Abstract
To initiate invasion of the mosquito midgut, Plasmodium ookinetes secrete chitinases that are necessary to cross the chitin-containing peritrophic matrix en route to invading the epithelial cell surface. To investigate chitinases as potential immunological targets of blocking malaria parasite transmission to mosquitoes, a monoclonal antibody (MAb) was identified that neutralized the enzymatic activity of the sole chitinase of Plasmodium falciparum, PfCHT1, identified to date. This MAb, designated 1C3, previously shown to react with an apical structure of P. falciparum ookinetes, also reacts with a discrete apical structure of P. gallinaceum ookinetes. In membrane feeding assays, MAb 1C3 markedly inhibited P. gallinaceum oocyst development in mosquito midguts. MAb 1C3 affinity isolated an ∼210-kDa antigen which, under reducing conditions, became a 35-kDa antigen. This isolated 35-kDa protein cross-reacted with an antiserum raised against a synthetic peptide derived from the P. gallinaceum chitinase active site, PgCHT1, even though MAb 1C3 did not recognize native or recombinant PgCHT1 on Western blot. Therefore, this affinity-purified 35-kDa antigen appears similar to a previously identified protein, PgCHT2, a putative second chitinase of P. gallinaceum. Epitope mapping indicated MAb 1C3 recognized a region of PfCHT1 that diverges from a homologous amino acid sequence conserved within sequenced chitinases of P. berghei, P. yoelii, and P. gallinaceum (PgCHT1). A synthetic peptide derived from the mapped 1C3 epitope may be useful as a component of a subunit transmission-blocking vaccine.
Malaria kills more than 2 million people each year, and the prevalence of drug-resistant malaria is increasing (8). At present there is no vaccine to prevent or ameliorate malaria. Therefore, alternative strategies for preventing and reducing the global burden of malaria are crucial. A key strategy for developing ways to prevent and treat malaria focuses on defining molecular targets, which can be used for drug or vaccine intervention. Recent studies have concentrated on transmission-blocking vaccines (1, 8), a strategy that targets antigens expressed by malaria parasites during transmission from humans to mosquitoes. Such vaccines are designed to induce antibodies in humans that, when ingested by the mosquito along with a Plasmodium-containing blood meal, interfere with the development of the parasite within the mosquito midgut.
The peritrophic matrix is the first physical barrier faced by the parasite in the mosquito midgut. Ultrastructural studies have demonstrated that Plasmodium ookinetes actively penetrate the peritrophic matrix, focally disrupting the matrix near the apical end of the parasite (21). The demonstrated presence of chitin in the peritrophic matrix (15), disruption of the peritrophic matrix during ookinete invasion (21), and the presence of chitinase in maturing Plasmodium gallinaceum ookinetes (7), suggested that a parasite-derived chitinase was degrading the peritrophic matrix, allowing the parasite access to cells of the midgut epithelium. The important role of chitinase in allowing the ookinete to traverse the peritrophic matrix was further supported by the observation that the presence of a chitinase inhibitor, allosamidin, in an infectious blood meal prevented oocyst formation (19), and more definitively by observations that mutations or deletion of the chitinase gene in P. falciparum and P. berghei impair infectivity of parasites for mosquitoes (3, 22). Since Plasmodium-secreted chitinases are critical to the parasite life cycle, this class of enzyme is an important potential target for blocking malaria parasite transmission from the vertebrate host to the mosquito vector (reviewed in reference 12).
Molecular studies to date have demonstrated the presence of only one chitinase gene in P. falciparum strain 3D7 (27) and P. gallinaceum (29). However, at least two chromatographically separable chitinase activities are present in P. gallinaceum ookinetes (13, 14, 29, 30), each associated with a different size protein as determined by Western immunoblots. Peak 1 contained an ∼60-kDa doublet protein encoded by a gene designated PgCHT1 (GenBank accession no. AF064079) (29). Peak 2 of chitinase activity contained an ∼210-kDa protein that under reducing conditions yielded two protein components: one with a molecular mass of ∼35 kDa (provisionally termed PgCHT2) and the other an ∼160-kDa protein; all of these proteins were recognized by antisera raised to synthetic peptides derived from other protozoal chitinase (family 18 glycohydrolase, EC 3.2.1.14) active sites, which are highly conserved (28, 29). While both peaks of chitinase activity hydrolyzed 4-methylumbelliferyl (MU) chitotrioside, peak 2 had a distinctly different pH activity profile and Km that were more similar to recombinant P. falciparum chitinase (PfCHT1) than to recombinant PgCHT1 (GenBank accession no. AF072442) (27, 29). These results suggested the possible presence of at least a second chitinase gene in the P. gallinaceum genome, provisionally called PgCHT2.
Because ookinete-secreted chitinases are potential targets for blocking malaria transmission, characterizing the complete complement of Plasmodium chitinases is of both fundamental and practical interest. Mechanisms by which ookinetes secrete chitinase and whether this enzyme is susceptible to neutralization may have future implications for the development of transmission blocking strategies for chitinase and other parasite molecules involved in infection of the mosquito. In the present study, we generated an anti-P. falciparum chitinase monoclonal antibody (MAb), designated 1C3, previously demonstrated to recognize PfCHT1 in Anopheles freeborni midgut-derived P. falciparum ookinetes (22). This MAb was used to detect and characterize a set of epitopes in P. gallinaceum ookinetes, which includes a 35-kDa protein with properties similar to the previously characterized putative chitinase, PgCHT2 (29). The results have implications for understanding details of Plasmodium cell biology and parasite-mosquito evolution and demonstrate the potential utility of chitinases as targets for blocking malaria parasite transmission to mosquitoes.
MATERIALS AND METHODS
The University of Texas Medical Branch Institutional Animal Care and Use Committee approved animal use in this study.
Preparation of P. gallinaceum ookinetes.
The 8A strain of P. gallinaceum was used to infect 4- to 6-week-old White Leghorn chickens. A gametocyte-producing line was maintained by mechanical subpassage in chickens and periodic passage through mosquitoes. Ookinetes were cultured from purified zygotes in serum- and protease-free M199 culture medium, and parasite lysates and extracts were obtained as described previously (10, 29).
Production of anti-PfCHT1 MAbs.
Recombinant P. falciparum chitinase (rPfCHT1) was expressed as previously described (27). Anti-rPfCHT1 MAbs were produced, by using SP2/0 myeloma cells, as previously described (6). Of the panel of MAbs obtained, MAb 1C3 (immunoglobulin G2b [IgG2b], κ chain) was selected for the present study. For some applications, MAb 1C3 and an IgG2b isotype control MAb of irrelevant specificity were purified from culture media with protein A-HiTrap Sepharose 1-ml columns (Amersham Pharmacia, Piscataway, N.J.). Culture supernatants were passed (1 ml/min) over protein A-Sepharose, with unbound protein washed from the column with 20 mM sodium phosphate buffer (pH 7.5). Bound MAbs were eluted with 0.1 M sodium citrate (pH 3.5). Purified MAb was immediately neutralized by collecting fractions directly into 0.1 M Tris-HCl (pH 9.0), and fractions were dialyzed extensively against phosphate-buffered saline (PBS) by using a Slide-A-Lyzer (10,000 molecular weight cutoff; Pierce).
