Abstract
Induction of T-cell memory by vaccination ensures long-term protection against pathogens. We determined whether on-going inflammatory responses during vaccination influenced T-cell priming. A preexposure of mice to Mycobacterium bovis BCG impaired their subsequent ability to prime T cells against Listeria monocytogenes. This was characterized by a decrease in L. monocytogenes-specific gamma interferon (IFN-γ)-secreting CD4+ and CD8+ T cells. The intensity of T-cell priming towards L. monocytogenes depended on the extent of L. monocytogenes expansion, and a cessation of this expansion caused by M. bovis BCG-induced inflammation resulted in impairment in T-cell priming. A challenge of M. bovis BCG-infected mice with a higher L. monocytogenes dose increased L. monocytogenes survival and restored T-cell priming towards L. monocytogenes. Impairment in T-cell priming towards L. monocytogenes due to M. bovis BCG-induced inflammation resulted in a compromised protective efficacy in the long term after mice were rechallenged with L. monocytogenes. Preexisting inflammation selectively impaired T-cell priming for replicating immunogens as CD8+ T-cell response to ovalbumin administered as an inert antigen (ovalbumin-archaeosomes) was enhanced by M. bovis BCG preimmunization, whereas priming towards ovalbumin administered as a live immunogen (L. monocytogenes-ovalbumin) was impaired. Thus, depending on the nature of the immunogen, the presence of prior inflammatory responses may either impede or boost vaccine efficacy.
Immune responses to intracellular pathogens are initiated by antigen-presenting cells that phagocytose the pathogen and subsequently process and present the antigenic peptides in the context of major histocompatibility complex (MHC) molecules to T cells (2, 51). This leads to clonal expansion and activation of antigen-specific T cells. Following this initial activation, some lymphocytes acquire an ability to persist for extended periods (43). These long-lived memory T cells are intrinsically programmed to proliferate and express their function more rapidly and provide protection against subsequent infection.
Listeria monocytogenes is a facultative intracellular pathogen that causes disease in immunocompromised individuals (11). In primary infections, bacterial growth is controlled during the first week of infection mainly by cells of the innate immune system involving macrophages, dendritic cells, NK cells, neutrophils and γδ-T cells (4, 11, 13, 46). However, protection against secondary infection is mediated mainly by CD8+ T cells through a gamma interferon (IFN-γ)-independent (10) and perforin (15)- and tumor necrosis factor alpha (TNF-α) (49)-dependent mechanism.
Secretion of the virulence factor listeriolysin by the pathogen inside an antigen-presenting cell ensures escape from the phagosomal vesicles (35), resulting in secretion of antigens into the cytosol and consequent presentation of antigenic peptides through the MHC class I processing pathway (3). Although multiple peptides are presented in the context of murine H-2Kd molecules, CD8+ T-cell response to the epitope encompassing residues 91 to 99 of listeriolysin has been shown to be dominant (48) and protective (9).
The intracellular pathogen Mycobacterium bovis BCG, in contrast to L. monocytogenes, induces a chronic infection in the host resulting in a persistent immune activation (8, 16, 17). Resistance and susceptibility to M. bovis BCG infection in mice have been shown to be controlled by the M. bovis BCG Ity/Lsh gene (47). Both BALB/c and C57BL/6 mice exhibit similar susceptibility to M. bovis BCG infection (8). Mycobacteria stimulate macrophages and dendritic cells to produce inflammatory cytokines and to express enhanced levels of costimulatory molecules (6, 12).
In this report, we addressed the interaction between preexisting inflammation and the development of T-cell response to subsequent immunogens. We show that, depending on the nature of the immunogen, potent preexisting inflammatory response, induced by M. bovis BCG, can either facilitate or impair T-cell priming.
MATERIALS AND METHODS
Bacterial strains.
Mycobacterium bovis BCG (Pasteur) was kindly provided by R. North (Trudeau Institute, Saranac Lake, N.Y.) and cultured at 37°C under constant shaking in 7H9 medium containing glycerol (0.2%), Tween 80 (0.05%), and albumin-dextrose supplement (ADC, 10%; Difco Laboratories, Detroit, Mich.). At mid-log phase (OD600 = 1.0), bacteria were harvested and frozen at −70°C (in 20% glycerol). CFU were determined by plating serial dilutions in PBS-T (0.025% Tween 80) on Middlebrook 7H10 solid medium containing glycerol (0.5%) and oleic acid-albumin-dextrose supplement (10%, Difco Laboratories).
A listeriolysin-positive, streptomycin-resistant strain of L. monocytogenes (10403S) was kindly provided by Wayne Conlan (Institute for Biological Sciences, NRC, Ottawa, Canada). The bacteria were grown in brain heart infusion (BHI) medium (Difco Laboratories, Detroit, Mich.), supplemented with 50 μg of streptomycin (Sigma-Aldrich Canada, Oakville, Canada) per ml. At mid-log phase (optical density at 600 nm [OD600] = 1.0), bacteria were harvested and frozen in 20% glycerol and stored at −70°C. CFU were determined by performing serial dilutions in 0.9% NaCl, which were spread on BHI-streptomycin agar plates.
For generation of recombinant L. monocytogenes expressing ovalbumin, the plasmid pJJD-OVA was replicated in Escherichia coli HB101 strain. Plasmid DNA was introduced in L. monocytogenes 10403S strain by electroporation (33). Chromosomal integration was selected by several passages at 42°C on BHI-agar with erythromycin (5 μg/ml; Sigma), at 37°C in BHI liquid culture without erythromycin, and again on BHI-agar with erythromycin (1 μg/ml). The loss of β-galactosidase activity was checked by growing the bacteria in the presence of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Bethesda Research Laboratories). The recombinant L. monocytogenes strain was grown at an OD600 = 0.4 as described above and aliquots were stored in 20% glycerol at −70°C. CFU were determined by plating 10-fold dilutions on BHI-agar (Difco Laboratories). Expression of ovalbumin partial protein was detected in the culture supernatant as a 31-kDa protein (data not shown).
Mice and immunizations.
