Abstract
Cryptococcus neoformans, an encapsulated yeast, is a common cause of life-threatening meningoencephalitis in immunosuppressed patients. We previously observed that administration of a monoclonal antibody (MAb) to the capsular polysaccharide to mice with pulmonary infection prolonged survival and enhanced granulomatous inflammation without reducing lung CFU. To understand the mechanism of MAb action, we studied leukocyte recruitment and cytokine profiles in lungs of A/JCr mice. B lymphocytes were the predominant cell type in lung infiltrates, comprising 15 to 30% of the leukocytes. Despite alterations in histological appearance, fluorescence-activated cell sorter analysis revealed no significant difference in total numbers of lung leukocytes in MAb-treated mice and controls. Differences in the immune response to C. neoformans between MAb-treated mice and controls included (i) an increase in the percentage of granulocytes among lung leukocytes on day 14, (ii) higher macrophage surface expression of CD86 on day 28, (iii) larger amounts of IL-10 in lung homogenates at day 7, (iv) a trend toward smaller amounts of gamma interferon mRNA and protein on day 7, and (v) a smaller increase in the levels of interleukin-4 mRNA and protein on day 7. Hence, the immune responses to C. neoformans infection in the presence and absence of specific antibody were qualitatively similar, and antibody administration was associated with several subtle quantitative differences in immune response parameters that could translate into enhanced survival. MAb may function partly by down-regulating the inflammatory response and reducing host damage. Our findings demonstrate unexpected complexity in the interaction between specific MAb and other components of the host immune response.
Cryptococcus neoformans is an encapsulated fungus that is both an intracellular and an extracellular pathogen (22, 34). The medical importance of this organism has increased in the past two decades as a consequence of the AIDS epidemic, with the incidence approaching 30% in patients with AIDS in parts of sub-Saharan Africa (14). The lung is the portal of entry of C. neoformans in humans. In immunocompetent hosts, infection is presumably contained in the lung and the majority of human infections are asymptomatic. However, in patients with impaired immunity, extrapulmonary infection is common (35). Currently available antifungal therapies do not eradicate infection in the severely immunocompromised host, and there is considerable interest in the development of both immunomodulatory therapy and an effective vaccine (12, 23). Toward this end, a phase I trial of a monoclonal antibody (MAb) that binds to the glucuronoxylomannan (GXM) component of the capsular polysaccharide (9) is nearing completion.
Pulmonary defense mechanisms against C. neoformans have been studied extensively in murine experimental infection (29, 33, 38). In response to C. neoformans infection, mice develop a heterogeneous tissue response that ranges from granuloma formation with intracellular yeast cells to a virtual absence of inflammation, in which yeast cells grow as cystic collections in the extracellular space (reviewed in reference 18). The mechanisms responsible for the development of the different tissue reactions are not fully understood. The effective tissue response for controlling C. neoformans infection is granulomatous inflammation (51). The inflammatory infiltrate seen in the lung following C. neoformans infection includes macrophages, lymphocytes, neutrophils, and eosinophils. We have previously observed that administration to experimentally infected mice of an anticapsular IgG1 MAb that is closely related to the one currently in clinical trial results in prolongation of survival and an enhanced granulomatous response (19, 21) without reducing the fungal burden. This suggests that a function of Ab-mediated immunity is to enhance the cellular response.
This study explored leukocyte recruitment and the cytokine response in A/JCr mice in response to C. neoformans infection in the presence and absence of capsule-specific IgG1 MAb. Consistent with the expanding list of host immune requirements required for demonstration of protection (5, 61), the results support the view that the mechanism of MAb action may involve pleotrophic effects.
MATERIALS AND METHODS
C. neoformans.
C. neoformans strain 24067 (serotype D) was obtained from the American Type Culture Collection (Manassas, Va.). Strain 24067 (also known as 52D) was selected for study because it was used in prior studies of Ab protection (19, 21) and by other investigators in models of cryptococcal pulmonary infection (13, 28). Cultures were grown in Sabouraud dextrose broth at 30°C overnight with moderate shaking. Cells were washed three times and suspended in sterile 0.02 M phosphate-buffered saline, pH 7.2 (PBS). Yeast cells were counted in a hemacytometer, and inocula were confirmed by plating.
Anticapsular MAb.
The capsule-binding MAb 2H1 (IgG1) has been described previously (10). The GXM epitope recognized by this MAb is present on C. neoformans strain 24067 (11). MAb-containing ascites was obtained by paracentesis of pristane-primed BALB/c mice following intraperitoneal (i.p.) injection of hybridoma cells. For all experiments, MAb was purified by protein G affinity chromatography and its concentration was determined by ELISA.
i.t. infection.
Specific-pathogen-free A/JCr mice were infected intratracheally (i.t.) with 104 organisms of C. neoformans ATCC 24067. This C5-deficient mouse strain is susceptible to C. neoformans infection and is the strain in which Ab-mediated protection to pulmonary infection was initially demonstrated (19, 45). One day prior to infection, mice received 1 or 1.5 mg of MAb 2H1 i.p. or the same volume of PBS. For some experiments, purified mouse IgG (Sigma) served as an additional control treatment. Mice were anesthetized with sodium pentobarbital and were infected by inoculation of 104 organisms in 0.05 ml of PBS into the trachea via a midline neck incision with a 26-gauge needle, as described previously (19). In some experiments, the right upper lobe of the lung was removed, fixed in 10% buffered formalin, and embedded in paraffin. Five-micrometer sections were stained with hematoxylin and eosin and imaged by light microscopy.
Preparation of single-leukocyte suspensions.