Immunofluorescence and immunoelectron microscopy with MAb 1C3.
Ookinete cultures at 24 h were centrifuged (10 min, 3,000 × g, 22°C), and ookinete pellets were resuspended in PBS containing 3% bovine serum albumin (BSA). Ookinetes, air dried and heat fixed to multichamber glass slides (PGC Scientific, Frederick, Md.), were incubated (1 h, 22°C) with PBS-3% BSA-3% Triton X-100 to block nonspecific binding sites and permeabilize parasites. Slides were incubated (30 min, 22°C) in a humidified chamber with primary MAbs (1C3 or IgG2b isotype control), washed with PBS (five times), incubated (30 min, 22°C) with fluorescein-conjugated goat anti-mouse IgG, IgM, or IgA (Kirkegaard & Perry, Gaithersburg, Md.) in PBS, washed with PBS (four times), and H2O (one time). All washes were for 5 min. Slides were mounted with Permafluor (Shandon, Pittsburgh, Pa.) and observed on a Zeiss Axiophot 2 immunofluorescence microscope.
For immunoelectron microscopy studies, in vitro-cultured P. gallinaceum ookinetes were fixed in PBS containing 1% glutaraldehyde and embedded in LR white resin (Polysciences, Warrington, Pa.). Sections were blocked (PBS, 5% nonfat dry milk, 0.01% Tween 20), incubated with protein A-purified MAb 1C3 or IgG2b isotype control of irrelevant specificity (∼70 μg/ml each), washed, incubated with affinity-purified colloidal gold-conjugated goat anti-mouse IgG or IgM (5 nm; Amersham Pharmacia, Piscataway, N.J.). Sections were stained (2% uranyl acetate in 50% methanol), rinsed (50% methanol), counterstained (Reynold's lead citrate), and carbon coated in a vacuum evaporator. Samples were examined and photographed with a Hitachi H-800 transmission electron microscope.
SDS-PAGE and Western immunoblotting.
For Western immunoblot (nonreducing, denaturing), rPfCHT1 (5 μg), rPgCHT1 (5 μg), P. gallinaceum ookinete culture supernatants (5 μg), or extracts (5 μg) were boiled (5 min) in sample buffer (25 mM Tris-HCl [pH 6.8], 2.2% sodium dodecyl sulfate [SDS], 15% glycerol, 0.001% bromophenol blue), centrifuged (10,000 × g, 5 min) to remove insoluble debris, and resolved in 4 to 20% Tris-glycine gradient gels (Invitrogen, Carlsbad, Calif.). Resolved proteins were electroblotted to nitrocellulose membranes by using the Novex Xcell Blot II module. Western blots were performed as described previously (16) and developed with BCIP/NBT alkaline phosphatase substrate (Kirkegaard & Perry). Primary antibodies were normal mouse serum, anti-rPfCHT1 (12), anti-rPgCHT1 (29), anti-PgCHT1 C terminus (29), anti-PgCHT1 active site (29), IgG2b isotype control (25 μg/ml), and 1C3 (25 μg/ml).
Effect of MAb 1C3 on chitinase activity and P. gallinaceum infectivity for Aedes aegypti mosquitoes.
Purified MAb 1C3 or isotype control antibody (IgG2b) (10 μg each) was incubated (1 h, 37°C) with purified rPfCHT1 or rPgCHT1 (0.50 μg), after which the chitinase substrate 4-MU-chitotrioside was added to determine whether 1C3 inhibited PfCHT1 enzymatic activity, as determined by microfluorimetry. Allosamidin (10 μg) or purified 1C3 or IgG2b (20 and 40 μg) was mixed with freshly drawn P. gallinaceum-infected (∼10% parasitemia) chicken blood (200 μl) containing heparin, and the mixtures were fed to overnight-starved Aedes aegypti mosquitoes through a membrane feeder. Midguts were removed from engorged mosquitoes at day 7 and stained with 1% (vol/vol) mercurochrome in water, and the numbers of oocysts per midgut were enumerated by light microscopy. Experimental and control groups were compared for oocyst numbers by the nonparametric Mann-Whitney U test.
Mapping of the epitope recognized by MAb 1C3.
rPfCHT1 was treated with enterokinase and isolated as described above but was further purified by hydrophobic interaction chromatography with phenyl Sepharose (Amersham Pharmacia). Enterokinase-cleaved rPfCHT1 (620 μg in 300 μl of PBS) was incubated (5 h, 37°C) with 1 M sodium bicarbonate (30 μl) containing Endoproteinase Glu-C (30 μg; Roche Molecular Biochemicals). The sample was then heated (100°C, 10 min) to destroy enzyme activity. Endoproteinase Glu-C-proteolyzed rPfCHT1 (248 μg) was subsequently injected onto a C18 reversed-phase high-pressure liquid chromatography (HPLC) column (2 mm by 25 cm) eluted with a linear gradient of 1% trifluoroacetic acid (TFA) (solvent A) and 0.8% TFA in neat acetonitrile (solvent B) at 0.2 ml/min. The gradient used was as follows: 100% solvent A for 15 min, 20 to 60% solvent B from 15 to 105 min, and 60 to 100% solvent B from 105 to 120 min. Fractions were collected every 2 min and assayed by dot blot for immunoreactivity with 1C3. Fractions (50 μl) were dotted onto a polyvinylidene difluoride membrane, and then the membrane was washed (three times, 200 μl/wash) with buffer A and fixed (20 min, 22°C) with 10% (vol/vol) acetic acid-25% (vol/vol) isopropanol. Membranes were probed (1 h, 22°C) with MAb 1C3 (25 μg/ml) or IgG2b isotype control MAb (25 μg/ml), and bound MAb was detected with alkaline phosphatase-conjugated goat anti-mouse IgG/M/A at 1:3,000 and BCIP/NBT phosphatase substrate. The 1C3 immunoreactive peptide fraction was analyzed by tandem mass spectrometry (MS/MS). Sequence information was determined by capillary reversed-phase liquid chromatography coupled to the electrospray ionization source of a Finnegan quadrupole ion trap mass spectrometer. The instrument was programmed to acquire successive sets of three scan modes consisting of full scan MS over the m/z range from 395 to 1,200, followed by two data-dependent scans on the most abundant ion in that full scan. These datum-dependent scans allowed the automatic acquisition of a high-resolution scan to determine the charge state, the exact mass, and the MS/MS spectra to establish the peptide sequence. In a separate experiment, the 1C3 immunoreactive fraction was applied to the biphasic column of a Hewlett-Packard G1005A, followed by a 1-ml wash with the manufacturer's sample loading solution. The purified peptide was then subjected to automated Edman degradation on an Applied Biosystems 494/HT Procise Sequencer in the University of Texas Medical Branch Protein Chemistry Core Facility.