Female BALB/c and C57BL/6 mice, 6 to 8 weeks of age, were obtained from Charles River Laboratory (St. Constant, Canada). Mice were maintained in the animal facility at the Institute for Biological Sciences (National Research Council of Canada, Ottawa, Canada) in accordance with the guidelines of the Canadian Council on Animal Care. For immunization with M. bovis BCG, frozen M. bovis BCG aliquots were thawed, washed once in phosphate-buffered saline plus 0.025% Tween 80 (PBS-T) and resuspended at 5 × 106 CFU/ml. Mice were inoculated with 106 M. bovis BCG (unless otherwise mentioned) suspended in 200 μl of PBS-T, via the lateral tail vein. Age-matched control mice were inoculated with 200 μl of PBS-T only (PBS mice).
For immunization with L. monocytogenes, frozen stocks were thawed and diluted in 0.9% NaCl to 2.5 × 104 CFU/ml. Mice were inoculated with 5 × 103 L. monocytogenes CFU (unless otherwise mentioned) suspended in 200 μl of 0.9% NaCl, via the lateral tail vein (unless otherwise mentioned). For immunization with a particulate antigen, C57BL/6 mice were injected intraperitoneally with ovalbumin (15 μg in 100 μl of PBS) entrapped in liposomal vesicles composed of archaebacterial lipids (archaeosomes) (19).
Experimental design.
To induce potent inflammation, mice were injected with M. bovis BCG as described above. On day 30, mice were challenged with L. monocytogenes, and the effects on L. monocytogenes clearance and T-cell priming were evaluated at subsequent time intervals. L. monocytogenes burden was evaluated on day 33 (3 days after L. monocytogenes challenge), and T-cell responses were evaluated at day 37 (7 days after L. monocytogenes challenge) and at day 60 (30 days after L. monocytogenes challenge) to monitor responses at effector and memory stages, respectively.
To address the effects of M. bovis BCG-induced inflammation on the development of T-cell response towards a live versus an inert antigen, we injected mice with PBS or M. bovis BCG on day 1 and challenged them on day 30 with a live immunogen (L. monocytogenes-ovalbumin) or an inert immunogen (ovalbumin-archaeosomes). Ovalbumin was used as a model antigen so that responses to the same antigen could be evaluated in the context of a live or an inert immunogen.
Archaeosomes represent a potent nonreplicating adjuvant system. We have previously reported that archaeosomes induce potent CD8+ T-cell responses to entrapped antigens (e.g., ovalbumin) via activation of macrophages and dendritic cells (20). Since the CD8+ T-cell epitope of ovalbumin residues 257 to 264 is H-2b restricted, these experiments were performed in C57BL/6 mice, which are also susceptible to M. bovis BCG infection and exhibit splenomegaly and inflammation similar to BALB/c mice (8).
Enumeration of listerial burden in spleen.
Spleens were harvested 3 days after the infection of mice with 5 × 103 L. monocytogenes. Single-cell suspensions were prepared by tweezing the spleens between the frosted ends of two sterile glass slides in RPMI 1640 (Gibco-BRL, Burlington, Ontario, Canada). Spleen cells were lysed with water for 10 s, and the suspension was evaluated for the number of bacteria. CFU were determined by performing serial 10-fold dilutions in 0.9% NaCl, which were spread on BHI-streptomycin (as above) agar plates. Plates were incubated for 24 h at 37°C, and colonies were counted visually.
Antigen preparations.
For preparation of L. monocytogenes antigen, the bacteria were grown in liquid culture (200 ml) as described, harvested by centrifugation (3,000 × g for 30 min), and washed and resuspended in 2.5 ml of ice-cold PBS. The cell suspension was disrupted by sonication on ice with a Sonifier cell disruptor 350 (Branson Sonic Power, SmithKline, Danbury, Conn.). This material was centrifuged at 1,900 × g for 10 min and filtered, and aliquots were stored at −80°C. The protein concentration was determined with a Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.) using bovine serum albumin (Sigma) as a protein standard. Ovalbumin entrapped in liposomal vesicles composed of archaeobacterial lipids (archaeosomes) was prepared as described elsewhere (19). Peptides 91 to 99 of listeriolysin and 257 to 264 of ovalbumin were synthesized at the peptide synthesis facility of our institute.
Cell lines.
P815 (H-2d), EL4 (H-2b), and WEHI 164-13 cells were obtained from the American Type Culture Collection (ATCC, Rockville, Md.) and maintained in RPMI 1640 medium (Life Technologies, Grand Island, N.Y.) supplemented with 8% fetal bovine serum (FBS) (HyClone, Logan, Utah) and 10 μg of gentamicin (Life Technologies) per ml. pHem3.3 cells (derivative of P815 cells) expressing peptide 91-99 of listeriolysin were obtained from M. Bevan (University of Washington, Seattle, Wash.). EG7 cells, a subclone of EL4 stably transfected with the gene encoding ovalbumin (26), were obtained from the ATCC. Both pHem3.3 and EG7 cells were cultured in RPMI plus 8% FBS, additionally containing 400 μg of G418 (Rose Scientific Ltd., Edmonton, Alberta, Canada) per ml.
Cell cultures.
Single-cell suspensions were prepared by tweezing the spleens between the frosted ends of two sterile glass slides in RPMI 1640 (Life Technologies). Cells were subsequently passed through Falcon 2360 cell strainers (Becton Dickinson Labware, Franklin Lake, N.J.). Erythrocytes were lysed in Tris-buffered ammonium chloride.
For measuring cytokine production in the supernatant, spleen cells were incubated at 2.5 × 106 cells/ml in the absence or the presence of various antigen preparations. Cells were cultured in 96-well round-bottomed tissue culture plates, and the culture medium consisted of RPMI 1640 with 8% FBS and 50 μg of gentamicin per ml (Life Technologies). Supernatants were collected at 72 h after the initiation of culture. TNF was measured by a bioassay using WEHI 164-13 cells, and IFN-γ was measured using a sandwich enzyme-linked immunosorbent assay (ELISA) (27, 39).
Enumeration of IFN-γ-secreting cells was done by Elispot assay (48). Briefly, spleen cells were incubated in coated Elispot plates in various proportions (in a final cell density of 5 × 105/well using feeder cells) in the presence of interleukin-2 (IL-2) (0.1 ng/ml) and medium or the various antigens described above. Incubation lasted either overnight or 48 h. The plates were subsequently blocked and incubated with the biotinylated secondary antibody (overnight) followed by avidin-peroxidase conjugate (2 h). Spots were revealed using diamidobenzidine as the substrate.
Cytokines, antibodies, and reagents.
