sodium chloride solution containing 0.6 mM EDTA, penicillin (100 U/ml), streptomycin (100 μg/ml), gentamicin (40 μg/ml), and amphotericin B (0.5 μg/ml). Approximately 7 to 8 ml of lavage fluid was obtained from each mouse. AMs were separated from the lavage fluid by centrifugation at 600 × g for 10 min. Red blood cells were lysed with 10 mM KHCO3 and 152 mM NH4Cl. Cells were washed three times with normal saline and resuspended in DMEM (BioWhittaker, Inc., Walkersville, Md.) with 10% heat-inactivated fetal bovine serum, glutamine (300 μg/ml), penicillin (100 U/ml), and 20 mM HEPES (pH 7.2). Cell preparations were demonstrated to be 98% pure AMs by routine Hema 3 staining, a Giemsa-like staining method (Biochemical Sciences, Inc., Bridgeport, N.J.), and to be greater than 95% viable by trypan blue exclusion. AMs were plated at a density of 2.5 × 105/100 μl/well on rat immunoglobulin G (IgG) (Calbiochem, San Diego, Calif.)-coated 96-well tissue culture plates, incubated for 24 h at 37°C in 5% CO2, and washed three times with antibiotic-free DMEM (200 μl/well) to remove unattached cells. The plates were stored at 4°C for 1 h prior to the attachment assay.
M. tuberculosis attachment assay.
Attachment of M. tuberculosis organisms to AMs was determined as described previously by our laboratory (10). Briefly, freshly isolated M. tuberculosis organisms were incubated for 18 h in 1 ml of serum-free DMEM and 200 μCi of sodium chromate [51Cr] (New England Nuclear, Boston, Mass.). The 51Cr-labeled M. tuberculosis organisms were centrifuged (3,800 × g, 15 min), the supernatant was discarded, and the pellet was washed four times to remove unincorporated 51Cr. A final concentration of 2.5 × 105 51Cr-labeled M. tuberculosis organisms in 100 μl of DMEM was added to each well of a 96-well tissue culture plate containing adherent AMs. After the incubation at 4°C, unbound organisms were removed by washing the monolayer cells three times with 200 μl of DMEM. The washes from each well were pooled and centrifuged at 3,000 × g for 30 min to separate unattached M. tuberculosis organisms from the supernatant. The supernatant was transferred to a separate tube. The adherent AMs in the 96-well plate containing bound M. tuberculosis were solubilized by the addition of 200 μl of 10% Triton X-100 (Sigma Chemical Co.). The radioactivity in each fraction was measured in a gamma counter, and percent attachment was expressed as follows: % attachment = (A/A + B) × 100, where A is 51Cr-labeled M. tuberculosis organisms bound to the AMs and B is unattached 51Cr-labeled M. tuberculosis organisms free in the medium. To determine percent injury to M. tuberculosis organisms, the percent release is expressed as follows: % release = (A/(A + B + C) × 100, where B is the number of counts per minute from 51Cr-labeled M. tuberculosis organisms bound to the AMs, A is the number of counts per minute from 51Cr-released into the medium, and C is the number of counts per minute from unattached 51Cr-labeled M. tuberculosis organisms free in the medium.
To determine the possible mechanisms involved in Fn-mediated attachment, 51Cr-labeled M. tuberculosis organisms were added to each well and incubated with the AMs at 4°C for 4 h. At 4°C only attachment (no phagocytosis) occurs. The attachment assay was performed to examine the effect of heparin (1.0 mM; Sigma Chemical Co.), RGDS (1.0 mM), RGES (1.0 mM), polyclonal antibodies to human Fn (100 μg/ml), and MAbs to human Fn (100 μg/ml; Chemicon, International) and in the presence or absence of appropriate isotype control MAbs (Chemicon, International) on M. tuberculosis attachment to AMs.
Statistical analysis.
A minimum of three experiments conducted in triplicate were performed. The results are expressed as means ± standard errors of the means (SEM). For each experiment the differences between control and experimental data were compared using Student's t test or analysis of variance with paired comparisons. Data were accepted as significantly different if P was <0.05 (22).
RESULTS
125I-Fn binding to M. tuberculosis.