Synthetic peptides were designed based on the amino acid sequence of the 1C3 immunoreactive peptide of rPfCHT1. A 22-amino-acid peptide (LYDSYAYYGKKYDYVIIMGFTL) was synthesized, spanning the first 22 amino acids of the 47-amino-acid Endoproteinase Glu-C-proteolyzed peptide. Additionally, a 13-amino-acid peptide (LYDSYAYYGKKYD) specifically representing the predicted epitope was synthesized, followed by a sequential deletion of the carboxyl-terminus amino acid, generating the following peptides: LYDSYAYYGKKY, LYDSYAYYGKK, LYDSYAYYGK, and LYDSYAYYG. Synthetic peptides were synthesized and purified at the University of Texas Medical Branch Protein Chemistry Core Facility. Immunolon 1 96-well plates (Nunc) were coated with each synthetic peptide (2 μg/ml in guanidine HCl, 50 μl/well), nonspecific binding sites were blocked with Tris-buffered saline (TBS)-1.5% BSA and incubated (1 h, 37°C) with MAb 1C3 (25 μg/ml) or IgG2b isotype control MAb (25 μg/ml). Plates were washed 10 times with TBST-1.5% BSA. Bound MAb was detected with alkaline phosphatase goat anti-mouse IgG/M/A and p-nitrophenyl phosphate substrate (Sigma, St. Louis, Mo.). Absorbance for each well was determined at 405 nm, and an A405 value that was two times greater than that of the isotype control MAb was considered positive.
Immunoaffinity isolation of P. gallinaceum ookinete antigen recognized by 1C3.
Protein A-purified 1C3 was coupled to protein A-Sepharose (Amersham Pharmacia) according to a previously described procedure (6). Briefly, after incubation (2 h, 22°C) of protein A matrix (0.25 g = 1 ml reconstituted) with 1C3 (3 mg) in PBS, the matrix was washed with 0.2 M sodium borate buffer (pH 9.0) and incubated (30 min, 22°C) in the same sodium borate containing 20 mM dimethylpimelidate. Next, the matrix was washed and incubated (2 h, 22°C) with 0.2 M ethanolamine, followed by washing in PBS containing 0.1% thimerosal. After determination of the optimal conditions for binding and elution, the column was used for preparative isolation of P. gallinaceum antigens bound by anti-P. falciparum MAb, 1C3, as follows. A combination of P. gallinaceum ookinete extracts and/or supernatants, in PBS, were run over (0.5 ml/min) a MAb 1C3-coupled Sepharose column by using the AKTA Explorer Chromatography System (Amersham Pharmacia). After being washed with PBS, the bound material was eluted (10% triethanolamine, pH 11), immediately neutralized (0.1 M Tris-HCl; pH 6.8), concentrated 50-fold with Centriprep 10 (Amicon), dialyzed (10-kDa exclusion limit) against PBS, and stored at −20°C prior to use. Eluted proteins were characterized by SDS-PAGE. Samples were boiled (5 min) in sample buffer containing 2.5% (vol/vol) 2-mercaptoethanol for reduced samples, centrifuged (10,000 × g, 15 min) to remove insoluble material, and resolved in 4 to 20% gradient gels (Invitrogen). Gels were either stained with Coomassie blue or subjected to Western immunoblotting as described above.
RESULTS
MAb 1C3 delineates the apical end of the P. gallinaceum ookinete.
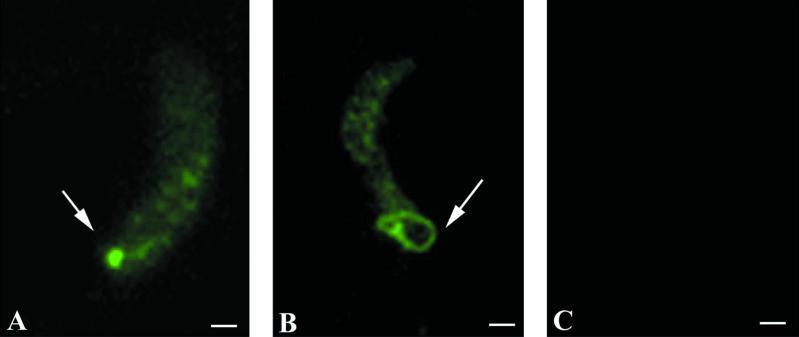
As detected by immunofluorescence microscopy, MAb 1C3 bound to ookinetes in a nondiffuse granular pattern throughout the parasite. At the apical end of the ookinete, dense focal concentrations were observed, manifesting either as a highly localized collection (Fig. 1A) or as ring-shaped formations (Fig. 1B). These two distinct immunofluorescence patterns were reproducibly observed in more than 100 ookinetes examined. In addition, MAb 1C3 reacted with amorphous antigen deposits pooled around morphologically mature ookinetes, a finding consistent with secreted material, as well as within incompletely transformed ookinetes (retorts) (not shown).
FIG. 1.
Immunofluorescence microscopy of P. gallinaceum ookinetes with MAb 1C3 (A and B) or isotype control MAb (C). The 1C3 epitope is concentrated in the apical end (A) and ring-like structure (B) of the parasite (arrows). Bar, 0.5 μm.
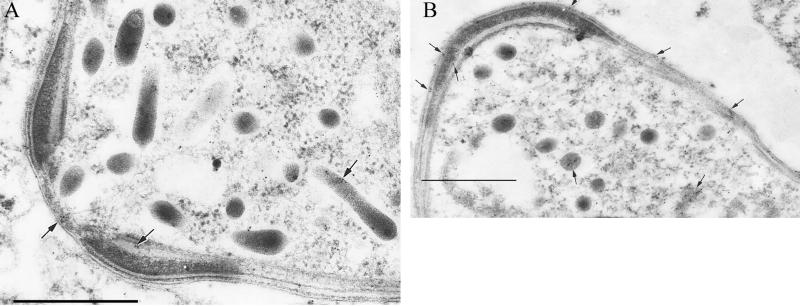
At the ultrastructural level, the MAb 1C3 epitope was found within the ookinete cytoplasm, within apically distributed micronemes, and on the plasma membrane on the anterior aspect of the ookinete in a configuration consistent with the immunofluorescence-delineated ring-shaped structure (Fig. 2). The electron microscopy-delineated location of the 1C3 epitope within the cytoplasm and micronemes at the apical end of the ookinete is also consistent with the immunofluorescence findings (Fig. 1). The plasma membrane location of the 1C3 epitope suggests that this protein not only is secreted freely into the extracellular milieu but also is present on the cell surface. The cytoplasmic location of the 1C3 epitope could indicate either its site of synthesis or its route of intracellular trafficking after synthesis. The subcellular localization patterns delineated by immunoelectron microscopy were highly reproducible. No immunogold labeling with an isotype control MAb used at identical concentrations was observed (data not shown).
FIG. 2.
Immunoelectron microscopy of P. gallinaceum ookinete with MAb 1C3. Labeling of the 1C3 epitope is present within micronemes, the apical tip, laterally along the conoid collar (arrows), and extracellularly. Bar, 0.5 μm.
Recognition of P. gallinaceum cell-associated and secreted proteins by MAb 1C3.