Recombinant mouse IL-2 was obtained from ID Labs (London, Ontario, Canada). Purified anti-mouse CD32/CD16 (FcγII/III receptor, 2.4G2 Fc Block), phycoerythrin (PE)-labeled anti-mouse CD11c, PE-labeled anti-mouse Mac1α, PE-labeled anti-mouse F4/80, PE-labeled anti-mouse γδ T-cell receptor (TCR), PE-labeled anti-mouse CD4, PE-labeled anti-mouse CD8, and PE-labeled anti-mouse DX5 were obtained from PharMingen (San Diego, Calif.). Sodium chromate (51Cr, 250 to 500 mCi/mg of Cr) was obtained from Amersham (Oakville, Ontario, Canada).
Cytotoxicity assays.
Single-cell suspensions from pooled spleens (n = 2 to 3) of immunized mice were prepared as described above. After washing, spleen cells (various numbers) from various experimental groups were incubated with 5 × 105 irradiated (10,000 rads) antigen-bearing target cells (pHem3.3 cells for cytotoxic T lymphocytes [CTL] against listeriolysin peptide or EG7 cells in case of CTL against ovalbumin peptide) in 10 ml of RPMI plus 8% FBS. Cultures contained 0.1 ng of IL-2 per ml and were placed in 25-cm2 tissue culture flasks (Falcon, Becton Dickinson, Franklin Lakes, N.J.), kept upright. After 5 days (37°C, 8% CO2), cells were harvested from the flasks, washed, counted, and used as effectors in a standard 51Cr release CTL assay.
P815 target cells (106/ml) were incubated with either medium or listeriolysin peptide 91-99 (LLO91-99, 10 μg/ml) for 2 h before labeling with radioactive chromium. Similarly, EL4 cells were also incubated with medium or with ovalbumin peptide 257-264 (OVA257-264). For labeling, 5 × 106 target cells (P815, P815 plus LLO91-99, EL4, and EL4 plus OVA257-264) were incubated with 50 μl of 51Cr (100 μCi) and 25 μl of RPMI plus 8% FBS medium. After 45 min, targets were washed twice, and various ratios of effectors and targets were cocultured for 4 h in 96-well round-bottomed tissue culture plates. The supernatants were collected, and radioactivity was detected by γ-counting. The percent cytotoxicity was calculated using the formula 100 × [(experimental cpm − spontaneous cpm)/(total cpm − spontaneous cpm)].
Flow cytometric analysis.
Spleen cell suspensions were prepared as described above from mice 30 days after injection with either PBS or M. bovis BCG. To obtain adherent cells, replicates of 50 × 106 spleen cells were placed in Falcon 3003 tissue culture dishes (Becton Dickinson Labware) in 10 ml of RPMI plus 8% FBS at 37°C in 8% CO2. After 2 h, nonadherent cells were removed by gentle washing, and the adherent cells were harvested after incubation in Ca2+-free PBS for 5 min at 37°C.
Adherent splenocytes (5 × 106 cells in 50 μl of RPMI 1640 plus 1% FBS) were incubated with rat anti-mouse CD32/CD16 (FcγII/III receptor). After 30 min, aliquots (106) were washed and incubated separately in 50 μl of RPMI plus 1% FBS with the following antibodies: PE-labeled anti-mouse CD11c, PE-labeled anti-mouse Mac1α, and PE-labeled anti-mouse F4/80. For evaluating NK, CD4+, CD8+, and γδTCR+ cells, nonfractionated spleen cells were incubated with the respective antibodies. All incubations were done (106 cells in 50 μl of RPMI 1640 plus 1% FBS) on ice and lasted for 30 min. After the last washing, cells were fixed in 1% formaldehyde in PBS and acquired on an Epics XL flow cytometer (Beckman Coulter, Hialeah, Fla.) and analysis was done using Expo software (Beckman Coulter). The number of cells expressing a particular marker was calculated by multiplication of percent positive cells with the total cell number.
RESULTS
Preexisting infection of mice with M. bovis BCG compromises T-cell priming for L. monocytogenes.
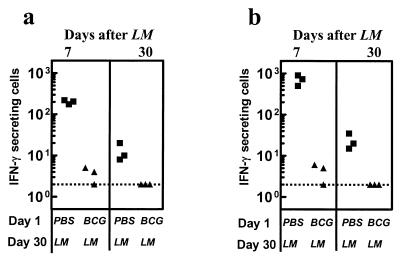
To evaluate the influence of inflammation on CD4+ T-cell priming, spleen cells were stimulated with the exogenous soluble L. monocytogenes antigen. Spleen cells of mice that received PBS initially (day 1) and L. monocytogenes on day 30 had a high frequency of IFN-γ-secreting cells (>102/106 spleen cells) on day 37 (Fig. 1a), indicating the early development of a strong Listeria-specific T-cell response. On the other hand, M. bovis BCG infection 30 days prior to infection with L. monocytogenes resulted in the development of a poor T-cell response towards L. monocytogenes that was characterized by reduced frequency of IFN-γ-secreting cells. Similar results were obtained when the response was measured at day 60 (30 days post-L. monocytogenes infection).
FIG. 1.
Preimmunization with M. bovis BCG impairs T-cell priming for L. monocytogenes. BALB/c mice were injected with PBS or M. bovis BCG on day 1, and 30 days later, mice were challenged with L. monocytogenes. Spleens were harvested at day 37 (7 days after L. monocytogenes [LM] challenge) and day 60 (30 days after L. monocytogenes challenge), and the number of IFN-γ secreting cells was enumerated after stimulating cells with (a) L. monocytogenes antigens or (b) LLO91-99. The number of IFN-γ secreting cells per 106 spleen cells is indicated. The dotted line indicates the threshold of detection.
We also measured the effects on the frequency of IFN-γ-secreting CD8+ T cells in response to the dominant CTL epitope 91-99 of the major virulence factor listeriolysin. Spleen cells of mice injected with PBS initially and challenged with L. monocytogenes on day 30 had a high frequency of IFN-γ-secreting cells on day 37 in response to LLO91-99 (Fig. 1b), indicating the induction of a potent Listeria-specific CD8+ T-cell response. However, an infection with M. bovis BCG 30 days prior to L. monocytogenes challenge resulted in a severely compromised CD8+ T-cell response towards LLO91-99. Similar results were obtained when response was measured at day 60 (30 days post-L. monocytogenes infection). As with IFN-γ, the production of TNF was also compromised by M. bovis BCG preimmunization (data not shown).