Five infected control (PBS-treated) mice and five MAb 2H1-treated mice were killed on days 7, 14, 21, and 28 after infection. Their lungs were dissected; washed in 50 ml of Ca2+-, Mg2+-, and phenol red-free Hanks balanced salt solution (HBSS) (Gibco); homogenized in lysis buffer (5% fetal calf serum, 10 mM HEPES, 50 μM β-mercaptoethanol, 20 mM l-glutamine, 0.2 U of dispase from Bacillus polymyxa [Calbiochem, San Diego, Calif.] per ml, 1 μg of DNase type 1 (Sigma) per ml, and 1% penicillin-streptomycin); and incubated at 37°C for 1 h in a shaker. The homogenates were then centrifuged at 290 × g for 10 min at 4°C, the supernatant was discarded, and homogenates were suspended in 5 ml of HBSS and passed through a 70-μm-pore-size filter. The filter was washed with an additional 5 ml of HBSS, cells were centrifuged as described above, and the supernatant was discarded. Cells were suspended in 5 ml of 0.17 M NH4Cl (pH 7.2) and incubated for 10 min at room temperature to lyse red blood cells. Forty-five milliliters of HBSS was slowly added to the suspension, which was then centrifuged. Cells were washed with an additional 50 ml of HBSS, pelleted, and suspended in 5 ml of HBSS, and 50 μl of this suspension was removed for quantitation of cells with trypan blue. The cells were then centrifuged and suspended in 90 μl of PBS with 1% bovine serum albumin (BSA) per 107 cells. Rat anti-mouse CD45-conjugated magnetic cell sorting (MACS) microbeads (Miltenyi Biotec, Inc., Auburn, Calif.) were added at a concentration of 10 μl per 107 cells, and cells were incubated at 4°C for 15 min. Cells were washed and suspended in 2 ml of (MACS) buffer (PBS [pH 7.2] with 0.5% BSA). MACS was performed using the possel-s program of the AutoMACS (Miltenyi Biotech, Inc.). For the MACS-positive sample, 50 μl was removed and suspended in a solution containing trypan blue, and cells were counted using a hemacytometer. The remainder of the suspension was distributed into five Eppendorf tubes and blocked with serum (either 10% goat serum or 10% goat serum with 10% hamster serum) for 20 min at room temperature. Fluorochrome- or biotin-conjugated MAbs to cell surface markers or isotype-matched controls were produced in rat, except where shown, and were obtained from Pharmingen (San Diego, Calif.) (CD8a-phycoerythrin [PE] [IgG2a], CD19-biotin [IgG2a], Mac-3-fluorescein isothiocyanate [IgG1], and NK-PE [IgM]), from Caltag Laboratories (Burlingame, Calif.) (CD3-fluorescein isothiocyanate [hamster IgG], CD4-allophycocyanin [IgG2a], CD80-biotin [IgG2a], CD45-allophycocyanin [IgG2b], IgM-biotin, IgG1-biotin, IgG2a-allophycocyanin, IgG2b-allophycocyanin, and hamster IgG-biotin), or Southern Biotechnology Associates (Birmingham, Ala.) (CD86-PE [IgG2a]). The rat hybridoma producing RB6-8C5 (IgG2b), which binds to murine granulocytes, was a generous gift from Robert Coffman (DNAX Research Institute of Molecular and Cellular Biology, Inc., Palo Alto, Calif.) (25). RB6-8C5 was purified from concentrated cell supernatants by protein G column chromatography, conjugated to Alexa-488 (Molecular Probes, Eugene, Oreg.) as per the manufacturer's instructions, and quantitated by ELISA. Following determination of optimal MAb concentrations in pilot experiments, cocktails comprised of MAbs to murine cell surface markers or isotype-matched controls were added to cell suspensions in the following combinations at the concentrations indicated: (i) 10 μg each of CD3, CD8a, CD19, and CD4 per ml; (ii) 20 μg of Mac-3 per ml, 10 μg of CD86 per ml (on days 21 and 28 only), 10 μg of CD80 per ml, and 2 μg of CD45 per ml; (iii) 2 μg of RB6 per ml and 10 μg each of NK, rat IgM, and CD45 per ml; (iv) 20 μg of Mac-3 and 10 μg each of rat IgG1 and rat IgG2b per ml; and (v) 10 μg each of CD3, CD86, hamster IgG, and rat IgG2a per ml. Cell suspensions were incubated for 20 min on ice, and then 1 ml of MACS buffer was added and cells were centrifuged for 5 min, suspended in 2 μg of streptavidin conjugated to peridinin chlorophyll protein (Pharmingen) per ml, and incubated on ice for 20 min. Cells were again washed in 1 ml of MACS buffer and suspended in 1 ml of MACS buffer. Fluorescence-activated cell sorter (FACS) analysis was performed using the FACSCalibur (Becton Dickinson, Franklin Lakes, N.J.). Analysis was performed using the CellQuest software program (Becton Dickinson). For samples containing anti-CD45 markers, cell samples were gated on a pan-leukocyte region based on forward and side scatter and on CD45. For the remaining samples, analysis was done using a pan-leukocyte gate.
RNA isolation, reverse transcription (RT), and RT-PCR.
In the first experiment, 12 mice were infected as described above, and 4 mice were killed 4, 7, or 14 days after infection (n = 2 mice each for the control and MAb 2H1-treated mice). Three additional mice received sham i.p. and i.t. inoculations with PBS, and one each was killed 4, 7, or 14 days after infection. In the second experiment, three control and two MAb 2H1-treated mice were studied. An additional mouse that had received neither i.p. injection nor i.t. inoculation (naive) was studied to determine basal cytokine expression. Data from the two experiments were pooled for statistical analysis. CFU determined from 10% of the lung confirmed infection. At each time, the lungs were homogenized and RNA was isolated using an RNA isolation kit (Stratagene, Inc., La Jolla, Calif.), according to the manufacturer's protocol. The RNA was quantitated by determining the optical density at 260 nm. The presence of RNA was confirmed by agarose gel electrophoresis of a portion of each sample.
For each 2 μg of RNA, reaction mixtures contained 2 μl of buffer containing 100 mM Tris, 500 mM KCl [pH 8.3], 4 μl of MgCl2, 1 mM deoxynucleoside triphosphates, 3.2 μg of random hexamers, 40 U of RNase inhibitor, and 0.8 μl of avian myeloblastosis virus reverse transcriptase (Boehringer Mannheim Corporation, Indianapolis, Ind.). Sterile water was added to adjust the volume to 20 μl per reaction mixture. The reaction mixtures were incubated at 25°C for 10 min, at 42°C for 1 h, and then at 99°C for 5 min. The samples were then diluted 1:5 in sterile water with 50 μg of glycogen per ml. For RT-PCR, 50-μl reaction mixtures contained 50 mM KCl; 10 mM Tris-HCl (pH 8.3); 1.5, 2, or 2.5 mM MgCl2; 200 μM deoxynucleoside triphosphates; 4 μM (each) 5′ and 3′ primers; 4 U of AmpliTaq DNA polymerase (Perkin-Elmer Life Sciences, Inc., Boston, Mass.); and 5 μl of diluted cDNA. Cycling conditions were 94°C for 40 s, 60°C for 20 s, and 72°C for 40 s. The number of cycles was 35 (hypoxanthine phosphoribosyltransferase [HPRT]), 40 (interleukin-2 [IL-2], IL-4, IL-10, and IL-12) or 45 (gamma interferon [IFN-γ]). A final extension step was performed at 72°C for 10 min.
Quantitative RT-PCR.
The amount of HPRT per 5 μl of diluted cDNA was determined using the polycompetitor plasmid insert PQRS described and constructed by Reiner et al. (48). The plasmid was transformed into and grown in MAX Efficiency DH5α competent cells (Life Technologies, Gibco BRL, Gaithersburg, Md.) and then isolated using an alkaline sodium dodecyl sulfate miniprep protocol. The polycompetitor insert was digested with SfiI and NotI (Promega, Madison, Wis.), purified using the Qiaex II gel extraction kit (Qiagen, Valencia, Calif.), and quantitated by gel electrophoresis by comparison to quantitated λ DNA HindIII fragments (Gibco). Reactions were first performed with mixtures containing serial 10-fold dilutions of the plasmid insert, and the amplified products were electrophoresed on ethidium bromide-stained agarose gels. After determination of the nearest dilution to the point of equivalence, reactions were performed with serial twofold dilutions of the polycompetitor, and the equivalent concentrations were determined by visual inspection of agarose gels following electrophoresis. Samples containing >2 amol of cDNA per μl were diluted in distilled water containing 50 μg of glycogen per ml. For samples containing <2 amol/μl, the cDNA was precipitated with 70% ethanol and suspended in a smaller volume. When samples contained between 1 and 2 amol of cDNA per μl, PCR amplification was performed using primers for IL-2, IFN-γ, IL-12, IL-4, or IL-10 (Table 1). For samples demonstrating positive reactions, quantitation of mRNA expression for the relevant cytokine was again performed by quantitative PCR with the polycompetitor insert. Results are expressed as the ratio of cytokine mRNA to that of HPRT. The limit of detection for cDNA was defined by determining the smallest quantity of insert that produced visible PCR product and was 0.02 amol/μl for IL-2 and IL-12, 0.004 amol/μl for IFN-γ, 0.002 amol/μl for HPRT, 0.0016 amol/μl for IL-10, and 0.0002 amol/μl for IL-4.