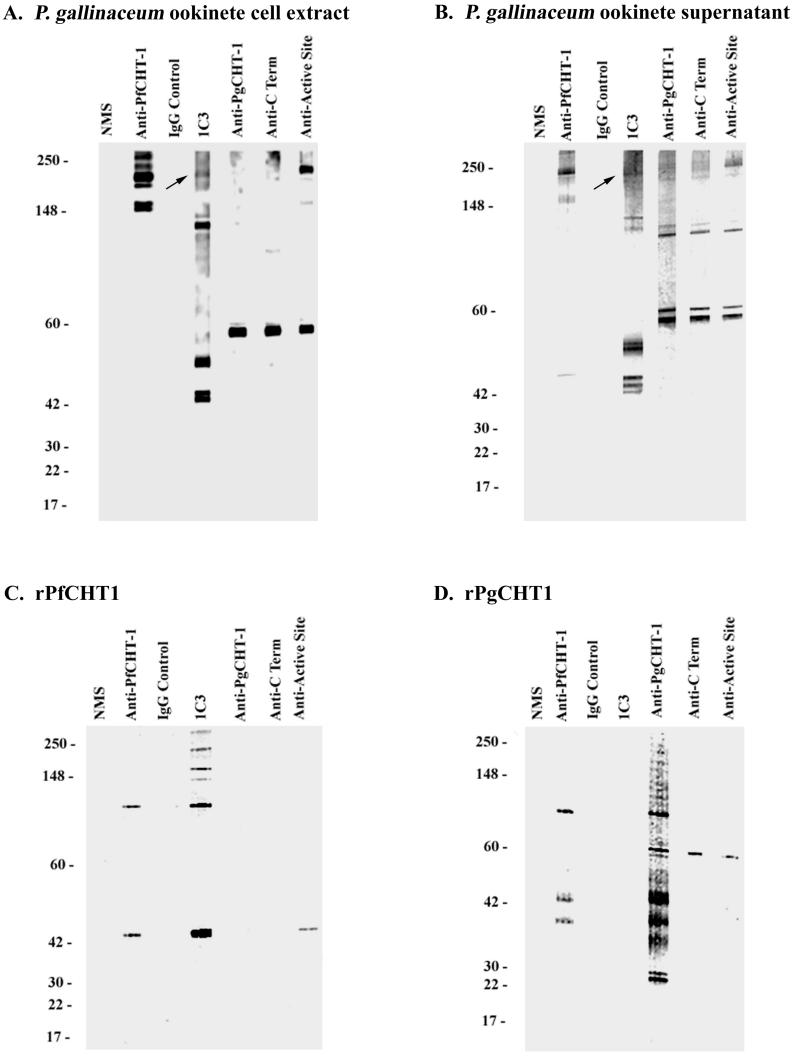
In Western immunoblots of native P. gallinaceum ookinete extracts and supernatants from axenically cultured ookinetes, MAb 1C3 recognized multiple proteins ranging from 42 to ∼210 kDa (Fig. 3A and B). These proteins are of identical molecular mass compared to the previously described PgCHT2 (29). As a positive control MAb 1C3 recognized rPfCHT1 at the predicted molecular mass of ∼42 kDa (Fig. 3C). Polyclonal anti-PfCHT1 antiserum cross-reacted with several high-molecular-weight bands in ookinete extracts and culture supernatants but not with PgCHT1, suggesting that this antiserum might be recognizing an ortholog of the PfCHT1 gene product in P. gallinaceum ookinetes. Only one of the bands recognized by the polyclonal anti-PfCHT1 antiserum comigrated with the high-molecular-weight epitope recognized by MAb 1C3. The higher-molecular-weight proteins recognized by polyclonal anti-PfCHT1 and MAb 1C3 on Western immunoblots of purified rPfCHT1 are multimers of rPfCHT1, since under reducing conditions the bands disappeared to yield a single ∼42-kDa protein upon Coomassie blue staining (data not shown). However, MAb 1C3 failed to recognize enzymatically active rPgCHT1 on Western immunoblot (Fig. 3D), indicating that 1C3 recognized a different, non-PgCHT1 micronemal protein secreted by P. gallinaceum ookinetes. Positive control antisera raised to the PgCHT1 chitinase active site bound to rPgCHT1 and rPfCHT1, confirming the presence of the proteins on the nitrocellulose membrane. The multiple bands recognized by polyclonal anti-PgCHT1 antiserum on the rPgCHT1 Western immunoblot are attributable to multiple conformers of the cysteine-rich recombinant protein produced in Escherichia coli.
FIG. 3.
Western immunoblot recognition of chitinases and the 1C3 epitope in P. gallinaceum ookinete cell extract (A), P. gallinaceum ookinete culture supernatant (B), recombinant PfCHT1 (C), and recombinant PgCHT1 (D). Proteins were separated by SDS-PAGE and electroblotted onto nitrocellulose. Individual lanes were probed as labeled above lanes in each panel. Note the ∼210-kDa 1C3 immunoreactive band comigrating with anti-PgCHT1 active-site immunoreactive bands in both types of native P. gallinaceum antigen (A and B, arrows).
1C3 neutralizes chitinase activity in vitro and inhibits P. gallinaceum oocyst formation in membrane feeding.
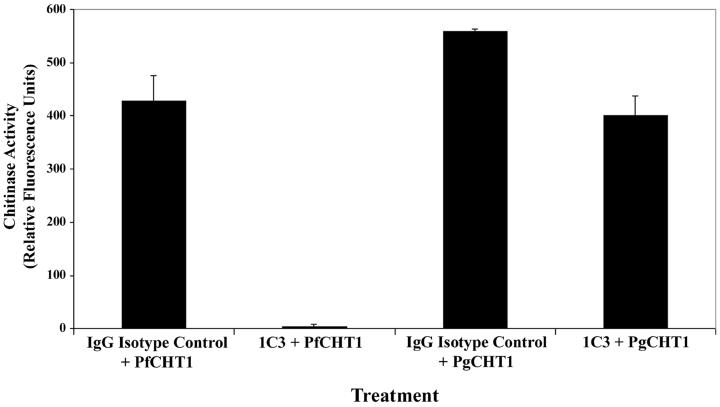
After in vitro incubation with MAb 1C3, rPfCHT1 enzymatic activity was completely neutralized, as determined by 4-MU-chitotrioside hydrolysis in a microfluorimetry assay (Fig. 4). However, rPgCHT1 enzymatic activity was not affected by treatment with MAb 1C3. The addition of MAb 1C3 to an infectious blood meal of P. gallinaceum fed to Aedes aegypti mosquitoes significantly inhibited the appearance of oocysts in a dose-dependent manner (Table 1).
FIG. 4.
Effect of MAb 1C3 on recombinant chitinase activity in vitro. MAb 1C3 or IgG2b isotype MAbs were incubated with either rPfCHT1 or rPgCHT1 and chitinase activity measured by using 4-MU-chitotrioside as the substrate. Error bars represent the standard deviations. Excitation was determined at 365 nm and emission at 460 nm to determine the relative fluorescent units (chitinase activity). The reduction of rPfCHT1 chitinase activity when treated with MAb 1C3 was statistically significant, while there was no significant effect of isotype control antibodies on rPfCHT1 or rPgCHT1, and no effect of MAb 1C3 on rPgCHT1.