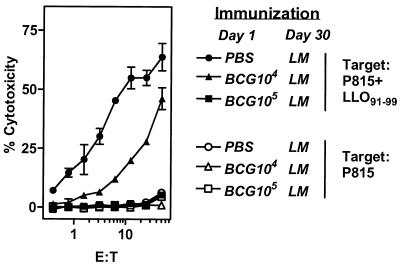
As cytolytic activity is considered to be one of the main functions of CD8+ T cells, we measured the influence of M. bovis BCG preimmunization on the development of cytolytic L. monocytogenes-specific CD8+ T cells. We also determined whether impairment in CD8+ T-cell response occurred even at reduced doses of M. bovis BCG. Mice were injected with PBS or with 104 or 105 M. bovis BCG organisms. On day 30, mice were challenged with L. monocytogenes, and 7 days later LLO91-99-specific cytolytic activity of CD8+ T cells was tested. Potent cytotoxic activity towards LLO91-99-pulsed targets was observed from the spleen cells of mice that received PBS initially and L. monocytogenes on day 30. M. bovis BCG preinfection impaired this cytotoxic activity in a dose-dependent manner, with no cytotoxic activity detectable at a dose of 105 M. bovis BCG (Fig. 2). None of the experimental groups showed any appreciable cytotoxicity towards the targets in the absence of the peptide.
FIG. 2.
M. bovis BCG inhibits the development of cytolytic CD8+ T-cell response to L. monocytogenes in a dose-dependent manner. BALB/c mice were injected with PBS or with 104 M. bovis BCG or 105 M. bovis BCG. Thirty days later mice were injected with L. monocytogenes (LM). On day 37, pooled spleen cells from various groups of mice were restimulated with irradiated pHem3.3 cells (expressing LLO91-99) for 5 days in the presence of IL-2 (0.1 ng/ml) as described in the Materials and Methods section. Cells were harvested and washed, and effectors were tested for their cytolytic activity on 51Cr-labeled P815 (open symbols) or P815+LLO91-99 (solid symbols) target cells. Means ± standard deviations for triplicate cultures are shown.
M. bovis BCG infection induces a potent inflammatory response.
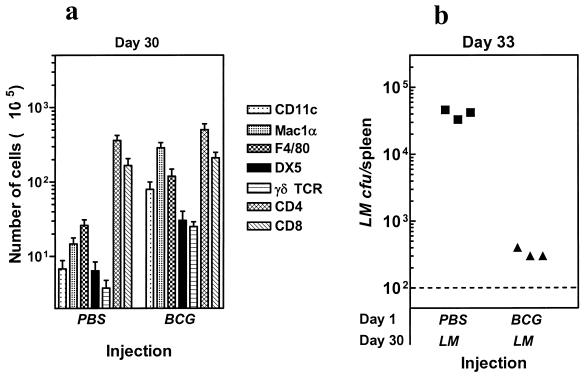
To address the mechanism(s) responsible for poor T-cell priming for L. monocytogenes after M. bovis BCG preimmunization, we evaluated the phenotype of various cell populations in the spleens of M. bovis BCG-infected mice on day 30. In comparison to PBS controls, mice infected with M. bovis BCG had strongly elevated numbers of cells expressing CD11c, Mac1α, F4/80, DX5, and γδ-TCR (Fig. 3a), indicating an accumulation of dendritic cells, macrophages, NK, and γδ-Τ cells, respectively. There was only a modest increase in the numbers of CD4+ and CD8+ T cells in the spleens of M. bovis BCG-infected mice at day 30.
FIG. 3.
M. bovis BCG infection results in an accumulation of inflammatory cells and causes increased clearance of L. monocytogenes. BALB/c mice were injected with PBS or M. bovis BCG. On day 30, spleen cells were isolated, and the numbers of cells expressing various markers were analyzed as described in the Materials and Methods section (a). Mice injected with PBS or M. bovis BCG were also challenged with L. monocytogenes (LM) on day 30, and the numbers of viable L. monocytogenes in the spleens of mice were enumerated on day 33 (b). Each symbol represents the data for an individual mouse. The dotted line indicates the threshold of detection.
To determine whether the accumulation of such inflammatory cells by M. bovis BCG infection results in acceleration of the clearance of L. monocytogenes, we challenged PBS or M. bovis BCG-injected mice on day 30 with L. monocytogenes. Since the growth of L. monocytogenes peaks around 72 h after infection, we harvested spleens on day 33, and evaluated the numbers of viable bacteria. As is evident from Fig. 3b, L. monocytogenes expanded extensively in the spleens of mice that were injected previously with PBS. However, there was a >100-fold reduction of L. monocytogenes burden in mice preimmunized with M. bovis BCG.
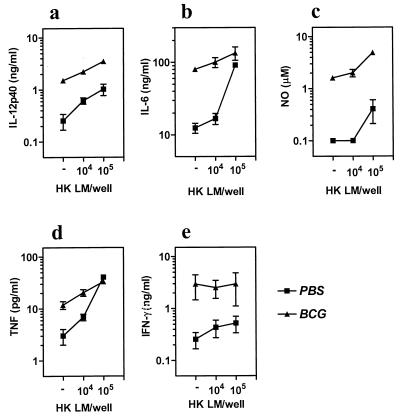
To determine whether M. bovis BCG-infected mice were secreting higher levels of inflammatory cytokines, we stimulated the spleen cells from PBS-treated or M. bovis BCG-infected mice on day 30 with L. monocytogenes (heat killed) in vitro. As is evident in Fig. 4a to e, spleen cells from M. bovis BCG-infected mice, even without stimulation, produced higher levels of IL-12, IL-6, nitric oxide, TNF, and IFN-γ, indicating the presence of a strong inflammatory response in such mice.
FIG. 4.
Potent inflammatory response in M. bovis BCG-infected mice. PBS or M. bovis BCG was injected into BALB/c mice on day 1. On day 30, spleen cells from the PBS (solid squares) or M. bovis BCG (solid triangles)-injected mice were incubated (5 × 105/well) with medium or with various numbers of heat-killed L. monocytogenes (HK LM) in vitro, and the production of various molecules was determined in 72-h supernatants. Means ± standard deviations for triplicate cultures are shown.
Impairment in T-cell priming by M. bovis BCG is due to accelerated clearance of L. monocytogenes.