125I-Fn binding to M. tuberculosis was time dependent, and this binding was saturated after 60 min (24.0 ± 2.5 ng). Fn binding was also concentration dependent, and saturation of binding was observed at or above Fn concentrations of 10 μg/ml. Further, 125I-Fn binding to M. tuberculosis was calcium concentration dependent, with saturation of binding occurring at or above 2 mM Ca2+ (51.3 ± 2.3 ng). These results indicate that 125I-Fn binds to M. tuberculosis in time-, Fn concentration-, and Ca2+ concentration-dependent manners.
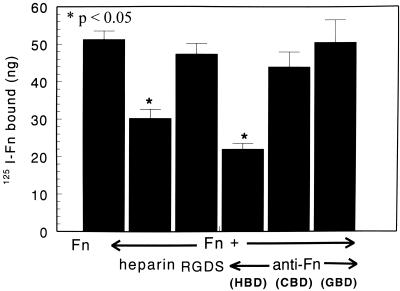
To determine the binding mechanism, 125I-Fn and M. tuberculosis organisms were incubated in the presence or absence of Fn peptide (RGDS), heparin, and various MAbs directed against different sites on Fn (Fig. 1). Heparin and a MAb specific to the HBD of Fn significantly decreased Fn binding to M. tuberculosis organisms from 51.3 ± 2.3 ng to 30.9 ± 2.4 ng and to 21.9 ± 1.5 ng, respectively (P < 0.05) (Fig. 1). However, the binding of Fn to M. tuberculosis (51.3 ± 2.3 ng) in the presence of RGDS (47.4 ± 2.8 ng [P > 0.05]) and MAbs specific to either CBD (43.9 ± 3.2 ng [P > 0.05]) or GBD (50.5 ± 6.0 ng [P > 0.05]) resulted in no significant change in Fn binding (Fig. 1). There was no significant effect on 125I-Fn binding to M. tuberculosis organisms in the presence of control isotype antibodies, IgG1 and IgM. Thus, these results suggest that the HBD, but not the CBD or GBD of Fn, is involved in Fn binding to M. tuberculosis organisms.
FIG. 1.
Binding mechanism of 125I-Fn binding to M. tuberculosis. Effect of RGDS, heparin anti-Fn MAbs to specific domains on 125I-Fn binding to M. tuberculosis. 125I-Fn was incubated with M. tuberculosis organisms in the presence or absence of RGDS or heparin or in the presence or absence of anti-Fn MAbs specific to the HBD, CBD, and GBD. After incubation, the pellet containing bound 125I-Fn was subjected to evaluation in a gamma counter. The results indicate addition of heparin or MAbs to HBD significantly reduces 125I-Fn binding to M. tuberculosis; however, RGDS, MAb to CBD, and MAb to GBD did not affect Fn binding. Results are expressed as means + SEMs (error bars) of three experiments performed in triplicate.
Fn-mediated attachment of M. tuberculosis to AMs.
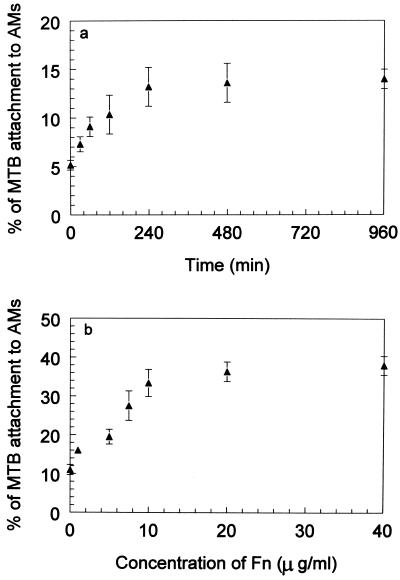
The attachment of M. tuberculosis to AMs in the absence of Fn increased as a function of time with maximal attachment occurring at 4 h (Fig. 2a). In the presence of Fn, attachment increased in a concentration-dependent manner (Fig. 2b), with maximal binding at approximately 10 μg/ml (33.5% ± 3.4%). The binding pattern of M. tuberculosis to AMs in the presence of Fn appears to be second order. There was no significant killing of M. tuberculosis as a result of Fn-mediated attachment to AMs, as measured by 51Cr release from M. tuberculosis (data not shown). Therefore, an Fn concentration of 10 μg/ml was used for all subsequent experiments.
FIG. 2.