TABLE 1.
Effect of MAb 1C3 on oocyst development of P. gallinaceum in Aedes aegypti mosquitoesa
| Treatment | No. of oocysts/gutb | Infectivity (%)c | No. infected/total no.d | Pe |
|---|---|---|---|---|
| PBS (−) cont.f | 1.68 | 100 | 14/19 | <1.000 |
| Allosamidin (+) cont.g | 0.15 | 8.8 | 2/21 | <0.001 |
| IgG2b ICh (20 μg) | 1.41 | 84 | 8/14 | <1.000 |
| IgG2b IC (40 μg) | 1.56 | 92 | 13/23 | >0.100 |
| 1C3 (20 μg) | 0.22 | 13 | 3/14 | <0.010 |
| 1C3 (40 μg) | 0.12 | 7 | 2/26 | <0.001 |
These data are representative of two experiments with similar results.
Geometric mean.
Infectivity if expressed as the percentage of mean oocysts per gut relative to that of the control group.
Number of mosquitoes infected/total number of mosquitoes dissected.
P values were determined by the Mann-Whitney U test.
Negative control.
Positive control.
IC, isotype control.
Mapping of the epitope recognized by MAb 1C3 delineates a hydrophobic amino acid stretch that diverges from homologous regions of non-P. falciparum chitinases.
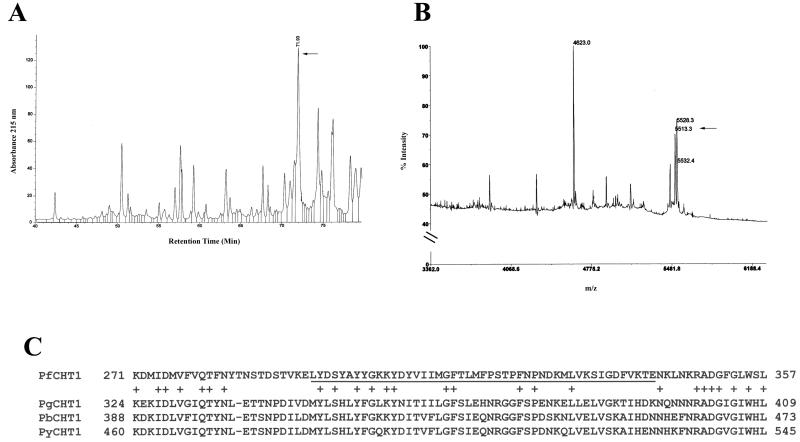
To identify the region of PfCHT1 bound by MAb 1C3, an epitope-mapping approach was used in which the highly purified recombinant protein was subjected to proteolysis, the resultant peptides were separated by HPLC, and fractions were tested for binding to MAb 1C3 by using a dot blot immunoassay. Several proteases were tested for their ability to digest rPfCHT1, including trypsin, Endoproteinase Lys-C, and Endoproteinase Glu-C. As previously observed, trypsin and Endoproteinase Lys-C were unable to cleave rPfCHT1 efficiently (27), whereas Endoproteinase Glu-C proteolyzed rPfCHT1 to yield a rich pattern of peptide fragments (Fig. 5A). As determined by a dot blot immunoassay of the HPLC-separated fractions, followed by Edman degradation and MS analysis of the positive fractions (indicated by the arrow in Fig. 5A and B), the rPfCHT1 epitope recognized by MAb 1C3 mapped to a hydrophobic peptide near the carboxy terminus of PfCHT1 (Fig. 5C, underlined portion of PfCHT1). A single amino acid sequence was obtained by Edman degradation analysis of the dot blot-positive HPLC fraction, which was consistent with the MS analysis. To confirm the identity of the MAb 1C3-recognized epitope, synthetic peptides based upon the Edman degradation-identified peptide sequence were tested for MAb 1C3 immunoreactivity. In an enzyme-linked immunosorbent assay, MAb 1C3 bound to the 13-residue peptide (LYDSYAYYGKKYD), as well as the four additional peptides containing sequential deletions of the C-terminal amino acid (LYDSYAYYGKKY, LYDSYAYYGKK, LYDSYAYYGK, and LYDSYAYYG) (data not shown).
FIG. 5.
(A) Mapping of 1C3 epitope within rPfCHT1 and homology analysis. rPfCHT1 was subjected to proteolysis by Endoproteinase Glu-C and peptides separated by HPLC. (B) Dot blot immunological analysis identified one HPLC fraction as reactive with MAb 1C3 (arrow). This fraction was analyzed by Edman degradation (see the text) and matrix-assisted laser desorption ionization-time of flight MS. The ion indicated by the arrow is consistent with the single amino acid sequence identified by peptide sequencing in the fraction, taking into account the enzymatic specificity of the protease. (C) The 1C3 epitope is underlined within the amino acid stretch from 271 to 357 of the PfCHT1 coding sequence (GenBank accession no. AAF63209). Compared to homologous regions from chitinases of P. gallinaceum (PgCHT1, GenBank accession no. AAF63208), P. berghei (PbCHT1, GenBank accession no. CAC40151), and P. yoelii (PyCHT1, The Institute for Genome Research website [http://www.tigr.org/tdb/edb2/pya1/htmls/PYA1.gene.list], Locus 1275.t00001, bp 3319 to 1157, assembly c2m1275, designated “endochitinase precursor”), the 1C3 epitope has <35% identity compared to a much higher homology shared among the latter three chitinases.
Sequence comparison of this PfCHT1-derived amino acid sequence with homologous regions of sequenced chitinases of P. gallinaceum (PgCHT1), P. berghei (PbCHT1), and P. yoelii (PyCHT1) indicated that the 1C3-recognized epitope of PfCHT1 showed <35% residue identity to the same region of PgCHT1 (Fig. 5C).
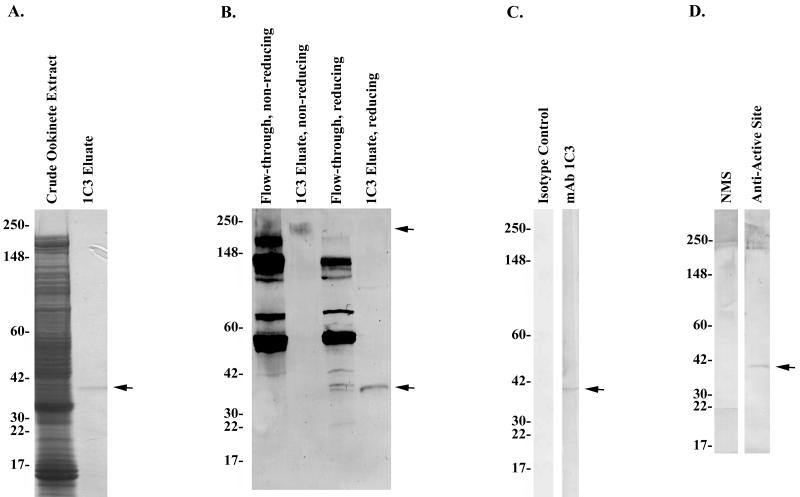
1C3 affinity chromatography isolated a reduced ∼35-kDa antigen cross-reactive with anti-PgCHT1 active-site polyclonal antiserum.