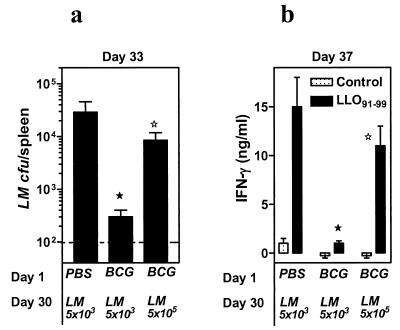
As described above, increased clearance of L. monocytogenes by M. bovis BCG correlated with impaired T-cell priming towards L. monocytogenes. We surmised that challenge of M. bovis BCG-infected mice with a higher L. monocytogenes dose that would increase L. monocytogenes survival might restore T-cell priming. We therefore challenged M. bovis BCG-preinfected mice with either the low dose (5 × 103), as described in Fig. 3b, or a 100-fold higher dose (5 × 105) of L. monocytogenes and compared the effects on L. monocytogenes clearance and IFN-γ production by CD8+ T cells.
As previously, PBS-injected mice allowed a greater L. monocytogenes expansion, whereas M. bovis BCG-infected mice strongly cleared (P < 0.05) the low-dose challenge of L. monocytogenes (Fig. 5a). Challenge of M. bovis BCG-infected mice with a 100-fold higher dose of L. monocytogenes resulted in a higher L. monocytogenes burden that was approximately similar (P = 0.32) to the L. monocytogenes burden in the spleens of PBS-injected mice. Furthermore, splenic IFN-γ production (on day 37) in response to LLO91-99 (Fig. 5b) was also restored. These results reveal a correlation between L. monocytogenes burden and T-cell priming.
FIG. 5.
Rapid clearance of L. monocytogenes by M. bovis BCG results in impairment in T-cell priming. BALB/c mice were injected with PBS or M. bovis BCG on day 1. On day 30, PBS-injected mice were challenged with 5 × 103 L. monocytogenes (LM), and the M. bovis BCG-injected mice were challenged with either 5 × 103 or 5 × 105 L. monocytogenes. Three days later (day 33), the number of viable L. monocytogenes organisms in the spleens of individual mice was enumerated (a). Dotted line indicates the threshold of detection. On day 37, spleen cells from the various experimental groups were incubated with medium (control) or with LLO91-99, and the production of IFN-γ was measured in 72-h supernatants (b). Means ± standard deviations for triplicate cultures are shown. Statistically significant values by Student's t test (P < 0.05) for PBS control mice versus M. bovis BCG-infected mice challenged with 5 × 103 L. monocytogenes (★) and for M. bovis BCG-infected mice challenged with 5 × 103 versus 5 × 105 L. monocytogenes (⋆).
Extent of T-cell memory development to L. monocytogenes correlates to the dose of L. monocytogenes.
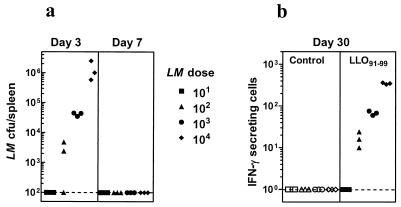
To further determine whether the dose of L. monocytogenes influences memory commitment, we injected mice with various doses of L. monocytogenes and measured L. monocytogenes persistence and T-cell memory development to LLO91-99. Figure 6a indicates that at day 3 after injection, the higher infection dose results in a higher bacterial load in the spleen. However, the persistence of L. monocytogenes in the spleen was short-lived, as no colonies were detectable in any of the groups at day 7. Increased L. monocytogenes burden during the initial period resulted in a correspondingly higher CD8+ T-cell memory (at day 30) as evidenced by higher numbers of LLO91-99-specific IFN-γ-secreting cells (Fig. 6b). This result further supports the notion that impairment in the expansion of L. monocytogenes during the initial period can limit T-cell priming.
FIG. 6.
Development of T-cell memory to L. monocytogenes correlates to the expansion of L. monocytogenes. BALB/c mice were infected with various numbers of L. monocytogenes (LM) as indicated in the figure. At days 3 and 7, the number of viable L. monocytogenes organisms in the spleens of individual mice in various experimental groups was enumerated (a). At day 30, the number of IFN-γ-secreting cells was enumerated after stimulating the spleen cells with medium (control) or with LLO91-99. The number of IFN-γ-secreting cells per 106 spleen cells in triplicates is indicated. The dotted line indicates the threshold of detection.
Impairment in the generation of T-cell memory compromises long-term protection.
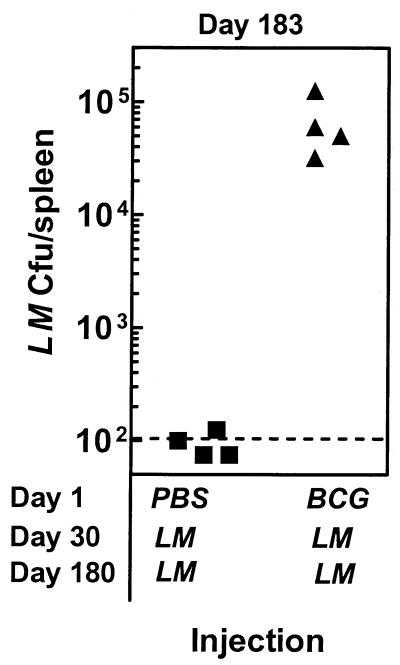
As memory T cells mediate long-term protection, we addressed the consequences of impairment in T-cell priming due to M. bovis BCG preimmunization. Mice were injected with PBS or M. bovis BCG and 30 days later challenged with L. monocytogenes. Mice were rechallenged on day 180 with L. monocytogenes. On day 183, spleens were removed and the number of viable L. monocytogenes was enumerated. Figure 7 shows that mice receiving a PBS injection on day 1 and L. monocytogenes injection on day 30 resisted a subsequent challenge with L. monocytogenes, and detectable colonies were noted in only one of four mice. On the other hand, mice that received an M. bovis BCG injection on day 1 and L. monocytogenes injection on day 30 had a considerable L. monocytogenes burden after a rechallenge with L. monocytogenes. These results indicate that the presence of strong inflammation before vaccination with a live immunogen can severely compromise long-term protection.
FIG. 7.
Impairment in the development of a T-cell response due to inflammation compromises long-term protection against a rechallenge with L. monocytogenes. BALB/c mice were injected with PBS or M. bovis BCG on day 1, and 30 days later mice were infected with L. monocytogenes (LM). On day 180, mice were rechallenged with L. monocytogenes, and the numbers of viable L. monocytogenes organisms in the spleens were enumerated at day 183. Each symbol represents data for an individual mouse. The dotted line indicates the threshold of detection.