Effects of time and Fn concentration on attachment of M. tuberculosis to AMs. (a) Attachment of 51Cr-labeled M. tuberculosis organisms to murine AMs was quantified at different time intervals (0 to 16 h) at 4°C, and at this temperature only attachment occurs but not phagocytosis. (b) The effect of Fn on attachment was examined in the presence of increasing concentrations of human Fn (0 to 40 μg/ml) at 4°C for 4 h. The attachment increased in the presence of increasing concentrations of Fn, and the maximum binding occurred at 10 μg of Fn/ml. The binding pattern of M. tuberculosis to AMs in the presence or absence of Fn appears to be second order. Experimental values were significantly different from the control values (P < 0.05). Results are expressed as means ± SEMs (error bars) of three experiments performed in triplicate.
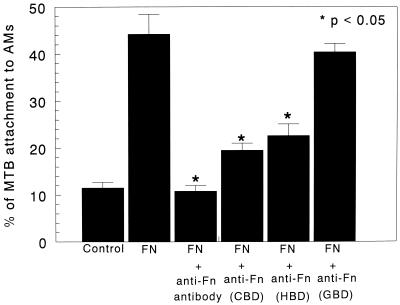
The specificity of Fn-enhanced attachment of M. tuberculosis to AMs was examined in the presence of Fn and polyclonal antibodies to Fn. Addition of anti-Fn polyclonal antibodies to the attachment assay significantly decreased the Fn-enhanced attachment from 44.2% ± 4.2% to 10.8% ± 1.2% (P < 0.05) (Fig. 3). Use of nonspecific IgG or control isotype antibodies IgG1 and IgM did not affect M. tuberculosis attachment to AMs (data not shown).
FIG. 3.
Effects of anti-Fn polyclonal antibodies and MAbs on in vitro attachment of M. tuberculosis to AMs. Fn-enhanced attachment of M. tuberculosis to AMs was examined in the presence of Fn and in the presence or absence of anti-Fn polyclonal antibodies and MAbs directed against the CBD, HBD, and GBD of Fn. Anti-Fn polyclonal antibody significantly decreased the Fn-enhanced attachment of M. tuberculosis to AMs (P < 0.05). Addition of a MAb specific to either CBD or HBD significantly decreased the attachment of M. tuberculosis to AMs (P < 0.05), whereas MAbs specific to GBD showed no effect on attachment of M. tuberculosis to AMs. Results are expressed as means + SEMs (error bars) of three experiments performed in triplicate.
To determine whether the attachment of M. tuberculosis to AMs is Ca2+ dependent, the attachment assay of M. tuberculosis to AMs was repeated in the presence or absence of EDTA and EGTA. Both EDTA and EGTA significantly decreased Fn-enhanced attachment of M. tuberculosis to AMs from 44.2% ± 4.2% to 8.4% ± 1.2% and 9.7% ± 0.6% (P < 0.05, both comparisons), respectively, indicating the requirement of Ca2+ for the Fn-enhanced attachment of M. tuberculosis to murine AMs. As the CBD and HBD sites for Fn are known to be Ca2+ dependent (15, 34), the Ca2+ dependency of M. tuberculosis binding to AMs is clearly multifactoral.
MAbs directed against the HBD, CBD, and GBD were added to the assay to determine the site(s) on the Fn molecule responsible for the Fn-mediated attachment of M. tuberculosis to AMs. Addition of a MAb specific to HBD significantly decreased the attachment of M. tuberculosis to AMs from 44.2% ± 4.2% to 19.5% ± 1.2% (P < 0.05). In addition, the MAb to CBD of Fn decreased attachment of M. tuberculosis to AMs from 44.2% ± 4.2% to 22.6% ± 2.2% (P < 0.05), respectively, whereas MAb specific to GBD showed no significant effect on M. tuberculosis attachment to AMs (40.5% ± 1.8% [P > 0.05]) (Fig. 3). Thus, these results suggest that Fn-enhanced attachment of M. tuberculosis to AMs is mediated, in part, by the HBD of the Fn molecule recognizing M. tuberculosis and the CBD of Fn recognizing a surface receptor on AMs (27).