To begin to characterize proteins recognized by MAb 1C3, immunoaffinity chromatography was used to isolate proteins from ookinete cell extracts. Coomassie blue staining of eluted fractions performed under reducing conditions demonstrated an ∼35-kDa band (Fig. 6A, lane 2). MAb 1C3 was only able to bind to a single high-molecular-mass protein under native conditions, and several MAb 1C3-reactive proteins were present in the flowthrough material not bound by the antibody. Under reducing conditions the high-molecular-mass protein was no longer observed, but an ∼35-kDa antigen appeared. By Western immunoblot (Fig. 6B, lane 4, and C, lane 2), MAb 1C3 recognized a protein that comigrated with the Coomassie blue-stained band from the reduced 1C3 column eluate (Fig. 6A, lane 2). The reduced ∼35-kDa protein eluted from the MAb 1C3 immunoaffinity column was immunoreactive with a previously characterized anti-PgCHT1 active-site antiserum (29) by Western immunoblot, a finding consistent with the possibility that this MAb 1C3-recognized protein is a putative second chitinase of P. gallinaceum, PgCHT2 (Fig. 6D). As an additional negative control, the polyclonal antiserum raised against a peptide sequence from the carboxy terminus of PgCHT1 within the putative chitin-binding domain (16) failed to react with the 1C3-affinity isolated protein (data not shown).
FIG. 6.
Analysis of MAb 1C3 affinity column-isolated P. gallinaceum antigen. (A) Coomassie blue-stained SDS-4 to 20% PAGE gel of P. gallinaceum ookinete extract or supernatant column starting material (lane 1) and 1C3 affinity-isolated ∼35-kDa antigen (lane 2). The arrow indicates the MAb 1C3-isolated protein. (B) MAb 1C3 Western immunoblot of flowthrough fractions or MAb 1C3 affinity-isolated proteins from 1C3 affinity chromatography. Western blots were performed after the proteins were separated under either nonreducing or reducing SDS-PAGE conditions as noted above the membrane. Arrows indicate the isolated proteins analyzed under either reducing or nonreducing SDS-PAGE conditions. (C) Western immunoblot recognition of isolated P. gallinaceum antigens by isotype control MAb (lane 1) and MAb 1C3 (lane 2). (D) Identification of 1C3 epitope-containing protein as cross-reactive with an antiserum raised against a peptide derived from the conserved Plasmodium chitinase active site (29). A total of 5 μg of P. gallinaceum ookinete extract and/or supernatants and 8 μg of 1C3 affinity-isolated ookinete antigens was loaded per lane, respectively. NMS, normal mouse serum.
DISCUSSION
The results described here indicate that an epitope found in P. gallinaceum ookinetes reactive with a MAb raised against the P. falciparum chitinase, PfCHT1, is a target for blocking malaria transmission. A substantial amount of indirect evidence presented here and elsewhere (29) supports the hypothesis that MAb 1C3 blocks parasite infectivity for the mosquito midgut by interacting with a putative second chitinase, PgCHT2, expressed by P. gallinaceum ookinetes. It is possible that MAb 1C3 inhibits ookinete infectivity for the mosquito not only with a soluble, secreted protein but also by interacting with a protein associated with the surface of the parasite, as suggested by immunoelectron microscopy (Fig. 2B). However, definitive localization of the 1C3 epitope to the ookinete plasma membrane remains to be determined. The observation that MAb 1C3 alone is effective in blocking oocyst development by itself suggests that both the protein recognized by this antibody (putatively a chitinase) and PgCHT1, the well-defined chitinase secreted by P. gallinaceum ookinetes, are both important for parasite invasion of the mosquito midgut. However, it also appears that the ∼35-kDa protein immunoaffinity isolated by MAb 1C3 that has been provisionally named PgCHT2 may be strongly interacting with other proteins, giving rise to multiple protein bands recognized on Western immunoblots both under nonreducing and reducing conditions. The composition of the protein complexes containing the 1C3 epitope remains to be investigated. It is possible that MAb 1C3 also fortuitously reacts with nonchitinase ookinete proteins, as recognized by MAb 1C3 on Western blots of the flowthrough material from the 1C3 affinity column (Fig. 6B), which could contribute to its transmission-blocking properties. However, given the specific and unique immunofluorescence staining patterns observed on ookinetes stained with MAb 1C3 and the Western immunoblot patterns (Fig. 3), it is reasonable to conclude that the mechanism by which MAb 1C3 inhibits P. gallinaceum infectivity is mediated by specific recognition of the 35-kDa protein immunoaffinity purified from P. gallinaceum ookinetes.
Previous data suggested that the P. falciparum chitinase was similar not to the single molecularly characterized P. gallinaceum chitinase reported to date, PgCHT1 (29), but to a second chitinase of P. gallinaceum, provisionally termed PgCHT2 (22, 27, 29). Four new independent lines of evidence are consistent with the presence of a second putative chitinase, PgCHT2, secreted by P. gallinaceum ookinetes: (i) apical, micronemal, and extracellular localization of the 1C3 epitope; (ii) cross-reactivity of a non-PgCHT1 protein associated with chitinase activity in P. gallinaceum ookinetes with the anti-PfCHT1 MAb, 1C3; (iii) mapping of the 1C3 epitope to a region of PfCHT1 that diverges from other sequenced Plasmodium chitinases; and, critically, (iv) cross-reactivity of the immunoaffinity-isolated 1C3 target with a previously characterized (29) polyclonal antiserum against the conserved Plasmodium active-site peptide.
MAb 1C3 did not recognize native or recombinant PgCHT1 in Western immunoblots, despite the fact it specifically bound to P. gallinaceum proteins, as determined by immunofluorescence and immunoelectron microscopy. MAb 1C3 recognized several proteins in native P. gallinaceum ookinete extracts and supernatants. Of greatest interest is an immunoreactive ∼210-kDa protein (arrow, Fig. 3A and B) that comigrates with a protein recognized by antichitinase active site polyclonal antiserum but not with a protein recognized by an antiserum raised against the PgCHT1 carboxy-terminal domain (29). The additional bands recognized by polyclonal anti-PfCHT1 antiserum (Fig. 3A and B) and MAb 1C3 (Fig. 3A and B; Fig. 6) in P. gallinaceum ookinetes remain to be more fully characterized at the molecular level. Nonetheless, these data provide further evidence that MAb 1C3 may delineate a hitherto uncharacterized chitinase of P. gallinaceum, PgCHT2 that is potentially the ortholog of PfCHT1. The other proteins immunoreactive with MAb 1C3 could either be complexes of the target protein or different gene products containing a cross-reactive epitope. These possibilities will be addressed in the future. A P. gallinaceum ortholog of PfCHT1, which lacks a chitin-binding domain, could explain why the protein recognized by MAb 1C3 is recognized by anti-PgCHT1 active-site antibodies but not by anti-PgCHT1 C-terminus antibodies, as is the case with PfCHT1 (Fig. 3). Previous results suggested the presence of a second additional chitinase in P. gallinaceum when two peaks of chitinase activity were separated by HPLC of ookinete cell extracts (15). The second peak of chitinase activity contained an ∼210-kDa antigen that was reactive with anti-PgCHT1 active-site antiserum, but not anti-PgCHT1 carboxyl-terminus antiserum, as seen with the MAb 1C3 immunoreactive ∼210-kDa antigen. These findings are consistent with the hypothesis that MAb 1C3 recognizes the putative second chitinase of P. gallinaceum, PgCHT2, an ortholog of PfCHT1.