Impairment in T-cell priming is selective for replicating immunogen.
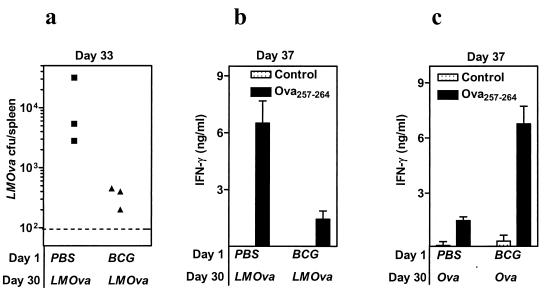
We determined whether the hyperactivated inflammatory response induced by M. bovis BCG influences CD8+ T-cell priming to immunogens that do not replicate. We therefore evaluated the effects of M. bovis BCG-induced inflammation on CD8+ T-cell priming towards OVA257-264 delivered in a replicating adjuvant system (L. monocytogenes expressing ovalbumin) or nonreplicating adjuvant system (ovalbumin-archaeosomes).
Challenge of M. bovis BCG-infected mice with L. monocytogenes-ovalbumin resulted in increased clearance of L. monocytogenes-ovalbumin (Fig. 8a) and consequently decreased OVA257-264-specific CD8+ T-cell response (Fig. 8b). Challenge of M. bovis BCG-infected mice with ovalbumin-archaeosomes, on the other hand, resulted in an increased CD8+ T-cell priming for OVA257-264 (Fig. 8c). Thus, M. bovis BCG-induced inflammation suppressed T-cell priming against a replicating immunogen, but not against a nonreplicating immunogen.
FIG. 8.
M. bovis BCG-induced inflammation has opposite effects on T-cell priming depending on the nature of the immunogen. C57BL/6 mice were injected with PBS or with M. bovis BCG. On day 30, mice were injected intraperitoneally with 5 × 105 L. monocytogenes-ovalbumin (LMOva) (a and b) or with 10 μg of ovalbumin-archaeosomes (Ova) (c). On day 33, the number of viable L. monocytogenes-ovalbumin in the spleens of individual mice was enumerated (a). Dotted line indicates the threshold of detection. On day 37, spleen cells were harvested and incubated with medium (control) or OVA257-264. The production of IFN-γ after injection with L. monocytogenes-ovalbumin (b) or with ovalbumin-archaeosomes (c) was measured in 72-h supernatants. Means ± standard deviations for triplicate cultures are shown.
DISCUSSION
Activation of T cells and generation of T-cell memory are important for vaccination. The development of a type 1 T-cell response characterized by IFN-γ production is crucial to the long-term eradication of various intracellular pathogens, including Leishmania major (44), Toxoplasma gondii (7), L. monocytogenes (14), and Mycobacterium tuberculosis (42). As multiple pathogens can infect susceptible hosts and the response to pathogens often involves inflammation, we determined the effects of preexisting inflammation on the development of T-cell responses to subsequent antigens.
Preimmunization of mice with M. bovis BCG has been shown to induce nonspecific protection against a challenge with L. monocytogenes (24, 34), which lasts only for a few months (34). Since this protection is nonspecific and does not last for a long time, it is conceivable that M. bovis BCG-induced memory T cells are not involved in mediating this cross-protection against L. monocytogenes. In fact, stimulation of the production of nitric oxide and inflammatory cytokines by M. bovis BCG was reported to be responsible for mediating rapid L. monocytogenes clearance (50). Furthermore, removal of macrophages from the M. bovis BCG-infected spleens abrogated this protection, indicating that inflammatory antigen-presenting cells, induced by M. bovis BCG, mediate accelerated clearance of L. monocytogenes (24). Our data support this interpretation, as increased numbers of inflammatory antigen-presenting cells were noted in M. bovis BCG-infected spleens.
The impact of inflammation on T-cell priming, even for replicating immunogens, may be largely governed by the relative timing between the generation of antigenic load and the induction of inflammation. In quiescent mice with no preexisting inflammation, vaccination with a replicating immunogen itself induces potent inflammation, but only after high antigen loads are already achieved. This would result in strong T-cell priming aided by the presence of inflammation (5, 18, 36, 37, 41). It is thus conceivable that the presence of inflammation before or after vaccination with a live immunogen would have contrasting effects on T-cell priming.
Since the extent of T-cell memory development is directly proportional to the intensity of primary response (28), the interplay between inflammation and the replicating immunogen may regulate T-cell activation and memory commitment. During the initial stages, minimal inflammation would allow substantial pathogen expansion resulting in the generation of high peptide levels sufficient to prime T cells. The progressive recruitment and activation of inflammatory cells (macrophages and NK cells) would restrict subsequent pathogen expansion and halt the generation of peptides.
Our data indicate that the development of potent CD8+ T-cell memory in the L. monocytogenes model correlates positively with the infection dose. This is true not only for LLO91-99-specific CD8+ T cells (Fig. 6); we have obtained similar results with OVA257-264-specific CD8+ T cells (data not shown). These results are consistent with the other reports on viral infection models, where viral dose correlates positively with T-cell memory development (21, 30, 31). The expansion of L. monocytogenes and the consequent induction of a detectable CD8+ T-cell memory by injection of 102 but not 10 L. monocytogenes organisms indicates a relatively low L. monocytogenes expansion threshold for induction of a detectable CD8+ T-cell memory. Thus, lack of any detectable CTL priming in M. bovis BCG-infected mice indicates a reduction in the expansion of L. monocytogenes well below these threshold levels.
Our results are in agreement with two reports which have addressed the influence of L. monocytogenes expansion on the generation of T-cell memory (25, 29). While both studies indicate that cessation of L. monocytogenes expansion by ampicillin impairs the generation of protective T cells, one study (29) suggests that protective memory T cells are generated continuously throughout the entire course of primary L. monocytogenes infection, whereas the other (25) indicates that priming occurs only within, but not after, the first 24 h of L. monocytogenes expansion. Our results are compatible with both of these studies, as the accelerated clearance of L. monocytogenes by M. bovis BCG preinfection is evident throughout the duration of primary L. monocytogenes infection, even within the first 24 h of L. monocytogenes infection (data not shown).