TABLE 1.
Primer sequences and sizes of the wild-type and competitor cDNAs in pPQRS, as described in reference 48.
| Gene | Primer sequences | cDNA (bp) Wild type | PQRS |
|---|---|---|---|
| HPRT | 5′ GTT GGA TAC AGG CCA GAC TTT GTT G | 352 | 450 |
| 3′ GAG GGT AGG CTG CCC TAT AGG CT | |||
| IFN-γ | 5′ CAT TGA AAG CCT AGA AAG TCT G | 267 | 320 |
| 3′ CTC ATG AAT GCA TCC TTT TTC G | |||
| IL-2 | 5′ TCC ACT TCA AGC TCT ACA G | 247 | 320 |
| 3′ GAG TCA AAT CCA GAA CAT GCC | |||
| IL-4 | 5′ CAT CGG CAT TTT CAA CGA GGT CA | 240 | 360 |
| 3′ CTT ATC GAT GAA TCC AGG CAT CG | |||
| IL-10 | 5′ CCA GTT TTA CCT GGT AGA AGT GATG | 324 | 440 |
| 3′ TGT CTA GGT CCT GGA GTC CAG CAG ACT CAA | |||
| IL-12 | 5′ ATG GCC ATG TGG GAG CTG GAG | 335 | 460 |
| 3′ TTT GGT GCT TCA CAC TTC AGG |
Cytokine protein concentrations.
In the first experiment, 21 mice (7 each) were injected i.p. with PBS, 1 mg of mouse IgG, or 1 mg of MAb 2H1. Forty-eight hours later, 15 mice (5 PBS treated, 5 IgG treated, and 5 MAb 2H1 treated) were infected with C. neoformans. Two additional mice in each treatment group mice received sham inoculations of PBS i.t. On day 7 after infection, mice were killed and their lungs were homogenized in PBS with 3% BSA. After three freeze-thaw cycles, the concentrations of IL-2, IL-4, IL-10, IL-12, and IFN-γ in filtered homogenate supernatants were determined by ELISA according to the instructions of the manufacturer (Endogen, Woburn, Mass.). The cytokine concentration in lung homogenates of two naive mice served as an additional control. According to the manufacturer, the limits of detection of these assays are <3 pg/ml for IL-2, <5 pg/ml for IL-4 and IL-12, and <10 pg/ml for IFN-γ. In a second experiment, 60 mice (20 PBS treated, 20 IgG treated, and 20 MAb 2H1 treated) were infected, and 10 from each group were killed on day 7 or 14. Two additional sham-infected mice with each i.p. treatment and one naive mouse were also included at both of these times.
Statistical analysis.
Statistical analysis was performed by the unpaired two-tailed Student t test or by Mann-Whitney rank sum test using SigmaStat (SPSS Science, Chicago, Ill.). Significance for single comparisons was determined by an alpha level of 0.05. When multiple comparisons were made, the Bonferroni correction was applied and the alpha level was adjusted accordingly.
RESULTS
Leukocyte recruitment and FACS analysis.
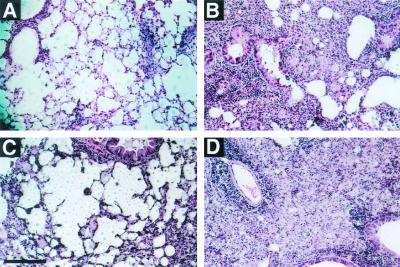
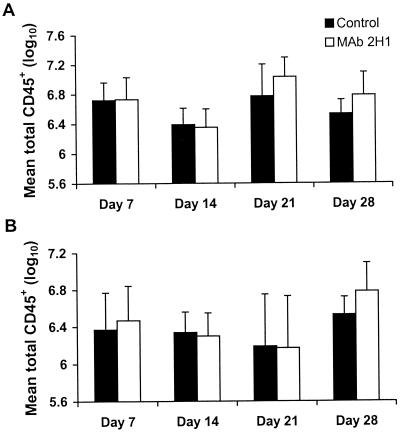
Administration of MAb 2H1 to A/JCr mice infected with C. neoformans is associated with a change in the appearance of the inflammatory response when lung tissue is viewed by light microscopy (Fig. 1). Specifically, MAb-treated mice tend to have a more organized granulomatous response associated with a larger proportion of internalized C. neoformans cells (21). Although in both control and MAb-treated mice, areas of infection are globally distributed throughout the lung, in MAb-treated mice, areas of infection are more focally contained (19). We hypothesized that the observed apparent enhancement of granuloma formation in MAb-treated mice might result from the presence of more leukocytes in the lung or differences in the cell populations present. Surprisingly, quantitation of total lung cells (pre-MACS) and total lung leukocytes (post-MACS) showed no difference in the number of cells present in the lungs of control and MAb-treated mice on days 7, 14, 21, or 28 after infection (Fig. 2). In preliminary experiments, too few leukocytes were obtained from the lungs of individual mice for reliable FACS analysis before 14 days after infection.
FIG. 1.
Histological appearance of the lungs of A/JCr mice 14 days after infection with C. neoformans. While in control mice (A and C), yeast filled the alveolar spaces, in mice treated with MAb 2H1 on the day prior to infection (B and D), yeast appear to be contained within regions of granulomatous inflammation. Bar, 0.1 mm.
FIG. 2.
Total number of leukocytes obtained from the lungs of mice at 7, 14, 21. and 28 days following infection with C. neoformans, as determined by counting of trypan blue-stained cell suspensions. Mice received MAb 2H1 or the same volume of PBS 1 day prior to infection. (A) Pre-MACS; (B) post-MACS. Error bars indicate standard deviations.
Analysis of cell samples following MACS showed that the mean percentage of CD45+ cells in the suspensions was 94.2 ± 2.8%. Of cells in the solution excluded by MACS, 1.8 ± 0.88% were CD45+, indicating that the samples used for FACS analysis were relatively pure populations of leukocytes and that the proportion of leukocytes lost during MACS was small. Analysis of unstained MACS-positive cell suspensions showed fluorescence in <0.5% of cells, indicating that autofluorescence was not occurring (data not shown). Staining of cells with isotype control MAbs yielded percentages of positive cells that were <1% for IgG2a and IgG2b, <1.6% for IgM, and <3% for hamster IgG. Although up to 8.4% of cells stained with the IgG1 isotype control, on day 28 1.2 ± 0.1% of cells from PBS-treated mice and 0.6 ± 0.2% from MAb 2H1-treated mice were fluorescent. Since these cells were different populations from Mac-3+ cells and the percentage of cells that stained with Mac-3 was significantly higher, labeling was considered to be specific. No reliable staining of cells with CD80 was found. Because we did not have an adequate positive control, we were unable to determine whether this reflected a systematic error or whether the amount of CD80 present on cells was too small to allow detection.