MAb 1C3 recognized a specific epitope shared between P. falciparum and P. gallinaceum. Comparison of the 1C3 epitope of rPfCHT1 with homologous regions of other Plasmodium chitinases demonstrated that this region diverges from the amino acid sequence just after the highly conserved catalytic domain. Of special note, this epitope is tyrosine-rich and contains highly hydrophobic and antigenic portions as determined by Kyte-Doolittle and Jameson-Wolf analysis (data not shown). Some difficulties were observed during synthesis and purification of the synthetic peptides used to confirm the 1C3 epitope, suggesting that it may be folding into β-pleated sheet structure(s). The 22-amino-acid peptide (LYDSYAYYGKKYDYVIIMGFTL) was difficult to solubilize. The shorter peptides (LYDSYAYYGKKYD, LYDSYAYYGKKY, LYDSYAYYGKK, LYDSYAYYGK, and LYDSYAYYG) were initialliy solubilized in acidic solutions and retained immunoreactivity with 1C3. This observation confirmed that the 1C3 epitope mapped to a peptide that retains its structure and function under harsh conditions. Retention of epitope function in harsh conditions could be critical for the protein to withstand proteolytic degradation within the milieu of the mosquito midgut. Amyloid proteins which contain large amounts of β-sheet structure are notoriously resistant to proteolysis. Identification of a hydrophobic epitope could indicate a high-avidity binding by 1C3, since single-amino-acid changes of hydrophobic residues in immunoglobulin-binding motifs often result in a loss of binding (20). Further, critical hydrophobic epitopes have been associated with some of the strongest immune responses known, such as peanut allergens (20). Therefore, the 1C3 epitope itself may be useful as a component of a subunit transmission-blocking vaccine. This possibility will be tested by membrane feeds in which antisera raised to a synthetic 1C3 epitope fused to a carrier protein will be assessed for the ability to prevent oocyst formation.
MAb 1C3 neutralized the enzymatic activity of rPfCHT, indicating that this antibody not only recognizes rPfCHT1 but also does so at a biologically important site. To test the hypothesis that cross-species neutralization of chitinase would reduce infectivity of P. gallinaceum for Aedes aegypti mosquitoes, standard membrane feeds were performed. MAb 1C3 significantly inhibited oocyst development in a dose-dependent manner. Protection provided by MAb 1C3 was comparable to that provided by allosamidin, a pseudo-oligosaccharide chitinase inhibitor previously shown to prevent the formation of Plasmodium oocysts in the mosquito midgut (19). These results are important because they show that inhibition of parasite-secreted chitinase activity in the mosquito midgut can reduce Plasmodium infectivity. Further, we have expanded on the strategy of transmission-blocking vaccines, demonstrating that ookinete-secreted chitinases are susceptible to immunological intervention. A potential concern of transmission-blocking vaccines directed against late-expressed ookinete proteins is that host antibodies taken up during the blood meal by the mosquito would be quickly degraded by proteases present in the mosquito midgut. Our findings, supported by the data of others (2, 11, 17, 18, 26), demonstrate that MAbs can successfully retain immunological neutralizing capabilities long after uptake into the mosquito. However, these previous studies have predominantly concentrated on gamete and/or zygote target proteins (which appear within minutes after the mosquito blood meal) and not ookinetes, which appear fully mature 15 to 36 h after blood meal ingestion. Therefore, antibodies targeting late-expressed ookinete proteins must retain function while withstanding harsh conditions for a much longer period of time. Few ookinete transmission-blocking studies have been reported; transmission-blocking studies have focused primarily on zygote and/or early ookinete-expressed surface proteins such as Ps25 and Ps28 (4, 5, 9, 23-25). Targeting a specific neutralization-sensitive epitope of an ookinete-expressed protein, as detailed in the present study, could enhance the efficacy of cocktail transmission-blocking vaccines. In contrast, the zygote or ookinete surface proteins Ps25 and Ps28 do not produce cross-species-protective antibody responses. The cross-species conservation of the 1C3 epitope suggests that it could be the basis for a pan-Plasmodium transmission-blocking vaccine. To test this hypothesis experimentally, identification of the chitinases of the other human malaria parasites, particular those of P. vivax, will be necessary.
The results reported here indicate that the epitope recognized by MAb 1C3 is an important target for Plasmodium transmission-blocking vaccines, particularly because this epitope is shared between two different Plasmodium species. Future studies will focus on molecular characterization of the 1C3 defined P. gallinaceum molecule, PgCHT2, to provide definitive confirmation of whether it is an ortholog of PfCHT1. The Plasmodium chitinases also will be useful molecules to study in delineating mechanisms of the cell biology of secretion in the Plasmodium ookinete. Finally, the results validate the strategy of targeting ookinete-secreted chitinases in the development of transmission-blocking vaccines.
Acknowledgments
We thank Abdel Razek el-Desouky for P. gallinaceum ookinete preparations, Steve Smith of the University of Texas Medical Branch Protein Chemistry Laboratory for peptide sequencing, Jingzhi Pan for excellent technical assistance in the HPLC work, and the Louisiana State University Health Science Center Core Lab for MS. We also thank Shosei Sakuda, Department of Applied Biological Chemistry, University of Tokyo, Tokyo, Japan, and Jon Mynderse of Eli Lilly, Indianapolis, Ind., for their kind gifts of allosamidin. Preliminary sequence data from the P. yoelii genome were obtained from The Institute for Genomic Research website (www.tigr.org). This sequencing program is carried on in collaboration with the Naval Medical Research Center and is supported by the U.S. Department of Defense.
This work was supported by U.S. Public Health Service training grant in Emerging and Reemerging Infectious Diseases T32-AI07536 (R.C.L.); NIH grants RO1 AI45999 (J.M.V.), KO2 AI50049 (J.M.V.), and CA 881317 (A.K.); a grant from the World Health Organization Tropical Diseases Research Molecular Entomology Program (J.M.V.); and an Advanced Research Program grant from the State of Texas Higher Education Coordinating Board (J.M.V.). J.M.V. is a Culpeper Medical Sciences Scholar supported by the Rockefeller Brothers Fund.