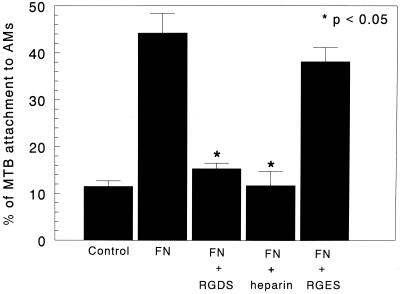
Attachment of M. tuberculosis to AMs was significantly decreased in the presence of heparin from 44.2% ± 4.2% to 11.8% ± 1.1% (P < 0.05) and in the presence of RGDS peptide to 15.3% ± 1.2% (P < 0.05) (Fig. 4). However, the false binding peptide, RGES, had no significant effect (40.5% ± 1.8% [P > 0.05]) on M. tuberculosis attachment to AMs (Fig. 4). Taken together, these data suggest that Fn may act as a bridge between the microorganisms and the AMs. These data suggest that the HBD of Fn binds to M. tuberculosis, whereas the CBD of Fn binds to the AMs.
FIG. 4.
Effects of RGDS, RGES, and heparin on in vitro attachment of M. tuberculosis to AMs. M. tuberculosis organisms were incubated with murine AMs at 4°C for 4 h in the presence or absence of tetrapeptide RGDS, a control tetrapeptide (RGES), and heparin. Addition of RGDS or heparin significantly decreased the attachment of M. tuberculosis to AMs (P < 0.05). However, RGES did not have a significant effect on the attachment. Results are expressed as means + SEMs (error bars) of three experiments performed in triplicate.
DISCUSSION
This study suggests that Fn binds to M. tuberculosis and that Fn can mediate attachment of M. tuberculosis to murine AMs. A MAb specific to the HBD of Fn, but not to the CBD or GBD of Fn, decreased binding of Fn to M. tuberculosis, suggesting that the HBD is the site on the Fn molecule responsible for Fn binding to M. tuberculosis. Fn-enhanced attachment of M. tuberculosis to murine AMs was decreased by the addition of MAbs specific to either the HBD or CBD or by RGDS, suggesting that the CBD of Fn likely plays a role in the Fn-mediated attachment of M. tuberculosis to AMs. Therefore, Fn may act as a bridge between M. tuberculosis and the AM. As Fn is a constitutively expressed protein in the lung (7) and is increased during inflammation (16), this interaction may represent a normal host response in the initial week of new infection in the alveolar spaces.
Fn is a large adhesive matrix protein with multiple binding domains known to mediate the adherence of many pathogenic microorganisms in a ligand receptor-mediated manner (17). Examples include E. coli, S. pyogenes, S. enterica serovar Dublin, C. albicans, S. aureus, T. pallidum, and P. carinii (14, 33, 35, 36, 39, 46, 47). For instance, previous studies from our laboratory have shown that P. carinii binds to Fn via the RGDS binding sites of Fn, and Fn appears to mediate the attachment of P. carinii to AMs with the RGDS binding site on the other arm of Fn molecule (34). As AMs are important in clearance of this extracellular pathogen from alveolar spaces (26), Fn likely is an important mediator in host defense in P. carinii pneumonia.
The present study reveals that Fn binds to M. tuberculosis via the HBD of the Fn molecule. Fn binding to M. tuberculosis was inhibited by a MAb specific to the HBD of the Fn molecule to levels comparable to that obtained with anti-Fn polyclonal antibodies. This indicates the importance of the HBD of the Fn molecule in Fn-mediated binding to M. tuberculosis. In contrast, MAbs to CBD and GBD had little effect on Fn binding to M. tuberculosis. Further, Fn binding to M. tuberculosis could be inhibited by heparin, suggesting the involvement of the HBD of the Fn molecule. A recent study suggests that the Mycobacterium bovis BCG interacts with Fn via the C-terminal region adjacent to the heparin binding domain of the Fn molecule (5). Since the type of proteins that bind to the HBD are proteoglycans (30), it is possible that the Fn-binding proteins present on the surface of M. tuberculosis are also proteoglycans.
Previous studies suggest that the BCG interacts with Fn via the C-terminal region adjacent to the HBD of the Fn molecule (5). α antigen, also known as 85B secreted protein, of mycobacteria interacts with either the C-terminal HBD or the more central CBD (29). In contrast, other studies have shown that gelatin but not heparin inhibited the binding of Fn to 85B protein (32). The gelatin binding site is located on the collagen binding domain of FN. These studies suggest that the 85B complex protein possesses multiple binding affinities for Fn.