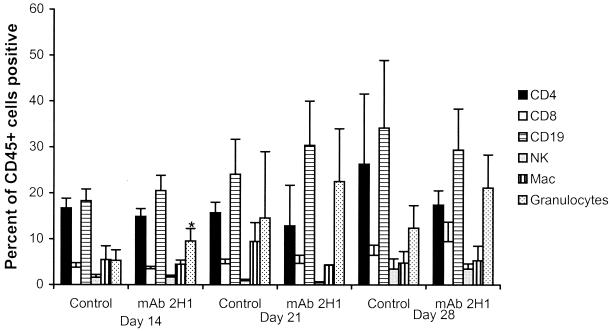
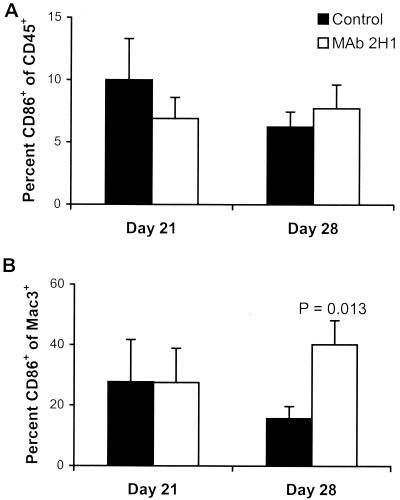
The cellular profiles obtained by FACS analysis are shown in Fig. 3, which shows marked similarity between the two groups. Surprisingly, B cells comprised, on average, the greatest proportion of leukocytes (15 to 30%) in the inflammatory response at all time intervals in both experimental groups (Fig. 3). For both MAb-treated and control mice, the leukocyte infiltrate consisted of CD4+ and CD8+ lymphocytes, B lymphocytes, macrophages, and granulocytes. However, two relatively subtle differences were found. First, in MAb-treated mice, there were significantly more RB6hi cells on day 14, a finding consistent with increased numbers of granulocytes. The second difference was that at 28 days after infection, the proportion of macrophages that were CD86+ was significantly higher in MAb-treated mice than in control mice (Fig. 4).
FIG. 3.
Cellular profiles of leukocyte populations in the lungs of mice infected with C. neoformans as determined by FACS analysis (n = 5 mice per group). Percentages represent means; error bars indicate standard deviations. The asterisk indicates a significant difference from control mice on day 14 of infection as determined by the unpaired two-tailed Student t test (P = 0.04).
FIG. 4.
Expression of costimulatory molecules in C. neoformans pulmonary infection, as indicated by detection of CD86 in the total white blood cell population (A) or on macrophages (B) (n = 5 mice per group). Percentages represent means; error bars indicate standard deviations. P values were determined by the unpaired two-tailed Student t test and compare control to MAb-treated mice.
MAb administration and the cytokine milieu.
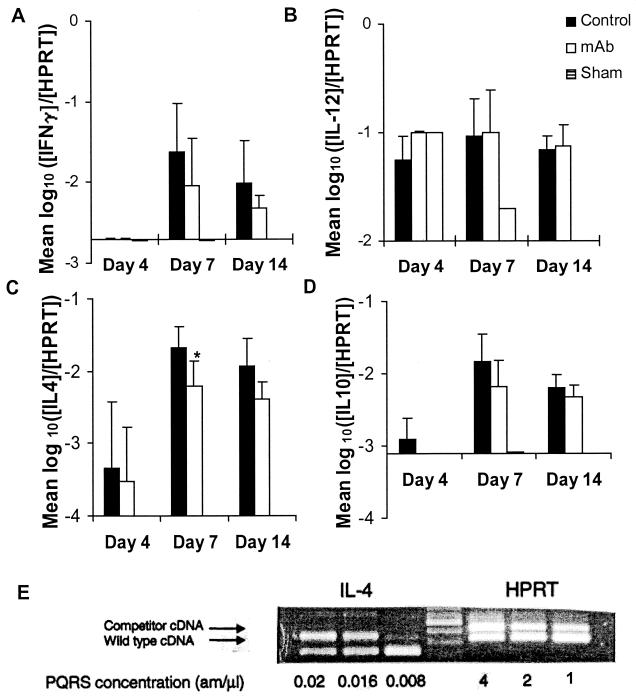
We explored the possibility that the observed differences in histological appearance reflected alterations in cytokine profiles of the cell populations by examining both cytokine mRNA and protein. For sham-infected and naive mice, the cytokine mRNA concentrations were at or below the limits of detection of the individual cytokines, except for IL-12 in the sham-infected mice on days 4 and 7 (Fig. 5). On day 4 of infection, concentrations of mRNA for IL-4, IL-10, and IL-12 in C. neoformans-infected mice were increased above the levels seen in naive or sham-infected mice. No qualitative difference was observed between control and MAb-treated mice. Compared to mice in the control group, there was a significantly smaller increase in the amount of IL-4 mRNA detected in the MAb 2H1-treated group at day 7 compared to PBS-treated mice (Fig. 5C). While a similar trend was apparent at day 14, this result was not statistically significant. A trend toward the presence of smaller amounts of mRNA for IFN-γ and IL-10 was observed, but these results were not statistically significant. mRNA for IL-2 was not detected in any mouse at any time studied, although the limit of detection for mRNA for this cytokine was higher than those of most of the rest.
FIG. 5.
Quantitative RT-PCR for expression of mRNA in mice treated with PBS or MAb 2H1 1 day prior to i.t. infection with C. neoformans. (A) IFN-γ; (B) IL-12; (C) IL-4; (D) IL-10. Numbers indicate means; error bars indicate standard deviations. Ratios compare the amount of mRNA for the indicated cytokine to the amount of HPRT in each sample. y axes cross at the limit of detection of the individual cytokines. When mRNA for a cytokine was not detected, the result is reported as the limit of detection. ∗, P = 0.045 compared to control group by the two-tailed Student t test. (E) Representative agarose gel showing determination of cytokine (IL-4) mRNA concentration. The concentration of competitor cDNA at which the wild-type band was of equivalent density defined the wild-type concentration. The amount of wild-type cDNA containing a fixed HPRT concentration (2 am/μl) was used for all measurements reported in panels A through D.