We have previously shown that IL-2 and IL-4 can impair responsiveness of CD8+ T cells (38, 40). IL-2 induces apoptosis in activated T cells (23) and IFN-γ can also restrict CD8+ T-cell expansion (1) and impair responsiveness of CD8+ T cells (S. Sad and T. R. Mosmann, unpublished observations). Recently, IL-6 has been reported to impair CD8+ T-cell responses (22). These inhibitory mechanisms may operate during M. bovis BCG infection and influence maintenance of T-cell responses.
We have addressed the effects specifically on T-cell priming, but not on maintenance of T-cell responses. We have not been able to observe any inherent suppressive activity in M. bovis BCG-infected spleens, as spleen cells from M. bovis BCG-infected mice fail to suppress cytokine production by antigen-specific CD4+ and CD8+ T cells (J.-P. Vasconcelos and S. Sad, manuscript in preparation). Furthermore, the restoration of T-cell priming against L. monocytogenes, in M. bovis BCG-infected mice, after challenge with a higher L. monocytogenes dose and enhancement of CD8+ T-cell priming against a nonreplicating immunogen (ovalbumin-archaeosomes) in M. bovis BCG-preimmunized mice, indicates that M. bovis BCG-induced suppression of T-cell priming against L. monocytogenes occurs mainly due to accelerated L. monocytogenes clearance.
It is worth noting that both L. monocytogenes-ovalbumin and ovalbumin-archaeosomes are particulate in nature and induce potent activation of dendritic cells and macrophages; however, the induction of CD8+ T-cell response to the former but not the latter is highly dependent on the growth of the immunogen. Thus, the same inflammatory environment can have completely different outcomes depending on the nature of the immunogen.
For evaluation of CD4+ T-cell responses, spleen cells were stimulated with the exogenous soluble L. monocytogenes antigen, as exogenous soluble antigens gain access mainly to the MHC class II processing pathway (2). Further, stimulation of spleen cells with the exogenous soluble L. monocytogenes antigen preparation has been shown to stimulate CD4+ but not CD8+ T cells in vitro (45). Although it is possible that other non-T cells may produce IFN-γ during such stimulation, the proportion of IFN-γ produced by non-CD4+ T cells at low antigen concentrations is minimal (our unpublished results). On the other hand, stimulation of spleen cells with the CTL epitope of listeriolysin has been shown to selectively induce cytokine production only by the CD8+ T cells, as peptide 91-99 is H-2Kd restricted (32). Similarly, cytolytic activity of spleen cells towards LLO91-99 has been shown to be mediated by CD8+ T cells (32). Furthermore, depletion of CD8+ T cells abrogates CTL activity towards LLO91-99 (our unpublished results).
Hyperactivated inflammation may benefit the host in the short term, as the proliferation of subsequent heterologous pathogens is restricted. However, since T-cell priming is impaired, long-term protection against the same heterologous pathogens may be compromised. The influence of inflammation on T-cell priming described in this study may have implications when one considers vaccination in areas of endemic chronic infections. A preexisting proinflammatory immune status induced in the host, particularly in areas of endemic chronic infections, may compromise the efficacy of live vaccine immunogens by inhibiting growth and T-cell priming.
Our results thus provide some scientific understanding for the long-held clinical practice of avoiding vaccinations during fever associated with ongoing infections. Further, our data also suggest that preexisting inflammation may be beneficial for vaccination with subunit or inert vaccines, implying that preexisting inflammation can be exploited to facilitate T-cell priming and hence protection.
Acknowledgments
We acknowledge from our institute the generous gift of ovalbumin-archaeosomes by Dennis Sprott. Synthesis of peptides by Gordon Willick is also appreciated. We also thank Joao Pedras-Vasconcelos for helpful discussions and critical reading of the manuscript.
Editor: R. N. Moore
Footnotes
This is publication no. 42444 from NRC.
REFERENCES
- 1.Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T-cell homeostasis by perforin and interferon-gamma. Science 290:1354-1358. [DOI] [PubMed] [Google Scholar]
- 2.Bevan, M. J. 1995. Antigen presentation to cytotoxic T lymphocytes in vivo. J. Exp. Med. 182:639-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunt, L. M., D. A. Portnoy, and E. R. Unanue. 1990. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J. Immunol. 145:3540-3546. [PubMed] [Google Scholar]
- 4.Dunn, P. L., and R. J. North. 1991. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect. Immun. 59:2892-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkins, K. L., J. T. Rhinehart, S. Stibitz, J. S. Conover, and D. M. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. [PubMed] [Google Scholar]
- 6.Flesch, I. E., J. H. Hess, S. Huang, M. Aguet, J. Rothe, H. Bluethmann, and S. H. Kaufmann. 1995. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon gamma and tumor necrosis factor alpha. J. Exp. Med. 181:1615-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 8.Gros, P., E. Skamene, and A. Forget. 1981. Genetic control of natural resistance to Mycobacterium bovis (M. bovis BCG) in mice. J. Immunol. 127:2417-2421. [PubMed] [Google Scholar]
- 9.Harty, J. T., and M. J. Bevan. 1992. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J. Exp. Med. 175:1531-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harty, J. T., R. D. Schreiber, and M. J. Bevan. 1992. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc. Natl. Acad. Sci. USA 89:11612-11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harty, J. T., A. R. Tvinnereim, and D. W. White. 2000. CD8+ T-cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275-308. [DOI] [PubMed] [Google Scholar]
- 12.Henderson, R. A., S. C. Watkins, and J. L. Flynn. 1997. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 159:635-643. [PubMed] [Google Scholar]
- 13.Hiromatsu, K., Y. Yoshikai, G. Matsuzaki, S. Ohga, K. Muramori, K. Matsumoto, J. A. Bluestone, and K. Nomoto. 1992. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J. Exp. Med. 175:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh, C. S., S. E. Macatonia, C. S. Tripp, S. F. Wolf, A. O'Garra, and K. M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260:547-549. [DOI] [PubMed] [Google Scholar]