Attachment of M. tuberculosis to murine AMs was enhanced in the presence of exogenous Fn. This enhanced attachment was competitively inhibited by the addition of the synthetic peptide RGDS, the tetrapeptide sequence for the CBD of the Fn molecule. Further, addition of MAbs directed against the CBD and HBD decreased Fn-enhanced attachment, indicating that the CBD and HBD of the Fn molecule are involved in the M. tuberculosis attachment to AMs. As expected, attachment of M. tuberculosis to AMs was Ca2+ dependent, a property required for the functioning of both the HBD and CBD of the Fn molecule. Others have suggested that Fn may mediate mycobacterial attachment. For instance, mycobacteria have been shown to attach via Fn to epithelial and Schwann cells (44). Further, in vivo studies have indicated that Fn may facilitate the uptake of BCG by bladder epithelium (20). Fn-mediated interactions may involve different sites on the Fn molecule. Our study shows Fn can increase attachment of M. tuberculosis to AMs; however, increased attachment does not necessarily mean there is an increase in phagocytosis by AMs (14).
In all likelihood M. tuberculosis attachment and phagocytosis are multifactoral, involving multiple mechanisms and receptors (12, 40). M. tuberculosis organisms have developed a number of mechanisms to gain entry into the macrophages using specific cell surface receptors (12). Like other microorganisms, M. tuberculosis binds to CR1, CR3, and CR4, which in turn results in the phagocytosis of the bacilli and entry into the phagosomes (42). M. tuberculosis can enter macrophages through the mannose receptors (41). Surfactant apoproteins such as surfactant protein A (SP-A) or SP-D may also facilitate attachment and/or phagocytosis of M. tuberculosis to macrophages (13). Our study clearly demonstrates that Fn enhances attachment of M. tuberculosis to AMs. The lung is a rich source of Fn, and Fn production increases in response to infection or inflammation (7). Multiple studies suggest that Fn plays a role in recognition of various species of mycobacteria. No studies have yet delineated which of these several mechanisms predominate in vivo. Because mycobacteria are intracellular pathogens, some mechanisms of attachment may enhance infection, whereas others may facilitate clearance of mycobacteria (4, 8, 31).
In summary, these Fn binding studies suggest that Fn binds to the M. tuberculosis organisms, likely through its HBD, whereas Fn-mediated binding of M. tuberculosis to the AM likely occurs through the CBD of the Fn molecule. Thus, Fn may promote the attachment of M. tuberculosis to AMs by acting as a bridge between the organism and the host cell. Further insights into the mechanisms of M. tuberculosis attachment to AMs may provide important information in the pathogenesis of this disease and may permit the development of therapeutic strategies to modulate this infectious process.
Acknowledgments
We thank Julie Valente and Diane L. Kachel for their contribution in the preparation and review of the manuscript.
This study was supported by National Institutes of Health grants R01 HL51962, R01 HL46647, and R01 HL43524.
REFERENCES
- 1.Abou-Zeid, C., T. Garbe, R. Lathigra, H. G. Wiker, M. Harboe, G. Rook, and D. B. Young. 1991. Genetic and immunological analysis of Mycobacterium tuberculosis fibronectin-binding proteins. Infect. Immun. 59:2712-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitige, L., C. Jagannath, A. Wanger, and S. Norris. 2000. Disruption of the genes encoding antigen 85A and antigen 85B of Mycobacterium tuberculosis H37Rv: effect on growth in culture and in macrophages. Infect. Immun. 68:767-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belisele, J. N., V. D. Vissa, T. Sievert, K. Takiyama, P. J. Brennan, and G. S. Besra. 1997. Role of major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420-1422. [DOI] [PubMed] [Google Scholar]
- 4.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, D. L., W. Shu, J. C. S. Choi, E. J. Margolis, M. J. Droller, and B. C. S. Liu. 1994. Bacillus Calmette-Guerin interacts with the carboxyl-terminal heparin binding domain of fibronectin: implications for the BCG-mediated antitumor activity. J. Urol. 152:1275-1280. [DOI] [PubMed] [Google Scholar]