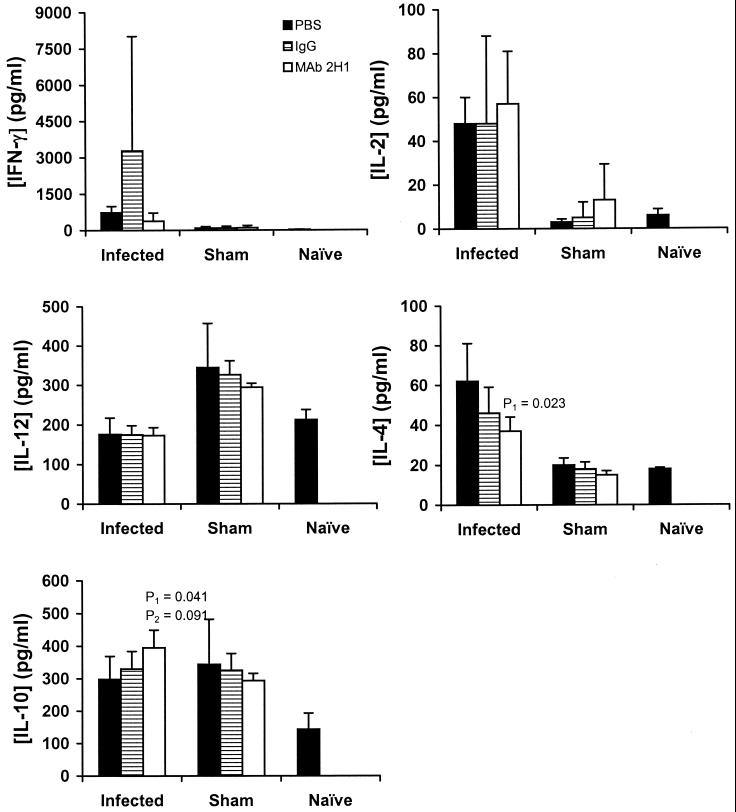
By cytokine protein ELISA, in the first experiment, infected mice treated with MAb 2H1 had significantly lower concentrations of IL-4 on day 7 than PBS-treated mice and a trend toward lower IL-4 concentrations than the IgG-treated mice (Fig. 6). In the second experiment, no difference was seen in IL-4 concentration at either day 7 or day 14, although on day 14 all mice (including sham-infected and naive mice) had substantially higher levels than in the first experiment (data not shown). Infected mice treated with MAb 2H1 had significantly higher concentrations of IL-10 than did infected PBS-treated mice, and again, although there was a trend toward higher levels than in the IgG-treated mice, this difference was not statistically significant. In the second experiment, MAb-treated mice had significantly higher concentrations of IL-10 than both PBS- and IgG-treated mice, and once more, the IgG group showed intermediate concentrations of IL-10. On day 14, there was no difference in IL-10 concentration for any of the treatment groups. For IFN-γ, in both experiments, there was a reduction in the average concentration in MAb-treated mice with a large variance within experimental groups. In the first experiment, there was a trend toward a reduction in the concentration of IFN-γ present in the lung. In the second experiment, on day 7, the concentration of IFN-γ in the lungs of PBS-treated mice was 867 ± 1,272 pg/ml, while that in MAb-treated mice was 63 ± 103 pg/ml (P < 0.001 by Mann-Whitney rank sum test). On day 14, although the mean concentration was again lower in the MAb-treated mice, statistical significance was not achieved (data not shown). For IL-2 and IL-12, there was no difference between the treatment groups.
FIG. 6.
Cytokine concentrations in lung homogenates 7 days after infection with C. neoformans (infected) or i.t. administration of PBS (sham) as determined by ELISA. The data shown are from experiment 1, as described in Results. Numbers represent means; error bars indicate standard deviations. Mice received IgG, MAb 2H1, or an equivalent volume of PBS (control) (n = 5 each in the control, IgG, and MAb 2H1 groups; n = 2 in each of the sham-infected groups). P values were determined by the two-tailed Student t test.
DISCUSSION
Pulmonary infection with C. neoformans in A/JCr mice induced an inflammatory response characterized by production of both TH1- and TH2-associated cytokines during the first 14 days of infection, without indicating polarization toward a TH1- or TH2-type response. Overall, the pattern of cytokine expression in A/JCr mice in response to pulmonary cryptococcal infection was consistent with those reported for other mouse strains (27, 33). This study attempted to explore the mechanism by which MAb to capsular polysaccharide prolongs survival and results in an apparent enhancement of granuloma formation in the lung. Although our original hypothesis was that MAb administration resulted in an increase in production of TH1-associated cytokines, the data obtained in this study do not support that view. Rather, we found a number of quantitative differences in cellular recruitment and in cytokine concentrations that were significant only at times but which, taken together, may contribute to the overall beneficial effect.
The experimental approach was to correlate cytokine production, as measured by both mRNA and protein, with the cellular composition of the inflammatory response. In general, we found good correlation between mRNA expression and protein concentrations. The experimental design included the comparison of groups receiving MAb 2H1 and groups receiving no Ab or polyclonal mouse IgG. The data suggest that there may be both specific and nonspecific components to the effect of Ab administration, as in some cases administration of polyclonal IgG gave results that differed from those for the PBS control or were intermediate between those for PBS- and MAb 2H1-treated mice. Administration of polyclonal IgG has wide-ranging immunomodulatory effects, including modulation of FcγRIIB expression (50), stimulation of NO production (52), and down-regulation of multiple cytokines by monocytes and T cells in response to stimulation (3, 4, 46). In retrospect, the choice of polyclonal Ab was almost certainly not an appropriate control for a MAb, since polyclonal immunoglobulin preparations may also contain antibodies to chemokine receptors and cytokine-neutralizing antibodies (8, 59). The issue of what is an appropriate control for specific Ab is extremely complex and depends largely on what question one is trying to answer. Since prior studies have shown that administration of irrelevant MAb has no effect on the course of infection (17, 19, 44) and since future studies of Ab efficacy in humans will almost certainly use a control group that receives no Ab, we feel that the relevant comparison is between the groups that received MAb 2H1 and PBS. Furthermore, the use of even “irrelevant” MAbs is treacherous, since it is extremely difficult to exclude reactivity with some host antigen. For example, we have noted that MAb 3665, which binds p-azophenylarsonate and is commonly used for a control, reacts with lung tissue (unpublished observation). Hence, further discussion is confined to results obtained with MAb 2H1, with the caveat that the effects observed may include both specific and nonspecific components.
One surprising observation was that B lymphocytes were the predominant cell type in the inflammatory response to C. neoformans at all time intervals. The function of B lymphocytes in the host defense against pulmonary C. neoformans infection is uncertain. Cryptococcal infection in A/JCr mice is not accompanied by a significant increase in serum antibodies to GXM (19), with the caveat that specific Ab, if made, would likely be sequestered in antigen-Ab complexes. Early studies had argued against an important role for this cell type by demonstrating no increase in susceptibility in B lymphocyte-depleted mice (43). However, a recent study using B lymphocyte-deficient mice suggests a role for B lymphocytes in protection against cryptococcal infection (1). The abundance of B lymphocytes in the lung infiltrate suggests the need to evaluate the contribution of this cell type to protection against and/or pathogenesis of cryptococcal infection.
Our original assumption was that the differences in histological appearance in lungs of MAb-treated and control mice with C. neoformans infection resulted from quantitative and qualitative differences in the cellular inflammatory infiltrate. To our surprise, we found no significant difference in the total number of leukocytes and only small effects on the relative proportion of inflammatory cells. We interpret this finding as indicating that similar numbers of inflammatory cells reach the lung in the presence and absence of Ab but that these cells achieve different distributions in and around infected tissues, producing differences in histological appearance. MAb administration could allow additional granulocyte recruitment, as seen on day 14, by lowering the tissue GXM concentration, as GXM exerts several effects that reduce neutrophil recruitment (15, 16, 37). Neutrophils or eosinophils might benefit the host immune response to C. neoformans by several mechanisms, including enhanced fungal killing, cytokine production, and alteration of the nature of the developing inflammatory pattern (20, 39-41, 49, 55).