REFERENCES
- 1.Carter, R. 2001. Transmission blocking malaria vaccines. Vaccine 19:2309-2314. [DOI] [PubMed] [Google Scholar]
- 2.Carter, R., P. M. Graves, D. B. Keister, and I. A. Quakyi. 1990. Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol. 12:587-603. [DOI] [PubMed] [Google Scholar]
- 3.Dessens, J. T., J. Mendoza, C. Claudianos, J. M. Vinetz, E. Khater, S. Hassard, G. R. Ranawaka, and R. E. Sinden. 2001. Knockout of the rodent malaria parasite chitinase pbcht1 reduces infectivity to mosquitoes. Infect. Immun. 69:4041-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffy, P. E., and D. C. Kaslow. 1997. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect. Immun. 65:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy, P. E., P. Pimenta, and D. C. Kaslow. 1993. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J. Exp. Med. 177:505-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 7.Huber, M., E. Cabib, and L. H. Miller. 1991. Malaria parasite chitinase and penetration of the mosquito peritrophic membrane. Proc. Natl. Acad. Sci. USA 88:2807-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaslow, D. C. 1997. Transmission-blocking vaccines: uses and current status of development. Int. J. Parasitol. 27:183-189. [DOI] [PubMed] [Google Scholar]
- 9.Kaslow, D. C., and J. Shiloach. 1994. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Bio/Technology 12:494-499. [DOI] [PubMed] [Google Scholar]
- 10.Kaushal, D. C., and R. Carter. 1984. Characterization of antigens on mosquito midgut stages of Plasmodium gallinaceum. II. Comparison of surface antigens of male and female gametes and zygotes. Mol. Biochem. Parasitol. 11:145-156. [DOI] [PubMed] [Google Scholar]
- 11.Kaushal, D. C., R. Carter, J. Rener, C. A. Grotendorst, L. H. Miller, and R. J. Howard. 1983. Monoclonal antibodies against surface determinants on gametes of Plasmodium gallinaceum block transmission of malaria parasites to mosquitoes. J. Immunol. 131:2557-2562. [PubMed] [Google Scholar]
- 12.Langer, R. C., and J. M. Vinetz. 2001. Plasmodium ookinete-secreted chitinase and parasite penetration of the mosquito peritrophic matrix. Trends Parasitol. 17:269-272. [DOI] [PubMed] [Google Scholar]
- 13.McCutchan, T. F., J. B. Dame, L. H. Miller, and J. Barnwell. 1984. Evolutionary relatedness of Plasmodium species as determined by the structure of DNA. Science 225:808-811. [DOI] [PubMed] [Google Scholar]
- 14.McCutchan, T. F., J. C. Kissinger, M. G. Touray, M. J. Rogers, J. Li, M. Sullivan, E. M. Braga, A. U. Krettli, and L. H. Miller. 1996. Comparison of circumsporozoite proteins from avian and mammalian malarias: biological and phylogenetic implications. Proc. Natl. Acad. Sci. USA 93:11889-11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrone, J. B., and A. Spielman. 1988. Time and site of assembly of the peritrophic membrane of the mosquito Aedes aegypti. Cell Tissue Res. 252:473-478. [DOI] [PubMed] [Google Scholar]
- 16.Perryman, L. E., D. P. Jasmer, M. W. Riggs, S. G. Bohnet, T. C. McGuire, and M. J. Arrowood. 1996. A cloned gene of Cryptosporidium parvum encodes neutralization-sensitive epitopes. Mol. Biochem. Parasitol. 80:137-147. [DOI] [PubMed] [Google Scholar]
- 17.Read, D., A. H. Lensen, S. Begarnie, S. Haley, A. Raza, and R. Carter. 1994. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. 16:511-519. [DOI] [PubMed] [Google Scholar]
- 18.Rener, J., P. M. Graves, R. Carter, J. L. Williams, and T. R. Burkot. 1983. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J. Exp. Med. 158:976-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahabuddin, M., T. Toyoshima, M. Aikawa, and D. C. Kaslow. 1993. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc. Natl. Acad. Sci. USA 90:4266-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin, D. S., C. M. Compadre, S. J. Maleki, R. A. Kopper, H. Sampson, S. K. Huang, A. W. Burks, and G. A. Bannon. 1998. Biochemical and structural analysis of the IgE binding sites on ara h1, an abundant and highly allergenic peanut protein. J. Biol. Chem. 273:13753-13759. [DOI] [PubMed] [Google Scholar]
- 21.Sieber, K. P., M. Huber, D. Kaslow, S. M. Banks, M. Torii, M. Aikawa, and L. H. Miller. 1991. The peritrophic membrane as a barrier: its penetration by Plasmodium gallinaceum and the effect of a monoclonal antibody to ookinetes. Exp. Parasitol. 72:145-156. [DOI] [PubMed] [Google Scholar]
- 22.Tsai, Y. L., R. E. Hayward, R. C. Langer, D. A. Fidock, and J. M. Vinetz. 2001. Disruption of Plasmodium falciparum chitinase markedly impairs parasite invasion of mosquito midgut. Infect. Immun. 69:4048-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuboi, T., Y. M. Cao, Y. Hitsumoto, T. Yanagi, H. Kanbara, and M. Torii. 1997. Two antigens on zygotes and ookinetes of Plasmodium yoelii and Plasmodium berghei that are distinct targets of transmission-blocking immunity. Infect. Immun. 65:2260-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuboi, T., D. C. Kaslow, Y. M. Cao, K. Shiwaku, and M. Torii. 1997. Comparison of Plasmodium yoelii ookinete surface antigens with human and avian malaria parasite homologues reveals two highly conserved regions. Mol. Biochem. Parasitol. 87:107-111. [DOI] [PubMed] [Google Scholar]
- 25.Tsuboi, T., D. C. Kaslow, M. M. Gozar, M. Tachibana, Y. M. Cao, and M. Torii. 1998. Sequence polymorphism in two novel Plasmodium vivax ookinete surface proteins, Pvs25 and Pvs28, that are malaria transmission-blocking vaccine candidates. Mol. Med. 4:772-782. [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeulen, A. N., T. Ponnudurai, P. J. Beckers, J. P. Verhave, M. A. Smits, and J. H. Meuwissen. 1985. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med. 162:1460-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinetz, J. M., S. K. Dave, C. A. Specht, K. A. Brameld, R. E. Hayward, and D. A. Fidock. 1999. The chitinase PfCHT1 from the human malaria parasite Plasmodium falciparum lacks proenzyme and chitin-binding domains and displays unique substrate preferences. Proc. Natl. Aca. Sci. USA 96:14061-14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinetz, J. M., and D. C. Kaslow. 1998. Plasmodium gallinaceum: use of antisera to degenerate synthetic peptides derived from the active site of protozoal chitinases to characterize an ookinete-specific chitinase. Exp. Parasitol. 90:199-202. [DOI] [PubMed] [Google Scholar]
- 29.Vinetz, J. M., J. G. Valenzuela, C. A. Specht, L. Aravind, R. C. Langer, J. M. Ribeiro, and D. C. Kaslow. 2000. Chitinases of the avian malaria parasite Plasmodium gallinaceum, a class of enzymes necessary for parasite invasion of the mosquito midgut. J. Biol. Chem. 275:10331-10341. [DOI] [PubMed] [Google Scholar]
- 30.Waters, A. P., D. G. Higgins, and T. F. McCutchan. 1991. Plasmodium falciparum appears to have arisen as a result of lateral transfer between avian and human hosts. Proc. Natl. Acad. Sci. USA 88:3140-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]