- 15.Kagi, D., B. Ledermann, K. Burki, H. Hengartner, and R. M. Zinkernagel. 1994. CD8+ T-cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur. J. Immunol. 24:3068-3072. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann, S. H. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 17.Kramnik, I., D. Radzioch, and E. Skamene. 1994. T-helper 1-like subset selection in Mycobacterium bovis bacillus Calmette-Guerin-infected resistant and susceptible mice. Immunology 81:618-625. [PMC free article] [PubMed] [Google Scholar]
- 18.Krieg, A. M. 2000. The role of CpG motifs in innate immunity. Curr. Opin. Immunol. 12:35-43. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan, L., C. J. Dicaire, G. B. Patel, and G. D. Sprott. 2000. Archaeosome vaccine adjuvants induce strong humoral, cell-mediated, and memory responses: comparison to conventional liposomes and alum. Infect. Immun. 68:54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan, L., S. Sad, G. B. Patel, and G. D. Sprott. 2000. Archaeosomes induce long-term CD8+ cytotoxic T-cell response to entrapped soluble protein by the exogenous cytosolic pathway, in the absence of CD4+ T-cell help. J. Immunol. 165:5177-5185. [DOI] [PubMed] [Google Scholar]
- 21.Kundig, T. M., M. F. Bachmann, S. Oehen, U. W. Hoffmann, J. J. Simard, C. P. Kalberer, H. Pircher, P. S. Ohashi, H. Hengartner, and R. M. Zinkernagel. 1996. On the role of antigen in maintaining cytotoxic T-cell memory. Proc. Natl. Acad. Sci. USA 93:9716-9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La, F. A., A. S. MacDonald, and E. J. Pearce. 2000. Role of IL-6 in directing the initial immune response to schistosome eggs. J. Immunol. 164:2419-2426. [DOI] [PubMed] [Google Scholar]
- 23.Lenardo, M. J. 1991. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature 353:858-861. [DOI] [PubMed] [Google Scholar]
- 24.Mackaness, G. B. 1969. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J. Exp. Med. 129:973-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T-cell populations responding to bacterial infection. J. Immunol. 165:6833-6839. [DOI] [PubMed] [Google Scholar]
- 26.Moore, M. W., F. R. Carbone, and M. J. Bevan. 1988. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 54:777-785. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann, T. R., and T. A. Fong. 1989. Specific assays for cytokine production by T cells. J. Immunol. Methods 116:151-158. [DOI] [PubMed] [Google Scholar]
- 28.Murali, K. K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 29.North, R. J., P. A. Berche, and M. F. Newborg. 1981. Immunologic consequences of antibiotic-induced abridgement of bacterial infection: effect on generation and loss of protective T cells and level of immunologic memory. J. Immunol. 127:342-346. [PubMed] [Google Scholar]
- 30.Ochsenbein, A. F., U. Karrer, P. Klenerman, A. Althage, A. Ciurea, H. Shen, J. F. Miller, J. L. Whitton, H. Hengartner, and R. M. Zinkernagel. 1999. A comparison of T-cell memory against the same antigen induced by virus versus intracellular bacteria. Proc. Natl. Acad. Sci. USA 96:9293-9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oehen, S., H. Waldner, T. M. Kundig, H. Hengartner, and R. M. Zinkernagel. 1992. Antivirally protective cytotoxic T-cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J. Exp. Med. 176:1273-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pamer, E. G., J. T. Harty, and M. J. Bevan. 1991. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature 353:852-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, S. F., and G. S. Stewart. 1990. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 34.Pelletier, M., A. Forget, D. Bourassa, P. Gros, and E. Skamene. 1982. Immunopathology of M. bovis BCG infection in genetically resistant and susceptible mouse strains. J. Immunol. 129:2179-2185. [PubMed] [Google Scholar]
- 35.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 37.Reis, A. Sher, and P. Kaye. 1999. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr. Opin. Immunol. 11:392-399. [DOI] [PubMed] [Google Scholar]
- 38.Sad, S., and L. Krishnan. 1999. Cytokine deprivation of naive CD8+ T cells promotes minimal cell cycling but maximal cytokine synthesis and autonomous proliferation subsequently: a mechanism of self-regulation. J. Immunol. 163:2443-2451. [PubMed] [Google Scholar]
- 39.Sad, S., R. Marcotte, and T. R. Mosmann. 1995. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity 2:271-279. [DOI] [PubMed] [Google Scholar]
- 40.Sad, S., and T. R. Mosmann. 1995. Interleukin (IL) 4, in the absence of antigen stimulation, induces an anergy-like state in differentiated CD8+ TC1 cells: loss of IL-2 synthesis and autonomous proliferation but retention of cytotoxicity and synthesis of other cytokines. J. Exp. Med. 182:1505-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scharton, T. M., and P. Scott. 1993. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T-cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 178:567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott, P., and S. H. Kaufmann. 1991. The role of T-cell subsets and cytokines in the regulation of infection. Immunol. Today 12:346-348. [DOI] [PubMed] [Google Scholar]
- 43.Sprent, J., and D. F. Tough. 1994. Lymphocyte life-span and memory. Science 265:1395-1400. [DOI] [PubMed] [Google Scholar]
- 44.Sypek, J. P., C. L. Chung, S. E. Mayor, J. M. Subramanyam, S. J. Goldman, D. S. Sieburth, S. F. Wolf, and R. G. Schaub. 1993. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J. Exp. Med. 177:1797-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szalay, G., C. H. Ladel, and S. H. Kaufmann. 1995. Stimulation of protective CD8+ T lymphocytes by vaccination with nonliving bacteria. Proc. Natl. Acad. Sci. USA 92:12389-12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unanue, E. R. 1997. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr. Opin. Immunol. 9:35-43. [DOI] [PubMed] [Google Scholar]
- 47.Vidal, S., M. L. Tremblay, G. Govoni, S. Gauthier, G. Sebastiani, D. Malo, E. Skamene, M. Olivier, S. Jothy, and P. Gros. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vijh, S., and E. G. Pamer. 1997. Immunodominant and subdominant CTL responses to Listeria monocytogenes infection. J. Immunol. 158:3366-3371. [PubMed] [Google Scholar]
- 49.White, D. W., and J. T. Harty. 1998. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-gamma but requires TNF-alpha. J. Immunol. 160:898-905. [PubMed] [Google Scholar]
- 50.Yang, J., I. Kawamura, and M. Mitsuyama. 1997. Involvement of inflammatory cytokines and nitric oxide in the expression of nonspecific resistance to Listeria monocytogenes in mice induced by viable but not killed Mycobacterium bovis BCG. Microb. Pathog. 22:79-88. [DOI] [PubMed] [Google Scholar]
- 51.Zinkernagel, R. M. 1996. Immunology taught by viruses. Science 271:173-178. [DOI] [PubMed] [Google Scholar]