We hypothesized that the observed differences in the inflammatory response reflected differences in costimulatory molecules on antigen-presenting cells (APCs). The best-studied costimulatory interactions are those involving CD80 (B7-1) and CD86 (B7-2), surface molecules found on macrophages and on other APCs. CD86 is constitutively expressed on macrophages and dendritic cells, and its expression is up-regulated after APC activation (24). We found that the percentage of macrophages on which we could detect CD86 was significantly higher in MAb-treated mice than in control mice late (28 days) after infection. This finding is in concert with studies of human peripheral blood mononuclear cells (PBMCs), which show that CD86 and, to a lesser extent, CD80 are critical for the development of the cellular immune response to C. neoformans (42). Our inability to detect CD80 at any time during infection may reflect too low a level of expression for detection in our system, although in the absence of a positive control, we could not exclude systematic error. Incubation of human PBMCs with acapsular, but not encapsulated, C. neoformans increases surface expression of both CD80 and CD86. Addition of MAb 2H1 increases the expression of these costimulatory molecules in response to encapsulated yeast (56). Although in some systems, CD80 appears to mediate TH1-like responses, while CD86 promotes TH2-associated responses, this correlation is not complete (24). In vitro, blockade of CD86 in cocultures of human PBMCs and autologous T cells decreases lymphoproliferation in response to C. neoformans via inhibition of IL-2 transcription and increases IFN-γ production (42). Hence, it is conceivable that the increase in CD86 expression observed in MAb-treated mice results in a more efficient cellular response.
We found higher concentrations of IL-10 in the lungs of mice treated with a protective MAb prior to infection. Antigen-Ab complexes induce monocyte production of IL-10 (6). However, this result was surprising, as IL-10−/− mice are more resistant to intravenous C. neoformans infection than their wild-type controls (5, 7), and IL-10 is viewed as an important mediator in GXM-induced immunosuppression that results in diminution of both effector and antigen presentation function (7, 58). In vitro, addition of MAb 2H1 to monocytes and lymphocytes significantly reduces production of IL-10 in response to encapsulated C. neoformans (57). Interestingly, MAb 2H1 does not prolong survival of C. neoformans-infected IL-10−/− mice (5). IL-10 has differential inflammatory effects depending upon the overall milieu, influencing inflammatory cell survival, effector function, and antigen presentation (60). The role of IL-10 in MAb-mediated protection is presently undefined, but higher levels of this cytokine might contribute to prolonged survival by reducing host damage through down-regulation of aspects of the pulmonary inflammatory response.
In each of the four experiments examining either protein or mRNA concentrations of IFN-γ, the mean concentration was lower in MAb-treated mice. IFN-γ is produced by TH1 and NK cells in response to infection with C. neoformans, and its production is stimulated by the regulatory cytokines IL-12 and IL-18 (31, 32, 47). IFN-γ is required for effective host killing of C. neoformans in pulmonary infection, and its effect is mediated through macrophage production of nitric oxide (26). The essential role for IFN-γ in the host response for a number of intracellular pathogens has been clearly demonstrated. However, an inability to down-regulate IFN-γ production can be detrimental to the host, as seen in infection with Toxoplasma gondii (36). In cryptococcal infection, mice that are genetically deficient in inducible nitric oxide synthase have increased susceptibility to C. neoformans that is associated with marked enhancement of inflammation and loss of protective efficacy of GXM-binding MAb (J. Rivera, J. Mukherjee, L. M. Weiss, and A. Casadevall, submitted for publication). Thus, MAb may be exerting its protective effect in part by reducing damage through down-regulation of the proinflammatory effects of IFN-γ.
IL-4 mRNA expression and IL-4 protein concentration (in one of two experiments) in the lung were reduced on day 7 of infection by pretreatment of mice with GXM-binding MAb. We had hypothesized that MAb administration resulted in enhancement of a TH1-like environment because of the apparent enhancement of granulomatous inflammation seen in prior studies (19, 21). The data suggest that MAb treatment may, in fact, result in the appearance of an enhanced TH1-like milieu through a reduction in the presence of this pivotal TH2-associated cytokine. Administration of Ab to IL-4 prolongs survival of i.t. infected mice (30), although the effect results from increased IFN-γ and enhancement of fungal killing, features not seen in our study. Further, the magnitude of the reduction of IL-4 in our system was small, and the results obtained were not consistent. Therefore, it is unlikely that reduction in IL-4 is the major mechanism by which MAb mediates protection, although it may play a contributory role.
Perhaps the most important outcome of this study was that which we did not find, a major difference between MAb-treated and control mice with regard to either the cellular inflammatory response and/or cytokine expression. Despite the administration of a potent opsonin in the form of capsule-binding MAb, there was remarkably little difference between the experimental and control groups for most of the parameters studied. Although the methods used in the present study may not be able to measure local differences in the lung, the histopathology of which demonstrates significant regional heterogeneity (22), the possibility also remains that the parameters selected for study do not include those with a striking effect that results in MAb-mediated protection. However, it is not an exaggeration to state that these results have significantly altered our conceptions of how Ab mediates protection. As noted above, the hypothesis that MAb administration polarizes the TH1-TH2 balance toward a TH1 response as a consequence of Fc receptor activation was supported by several in vitro studies showing that Ab-mediated phagocytosis was associated with production of proinflammatory cytokines (54, 57). Furthermore, we note discordance between some of our findings and results observed with cytokine-deficient mice, such as those deficient in IL-10 (5). Conceivably, Ab could be mediating its protective effect through actions that were not studied or that remain to be discovered. Alternatively, MAb-mediated protection may result from many small changes in the immune response which, in the aggregate, bestow help to the host that allows longer survival and clearance of infection in a significant number of mice (19). Some or all of these changes may result from removal of immunosuppressive effects of GXM.
We are aware of only one prior study where mediators of inflammation were measured in the presence and absence of specific Ab. Administration of Ab to herpes simplex virus to mice with corneal infection reduces mRNAs for several chemokines, leading to the suggestion that the mechanism of Ab-mediated protection involves inhibition of inflammatory mediators (53). These results, together with the findings presented here, suggest that Ab modulates inflammation, in addition to its classical roles as opsonin, agglutinin, and complement system activator.
In summary, our search for the mechanism by which GXM-binding MAb mediates protection in murine pulmonary cryptococcal infection revealed a variety of subtle changes, each of which may be contributing to the observed effect. The observation that MAb-mediated protection was associated with increased levels of IL-10 and reduced levels of IFN-γ is difficult to reconcile with present views of the effective host immune response against C. neoformans, which emphasize the critical role of TH1-associated cytokines as mediators of granulomatous inflammation. This apparent inconsistency may reflect the limitations of applying a strict view of the TH1-TH2 paradigm to the early phases of a chronic infectious process (2). The finding that containment of yeast within better-formed granulomas was associated with transiently increased levels of IL-10 and decreased IFN-γ suggests an overall down-regulatory role for specific MAb in its interaction with cell-mediated immunity. MAb may also be acting, in part, by enhancing antigen presentation (54) through more efficient phagocytosis and enhanced expression of CD86. Perhaps the enhanced granulomatous inflammatory response evident in MAb-treated mice reflects a more effective organization for the inflammatory cell infiltrate in the setting of a different cytokine milieu. The present study reveals many intriguing directions for exploration of the mechanism by which specific MAb prolongs survival in infectious disease.