- 6.Collinson, S., P. Doig, J. Doran, S. Clouthier, T. Trust, and W. Kay. 1993. Thin, aggregative fimbriae mediate binding of Salmonella enteritidis to fibronectin. J. Bacteriol. 175:12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean, D. C. 1989. Expression of the fibronectin gene. Am. J. Respir. Cell Mol. Biol. 1:5-10. [DOI] [PubMed] [Google Scholar]
- 8.Dennis, M. 1991. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates cell. Immunology 132:150-157. [DOI] [PubMed] [Google Scholar]
- 9.Dhople, A., A. Dhople, and M. Ibanzez. 1995. In vitro activities of 2,2′-bipyridyl analogues against Mycobacterium avium and Mycobacterium tuberculosis. Tuber. Lung Dis. 76:136-140. [DOI] [PubMed] [Google Scholar]
- 10.Downing, J. F., R. Pasula, J. R. Wright, H. L. Twigg III, and W. J. Martin II. 1995. Surfactant protein A promotes attachment of Mycobacterium tuberculosis to alveolar macrophages during infection with HIV. Proc. Natl. Acad. Sci. USA 92:4848-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. Raviglione. 1999. Global burden of tuberculosis. Estimated incidence, prevalence and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 12.Ernst, J. D. 1998. Macrophage receptors for Mycobacterium tuberculosis. Infect. Immun. 66:1277-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson, J. S. 1999. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J. Immunol. 163:312-321. [PubMed] [Google Scholar]
- 14.Forman, G., L. Switalski, A. Faris, T. Wadstrom, and M. Hook. 1984. Binding of Escherichia coli to fibronectin. J. Biol. Chem. 259:14899-14905. [PubMed] [Google Scholar]
- 15.Gailit, J., and E. Ruoslahti. 1988. Regulation of fibronectin receptor affinity by divalent cations. J. Biol. Chem. 263:12927-12932. [PubMed] [Google Scholar]
- 16.Gupta, S. K., P. G. Reinhert, and D. K. Balla. 1988. Enhancement of fibronectin expression in the rat lung by ozone and an inflammatory stimulus. Am. J. Physiol. 275:L330-L335. [DOI] [PubMed]
- 17.Hook, M., L. Switalski, T. Wadstrom, and M. Lindberg. 1989. Interactions of pathogenic microorganisms with fibronectin. Academic Press, New York, N.Y.
- 18.Hynes, R. O. 1989. Fibronectins, p. 113-175. Springer-Verlag, Inc., New York, N.Y.
- 19.Hynes, R. O. 1990. Methods for identification of fibronectin, p. 9-12. In A. Rich (ed.), Fibronectins. Springer-Verlag, Inc., New York, N.Y.
- 20.Kavoussi, L., E. Brown, J. Ritehey, and T. Ratliff. 1990. Fibronectin-mediated Calmette-Guerin Bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J. Clin. Investig. 85:62-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kornblihtt, A. B., K. Vibe-Pedersen, and F. E. Barelle. 1983. Isolation and characterization of cDNA clones for human and bovine fibronectins. Proc. Natl. Acad. Sci. USA 80:3218-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuebler, R. R., and H. Smith. 1976. Statistics. John Wiley and Sons, New York, N.Y.
- 23.Kuroda, K., E. J. Brown, W. B. Telle, D. G. Russell, and T. L. Ratliff. 1993. Characterization of the internalization of bacillus Calmette-Guerin by human bladder tumor cells. J. Clin. Investig. 91:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuusela, P., T. Vartio, M. Vuento, and E. Myhre. 1985. Attachment of Staphylococci and Streptococci on fibronectin, fibronectin fragments and fibrinogen bound to a solid phase. Infect. Immun. 50:77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laemmli, U. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 26.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Investig. 99:2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litvinov, R. I., O. D. Zinkevich, and L. D. Zubairova. 1983. Fibronectin receptors on the surfaces of alveolar macrophages. Tsitologiia 25:1185-1190. (In Russian.) [PubMed]
- 28.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. A. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 29.Naito, M., T. Fufida, and T. Yamada. 2000. The domain of human fibronectin mediating the binding of a antigen, the most immunopotent antigen of mycobacteria that induces protective immunity against mycobacterial infection. Biochem. J. 347:725-731. [PMC free article] [PubMed] [Google Scholar]
- 30.Ortega-Barria, E., and M. Periera. 1991. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell 67:411-421. [DOI] [PubMed] [Google Scholar]
- 31.Pasula, R., J. Wright, and W. J. Martin II. 1999. Surfactant protein suppresses reactive nitrogen intermediates by alveolar macrophages in response to Mycobacterium tuberculosis. J. Clin. Investig. 103:483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peake, P., A. Gooley, and W. J. Britton. 1993. Mechanism of interaction of the 85B secreted protein of Mycobacterium bovis with fibronectin. Infect. Immun. 61:4828-4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penn, C., and S. Klotz. 1994. Binding of plasma fibronectin to Candida albicans occurs through the cell binding domain. Microb. Pathog. 17:387-393. [DOI] [PubMed] [Google Scholar]
- 34.Pottratz, S. T., and W. J. Martin II. 1990. Mechanism of Pneumocystis carinii attachment to cultured rat alveolar macrophages. J. Clin. Investig. 86:1678-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pottratz, S. T., and W. J. Martin II. 1990. Role of fibronectin in Pneumocystis carinii attachment to cultured lung cells. J. Clin. Investig. 85:351-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Proctor, R. A., D. F. Mosher, and P. J. Olbrantz. 1982. Fibronectin binding to Staphylococcus aureus. J. Biol. Chem. 257:14788-14794. [PubMed] [Google Scholar]
- 37.Ratliff, T., R. McCarthy, W. Telle, and E. Brown. 1993. Purification of a mycobacterial adhesin for fibronectin. Infect. Immun. 61:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raviglione, M. C., D. E. Snider, Jr., and A. Kochi. 1995. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA 273:220-226. [PubMed] [Google Scholar]
- 39.Rocha, C., and V. Fischetti. 1999. Identification and characterization of a novel fibronectin-binding protein on the surface of group A streptococci. Infect. Immun. 67:2720-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roecklein, J. A., R. P. Swartz, and H. Yeager, Jr. 1992. Nonopsonic uptake of Mycobacterium avium complex by human monocytes and alveolar macrophages. J. Lab. Clin. Med. 119:772-781. [PubMed] [Google Scholar]
- 41.Schlesinger, L. 1993. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 150:2920-2930. [PubMed] [Google Scholar]
- 42.Schlesinger, L. S., and M. A. Horwitz. 1991. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J. Immunol. 147:1983-1994. [PubMed] [Google Scholar]
- 43.Schorey, J., M. Holsti, T. Ratliff, P. Allen, and E. Brown. 1996. Characterization of fibronectin-attachment protein of Mycobacterium avium reveals a fibronectin-binding motif conserved among mycobacteria. Mol. Microbiol. 21:321-329. [DOI] [PubMed] [Google Scholar]
- 44.Schorey, J. S., Q. Li, D. W. McCourt, M. Bong-Mastek, J. E. Clark-Curtiss, T. L. Ratliff, and E. J. Brown. 1995. A Mycobacterium leprae gene encoding a fibronectin binding protein is used for efficient invasion of epithelial cells and Schwann cells. Infect. Immun. 63:2652-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson, W., H. Courtney, and I. Ofek. 1987. Interactions of fibronectin with streptococci: the role of fibronectin as a receptor for Streptococcus pyogenes. Rev. Infect. Dis. 9:S351-S359. [DOI] [PubMed]
- 46.Speziale, P., M. Hook, L. Switalski, and T. Wadstrom. 1984. Fibronectin binding to a Streptococcus pyogenes strain. J. Biol. Chem. 157:420-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas, D., J. Baseman, and J. Alderete. 1985. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J. Exp. Med. 161:514-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wisniowski, P., R. Pasula, and W. J. Martin II. 1994. Isolation of Pneumocystis carinii gp120 by fibronectin affinity: evidence for manganese dependence. Am. J. Respir. Cell Mol. Biol. 11:262-269. [DOI] [PubMed] [Google Scholar]
- 51.Wyler, D., J. Sypek, and J. McDonald. 1985. In vitro parasite-monocyte interactions in human leishmaniasis: possible role of fibronectin in parasite attachment. Infect. Immun. 49:305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamada, K. 1989. Fibronectin domains and receptors, p. 47-121. In D. Mosher (ed.), Fibronectin. Academic Press, New York, N.Y.
- 53.Zimmerman, P. E., D. R. Voelker, F. X. McCormack, J. R. Paulsrud, and W. J. Martin II. 1992. 120-kD surface glycoprotein of Pneumocystis carinii is a ligand for surfactant protein A. J. Clin. Investig. 89:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]