Acknowledgments
M.F. is supported by NIH grant AI01341. A.C. is supported by NIH grants AI22774 and 13342 and a Burroughs Welcome Developmental Therapeutics Award.
We thank Dave Gebhardt of the Flow Cytometry Facility at the Albert Einstein College of Medicine for assistance with experimental design and Donna Bruno of the Graphic Arts Center of the Albert Einstein College of Medicine.
Editor:T. R. Kozel
REFERENCES
- 1.Aguirre, K. M. 1997. A role for B cells in resistance to Cryptococcus neoformans in mice. Infect. Immun. 65:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, J. E., and R. M. Maizels. 1997. Th1-Th2: reliable paradigm or dangerous dogma? Immunol. Today 18:387-392. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, J. P., and U. G. Andersson. 1990. Human intravenous immunoglobulin modulates monokine production in vitro. Immunol. 71:372-376. [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson, U., L. Bjork, U. Skansen-Saphir, and J. Andersson. 1994. Pooled human IgG modulates cytokine production in lymphocytes and monocytes. Immunol. Rev. 139:21-42. [DOI] [PubMed] [Google Scholar]
- 5.Beenhouwer, D. O., S. Shapiro, M. Feldmesser, A. Casadevall, and M. D. Scharff. 2001. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect. Immun. 69:6445-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger, S., H. Ballo, and H. J. Stutte. 1996. Immune complex-induced interleukin-6, interleukin-10 and prostaglandin secretion by human monocytes: a network of pro- and anti-inflammatory cytokines dependent on the antigen:antibody ratio. Eur. J. Immunol. 26:1297-1301. [DOI] [PubMed] [Google Scholar]
- 7.Blackstock, R., K. L. Buchanan, A. M. Adesina, and J. W. Murphy. 1999. Differential regulation of immune responses by highly and weakly virulent Cryptococcus neoformans isolates. Infect. Immun. 67:3601-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouhlal, H., H. Hocini, C. Quillent-Gregoire, V. Donkova, S. Rose, A. Amara, R. Longhi, N. Haeffner-Cavaillon, A. Beretta, S. V. Kaveri, and M. D. Kazatchkine. 2001. Antibodies to C-C chemokine receptor 5 in normal human IgG block infection of macrophages and lymphocytes with primary R5-tropic strains of HIV-1. J. Immunol. 166:7606-7611. [DOI] [PubMed] [Google Scholar]
- 9.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L.-A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadevall, A., J. Mukherjee, S. J. N. Devi, R. Schneerson, J. B. Robbins, and M. D. Scharff. 1992. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 165:1086-1093. [DOI] [PubMed] [Google Scholar]
- 11.Casadevall, A., J. Mukherjee, and M. D. Scharff. 1992. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J. Immunol. Methods 154:27-35. [DOI] [PubMed] [Google Scholar]
- 12.Casadevall, A., and L.-A. Pirofski. 2001. Adjunctive immune therapy for fungal infections. Clin. Infect. Dis. 33:1048-1056. [DOI] [PubMed] [Google Scholar]
- 13.Curtis, J. L., G. B. Huffnagle, G.-H. Chen, M. L. Warnock, M. R. Gyetko, R. A. McDonald, and G. B. Toews. 1994. Experimental murine pulmonary cryptococcosis. Differences in pulmonary inflammation and lymphocyte recruitment induced by two encapsulated strains of Cryptococcus neoformans. Lab. Investig. 71:113-126. [PubMed] [Google Scholar]
- 14.Desmet, P., K. D. Kayembe, and C. De Vroey. 1989. The value of cryptococcal serum antigen screening among HIV-positive/AIDS patients in Kinshasa, Zaire. AIDS 3:77-78. [DOI] [PubMed] [Google Scholar]
- 15.Dong, Z. M., and J. W. Murphy. 1997. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect. Immun. 65:557-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong, Z. M., and J. W. Murphy. 1996. Cryptococcal polysaccharides induce L-selectin shedding and tumor necrosis factor receptor loss from the surface of human neutrophils. J. Clin. Investig. 97:689-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dromer, F., J. Charreire, A. Contrepois, C. Carbon, and P. Yeni. 1987. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 55:749-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldmesser, M., S. Tucker, and A. Casadevall. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 9:273-278. [DOI] [PubMed] [Google Scholar]
- 19.Feldmesser, M., and A. Casadevall. 1997. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J. Immunol. 158:790-799. [PubMed] [Google Scholar]
- 20.Feldmesser, M., A. Casadevall, Y. Kress, G. Spira, and A. Orlofsky. 1997. Eosinophil-Cryptococcus neoformans interactions in vivo and in vitro. Infect. Immun. 65:1899-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldmesser, M., Y. Kress, and A. Casadevall. 1998. Effect of antibody to capsular polysaccharide on eosinophilic pneumonia in murine infection with Cryptococcus neoformans. J. Infect. Dis. 177:1639-1646. [DOI] [PubMed] [Google Scholar]
- 22.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleuridor, R., A. Lees, and L. Pirofski. 2001. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection. J. Immunol. 166:1087-1096. [DOI] [PubMed] [Google Scholar]
- 24.Harris, N. L., and F. Ronchese. 1999. The role of B7 costimulation in T-cell immunity. Immunol. Cell Biol. 77:304-311. [DOI] [PubMed] [Google Scholar]
- 25.Hestdal, K., F. W. Ruscetti, J. N. Ihle, S. E. Jacobsen, C. M. Dubois, W. C. Kopp, D. L. Longo, and J. R. Keller. 1991. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J. Immunol. 147:22-28. [PubMed] [Google Scholar]
- 26.Hoag, K. A., M. F. Lipscomb, A. A. Izzo, and N. E. Street. 1997. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am. J. Respir. Cell Mol. Biol 17:733-739. [DOI] [PubMed] [Google Scholar]
- 27.Hoag, K. A., N. E. Street, G. B. Huffnagle, and M. F. Lipscomb. 1995. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am. J. Respir. Cell. Mol. Biol. 13:487-495. [DOI] [PubMed] [Google Scholar]
- 28.Huffnagle, G. B., M. B. Boyd, N. E. Street, and M. F. Lipscomb. 1998. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 160:2393-2400. [PubMed] [Google Scholar]
- 29.Huffnagle, G. B., R. M. Strieter, L. K. McNeil, R. A. McDonald, M. D. Burdick, S. L. Kunkel, and G. B. Toews. 1997. Macrophage inflammatory protein-1α (MIP-1α) is required for the efferent phase of pulmonary cell-mediated immunity to a Cryptococcus neoformans infection. J. Immunol. 159:318-327. [PubMed] [Google Scholar]
- 30.Kawakami, K., Q. M. Hossain, T. Zhang, Y. Koguchi, Q. Xie, M. Kurimoto, and A. Saito. 1999. Interleukin-4 weakens host resistance to pulmonary and disseminated cryptococcal infection caused by combined treatment with interferon-gamma-inducing cytokines. Cell Immunol. 197:55-61. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami, K., M. H. Qureshi, T. Zhang, Y. Koguchi, K. Shibuya, S. Naoe, and A. Saito. 1999. Interferon-gamma (IFN-gamma)-dependent protection and synthesis of chemoattractants for mononuclear leucocytes caused by IL-12 in the lungs of mice infected with Cryptococcus neoformans. Clin. Exp. Immunol. 117:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawakami, K., M. H. Qureshi, T. Zhang, Y. Koguchi, S. Yara, K. Takeda, S. Akira, M. Kurimoto, and A. Saito. 2000. Involvement of endogenously synthesized interleukin (IL)-18 in the protective effects of IL-12 against pulmonary infection with Cryptococcus neoformans in mice. FEMS Immunol. Med. Microbiol. 27:191-200. [DOI] [PubMed] [Google Scholar]
- 33.Kawakami, K., M. Tohyama, X. Quifeng, and A. Saito. 1997. Expression of cytokines and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of interleukin-12. Infect. Immun. 65:1307-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levitz, S. M. 2001. Cryptococcus neoformans: intracellular or extracellular? Trends Microbiol. 9:417-418. [DOI] [PubMed] [Google Scholar]
- 35.Levitz, S. M. 1991. The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Rev. Infect. Dis. 13:1163-1169. [DOI] [PubMed] [Google Scholar]
- 36.Liesenfeld, O., H. Kang, D. Park, T. A. Nguyen, C. V. Parkhe, H. Watanabe, T. Abo, A. Sher, J. S. Remington, and Y. Suzuki. 1999. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 21:365-376. [DOI] [PubMed] [Google Scholar]
- 37.Lipovsky, M. M., G. Gekker, S. Hu, L. C. Ehrlich, A. I. M. Hoepelman, and P. K. Peterson. 1998. Cryptococcal glucuronoxylomannan induces interleukin (IL)-8 production by human microglia but inhibits neutrophil migration toward IL-8. J. Infect. Dis. 177:260-263. [DOI] [PubMed] [Google Scholar]
- 38.Lovchik, J., M. Lipscomb, and C. R. Lyons. 1997. Expression of lung inducible nitric oxide synthase protein does not correlate with nitric oxide production in vivo in a pulmonary immune response against Cryptococcus neoformans. J. Immunol. 158:1772-1778. [PubMed] [Google Scholar]
- 39.Mambula, S. S., E. R. Simons, R. Hastey, M. E. Selsted, and S. M. Levitz. 2000. Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect. Immun. 68:6257-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, M. F., and T. G. Mitchell. 1991. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect. Immun. 59:24-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monari, C., A. Casadevall, C. Retini, F. Baldelli, F. Bistoni, and A. Vecchiarelli. 1999. Antibody to capsular polysaccharide enhances the function of neutrophils from patients with AIDS against Cryptococcus neoformans. AIDS 13:653-660. [DOI] [PubMed] [Google Scholar]
- 42.Monari, C., T. R. Kozel, A. Casadevall, D. Pietrella, B. Palazzetti, and A. Vecchiarelli. 1999. B7 costimulatory ligand regulates development of the T-cell response to Cryptococcus neoformans. Immunology 98:27-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monga, D. P., R. Kumar, L. N. Mohapatra, and A. N. Malaviya. 1979. Experimental cryptococcosis in normal and B-cell-deficient mice. Infect. Immun. 26:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukherjee, J., L.-A. Pirofski, M. D. Scharff, and A. Casadevall. 1993. Antibody-mediated protection in mice with lethal intracerebral Cryptococcus neoformans infection. Proc. Natl. Acad. Sci. USA 90:3636-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60:4534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nachbaur, D., M. Herold, B. Eibl, H. Glassl, H. Schwaighofer, C. Huber, A. Gachter, M. Pichl, and D. Niederwieser. 1997. A comparative study of the in vitro immunomodulatory activity of human intact immunoglobulin (7S IVIG), F(ab′)2 fragments (5S IVIG) and Fc fragments. Evidence for post-transcriptional IL-2 modulation. Immunology 90:212-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qureshi, M. H., T. Zhang, Y. Koguchi, K. Nakashima, H. Okamura, M. Kurimoto, and K. Kawakami. 1999. Combined effects of IL-12 and IL-18 on the clinical course and local cytokine production in murine pulmonary infection with Cryptococcus neoformans. Eur. J. Immunol. 29:643-649. [DOI] [PubMed] [Google Scholar]
- 48.Reiner, S. L., S. Zheng, D. B. Corry, and R. M. Locksley. 1993. Constructing polycompetitor cDNAs for quantitative PCR. J. Immunol. Methods 165:37-46. [DOI] [PubMed] [Google Scholar]
- 49.Retini, C., A. Vecchiarelli, C. Monari, C. Tascini, F. Bistoni, and T. R. Kozel. 1996. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect. Immun. 64:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samuelsson, A., T. L. Towers, and J. V. Ravetch. 2001. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291:484-486. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz, D. A. 1988. Characterization of the biological activity of Cryptococcus infections in surgical pathology. The budding index and carminophilic index. Ann. Clin. Lab. Sci. 18:388-397. [PubMed] [Google Scholar]
- 52.Stangel, M., and A. Compston. 2001. Polyclonal immunoglobulins (IVIg) modulate nitric oxide production and microglial functions in vitro via Fc receptors. J. Neuroimmunol. 112:63-71. [DOI] [PubMed] [Google Scholar]
- 53.Su, Y. H., X. T. Yan, J. E. Oakes, and R. N. Lausch. 1996. Protective antibody therapy is associated with reduced chemokine transcripts in herpes simplex virus type 1 corneal infection. J. Virol. 70:1277-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Syme, R. M., T. F. Bruno, T. R. Kozel, and C. H. Mody. 1999. The capsule of Cryptococcus neoformans reduces T-lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect. Immun. 67:4620-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tateda, K., T. A. Moore, J. C. Deng, M. W. Newstead, X. Zeng, A. Matsukawa, M. S. Swanson, K. Yamaguchi, and T. J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 166:3355-3361. [DOI] [PubMed] [Google Scholar]
- 56.Vecchiarelli, A., C. Monari, C. Retini, D. Pietrella, B. Palazzetti, L. Pitzurra, and A. Casadevall. 1998. Cryptococcus neoformans differently regulates B7-1 (CD80) and B7-2 (CD86) expression on human monocytes. Eur. J. Immunol. 28:114-121. [DOI] [PubMed] [Google Scholar]
- 57.Vecchiarelli, A., C. Retini, C. Monari, and A. Casadevall. 1998. Specific antibody to Cryptococcus neoformans alters human leukocyte cytokine synthesis and promotes T-cell proliferation. Infect. Immun. 66:1244-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vecchiarelli, A., C. Retini, C. Monari, C. Tascini, F. Bistoni, and T. R. Kozel. 1996. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect. Immun. 64:2846-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wadhwa, M., A. Meager, P. Dilger, C. Bird, C. Dolman, R. G. Das, and R. Thorpe. 2000. Neutralizing antibodies to granulocyte-macrophage colony-stimulating factor, interleukin-1alpha and interferon-alpha but not other cytokines in human immunoglobulin preparations. Immunology 99:113-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, Z. Q., A. S. Bapat, R. J. Rayanade, A. S. Dagtas, and M. K. Hoffmann. 2001. Interleukin-10 induces macrophage apoptosis and expression of CD16 (FcgammaRIII) whose engagement blocks the cell death programme and facilitates differentiation. Immunology 102:331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan, R., A. Casadevall, J. Oh, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc. Natl. Acad. Sci. USA 94:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]